Abstract
A prominent theory of immune senescence holds that repeated antigenic stimulation and decreased production of naive cells combine to progressively exhaust the reserve of lymphocytes available to fight new pathogens, culminating in an accumulation of lymphocytes that achieved replicative senescence. A well-defined primate model of immune senescence in vivo would greatly facilitate testing of this theory. Here, we investigated phenotypic and functional T-cell aging in the rhesus macaques (RMs), currently the dominant primate model of AIDS. Our results show that sharp differences exist between the CD8 and CD4 T-cell subsets in (1) cell-cycle programs (as assessed by both in vitro proliferation and in vivo turnover measurement); (2) CD28 regulation on cell-cycle entry; and (3) accumulation of immediate effector cells among the CD28– cells, believed to be close to or at replicative senescence. These results further suggest poor reliability of CD28 as a marker for senescence. We suggest that some of the T-cell aging phenomenology in RMs can be ascribed to accentuation over time of the inherent differences in activation programs in CD8 and CD4 T cells.
Introduction
During aging, the immune system undergoes dramatic changes in both structure and function. Macroscopically, the most evident event is the involution of the thymus, which heavily diminishes the production of naive T cells. Consequently, peripheral lymphocyte subset composition gets shifted toward the memory phenotype, as an increasing proportion of naive T cells become exposed to foreign antigens (Ag's) over time. T-cell–receptor (TCR) diversity of the aging memory T-cell population is also frequently restricted as homeostatic mechanisms that maintain diverse clonal composition of the T-cell repertoire progressively break down.1-3 Findings suggest that this loss of T-cell homeostasis, reflected by a significant increase in CD8 T-cell numbers, an inverted CD4/CD8 ratio, and reduced T-cell diversity,4-6 is associated with higher mortality in the aging human.4 Indeed, clinical evidence clearly indicates the deterioration of T-cell immunity in the elderly.1 Moreover, numerous studies have demonstrated impaired T-cell activation, and findings have precisely defined multiple defects in TCR-mediated signaling in senescent rodent T cells.7-10 However, the mechanisms linking specific age-related changes in T-cell–subset distribution and function to the age-related immunodeficiency are still incompletely understood.
Most of the existing data describing the age-related changes in T-cell function come from studies of the rodent immune system. Although these models have been invaluable in elucidating various fundamental facets of the immune system, including its (dys)regulation in aging, there are reminders that the results from rodent models do not automatically translate to humans. This lack of translation includes failure in humans of vaccine approaches that were validated in rodents.11 Moreover, in the specific case of aging, there are additional overriding reasons for which rodent models are likely to imperfectly approximate the human situation. First, the span of chronologic aging is widely different between rodents and humans (differences in median and maximal life span between mice/rats and humans are ∼30-fold). Second, inbred laboratory rodents are maintained under specific pathogen-free (SPF) conditions that are very different from the microbial environment that shapes the state of activation of the outbred human immune system. Finally, evolutionary tables teach us that rodents and humans diverged from the common ancestor approximately 210 million years ago.12,13
The nonhuman primate (NHP) model scores much better on all of these points: difference in life spans is less than 3-fold, outbred genetics and exposure to environmental pathogens are comparable to humans, and an extremely close evolutionary relationship exists with an approximate divergence of 30 million years between the old-world monkeys and humans. Moreover, shared markers of T-cell phenotype differentiation and function14 between NHPs and humans further indicate that NHPs should represent highly desirable models for immunogerontologic studies, with direct relevance for human T-cell senescence. Moreover, validation in NHPs of the observations in rodents would provide a stringent, “real-life” test for such observations. With that in mind, we set out to develop and validate a comprehensive map of phenotypic and functional aging of the immune system in rhesus macaques (RMs).
We used CD95 and CD28 as the most consistent surface antigenic markers of end-stage memory differentiation in humans15 and RMs14 to analyze the representation and function of naive and memory-phenotype T cells over the entire life span of RMs. Overall, we found that the pattern of age-related T-cell changes was remarkably different in CD4 and CD8 subsets. These results highlight fundamental differences in the biology of the 2 T-cell lineages as one of the underlying causes for changes observed in immune senescence, and possibly in immune exhaustion, and also urge caution in using CD28 status as a marker for replicative senescence.
Materials and methods
Animals
All animals used in this study were colony-bred RMs (Macaca mulatta) of Indian origin maintained and used in accordance with guidelines of the Animal Care and Use Committee at the Oregon National Primate Research Center and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Healthy animals of either sex were selected to represent the life span of the RM. Groups included 6 neonates (mean age, 1 day; range, 1 day), 4 juveniles (mean, 3 years; range, 2.0-4.4 years), 7 adult (mean, 6.9 years; range, 5.5-9.3 years), and 8 old (mean, 20.5 years; range, 15.4-24.7 years) animals. No systematic sex differences were observed among the animals for the parameters analyzed. Tissues were obtained from animals undergoing necropsy, through the Oregon National Primate Research Center's Tissue Distribution Program. Animals with tumors or verifiable disease were excluded from the study. A group of 14 animals (aged 6-34 years) used for the in vivo 5-bromodeoxyuridine (BrdU)–labeling experiments partially overlapped with a cohort used for T-cell phenotype and in vitro functional studies.
Cell preparation
Splenocytes were obtained by mechanical dissociation, resuspended in complete medium (CM; RPMI 1640; Gibco BRL [Grand Island, NY], supplemented with l-glutamine, 10% heat-inactivated fetal calf serum [FCS], and penicillin and streptomycin at 100 μg/mL each), and red blood cells (RBCs) were lysed using NH4Cl. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by density gradient sedimentation using Ficoll-Hypaque (Histopaque-1077; Sigma-Aldrich, St Louis, MO).
CD28+ cells were isolated using the magnetic cell sorting (MACS) system (Miltenyi Biotec, Bergisch-Gladbach, Germany) with MS columns and OctoMACS magnet per manufacturer's instructions. The resulting cells were more than 95% CD28+ as determined by flow cytofluorimetric (FCM) analysis with directly labeled monoclonal antibody (mAb).
CFSE labeling for analysis of cell division
Cells were labeled with 5,6-carboxyfluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) as previously described.16 Briefly, whole spleen cells or CD28+-purified cells were resuspended in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) (2 × 107 cells/mL), and incubated with CFSE at a final concentration of 2.5 μM for 10 minutes at 37°C. Unbound CFSE was quenched by adding an equal volume of FCS, and the cells were washed 2 times with PBS/10% FCS.
T-cell stimulation
For analysis of tumor necrosis factor α (TNF-α) production, splenocytes were stimulated at 4 × 105 cells/well in 96-well plates with a predetermined optimal concentration of plate bound anti-CD3 mAb (FN-18; Biosource International, Camarillo, CA) in 200 μL complete medium. The cultures were incubated at 37°C in a humidified atmosphere for 6 hours in the presence of 5 μg/mL brefeldin A (Sigma-Aldrich) and stained and analyzed as described in “Immunofluorescent staining and FCM analysis.”
To track cell division or modulation of CD28 surface expression on dividing cells, CFSE-labeled whole spleen cells (4 × 105/well) or CD28+-enriched cells (2 × 105/well) were incubated with plate-bound anti-CD3 mAb for 96 hours, in 200 μL complete media alone, or in the presence of soluble anti-CD28 mAb (no. 555726; BD PharMingen, San Jose, CA) as indicated and analyzed by FCM. Cells cultured in the absence of anti-CD3 exhibited no spontaneous proliferation.
Immunofluorescent staining and flow cytofluorimetric (FCM) analysis
For cell surface staining, 0.5 to 1 × 106 cells were incubated with predetermined concentrations of fluorochrome-conjugated mAbs CD3-FL (no. 556611), CD8b-phycoerythrin (PE; no. PNIM2217), CD4-peridinin chlorophyll protein (PerCP; no. 550631), CD28-PE (no. 55729), CD28-CyChrome (no. 555730), or CD95-allophycocyanin (APC; no. 558814) from BD PharMingen. Following 25 minutes of incubation on ice, cells were washed in Hanks balanced salt solution (HBSS; Ca2+/Mg2+-free; Gibco), and resuspended in 200 μL of 1% paraformaldehyde in PBS.
For intracellular cytokine staining (ICS), 1 to 2 × 106 cells were first incubated with directly conjugated mAbs to surface T-cell markers CD4, CD8, CD28, or CD95. After incubation (25 minutes at 4°C) cells were washed (PBS/5% FCS), permeabilized (Cytofix-Cytoperm kit; BD PharMingen), and incubated with anti–TNF-α–APC (no. 553514; BD PharMingen). Following washing, cells were fixed (2% paraformaldehyde/PBS) and analyzed by FCM.
For BrdU analysis, cells were stained for surface markers, fixed (10 seconds at room temperature in FASCLyse solution; BD Biosciences), and then permeabilized for 12 to 14 hours in fixation/permeabilization solution (BD Biosciences, San Jose, CA) at 4°C. After washing (cold PBS/0.1% BSA), cells were incubated on ice (protected from light) with directly conjugated mAbs specific for BrdU and Ki-67 in the presence of 0.28 mg bovine pancreas-derived DNase 1 (Sigma-Aldrich) for 30 minutes. After staining, cells were resuspended in 2% paraformaldehyde in PBS.
FCM analysis was performed using the FACSCalibur instrument equipped with CellQuest software (BD Biosciences). CD4 and CD8 T-cell subsets were analyzed based on live lymphocyte light scatter profile and surface expression of CD3, CD4, and CD8β molecules. CD8, CD4, CD8+/CD28–, CD4+/CD28–, CD4+/CD28+, and CD8+/CD28+ were analyzed using one-way analysis of variance (ANOVA). Homogeneity of variances was tested using the Levene test, and pairwise comparisons of groups were performed using the Tukey-Kramer method when the homogeneity of variances was indicated and the Games-Howell method when the homogeneity of variances was not indicated. P ≤ .05 is considered statistically significant.
Results
Accumulation of memory-phenotype CD8+ T cells with immediate effector function in aged RMs
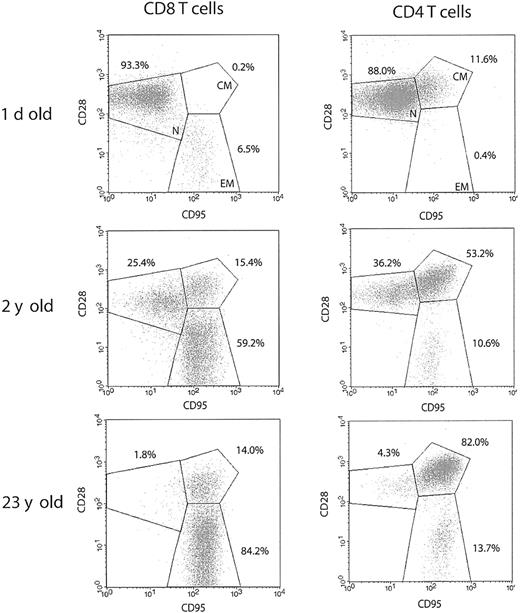
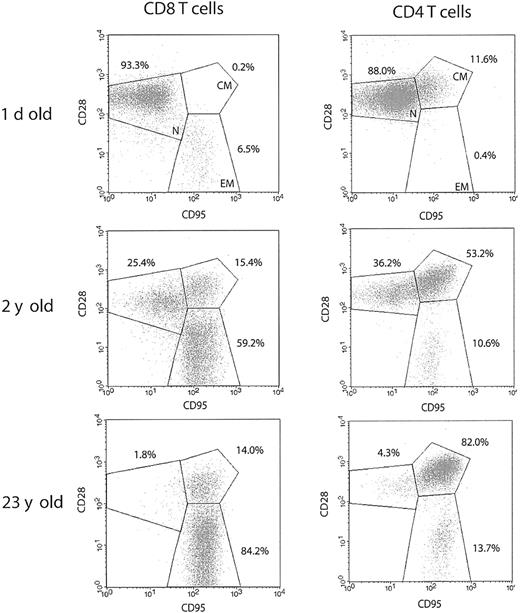
To investigate population dynamics of T-lymphocyte subsets in the course of RM aging, we analyzed splenic T-cell phenotypes by 4-color FCM. Animals of different age groups, representing the whole life span of RMs, were selected to perform a cross-sectional analysis of T-cell phenotype and effector function during the period of postnatal development, as well as in the course of advanced aging. To define distinct T-cell subsets, we used surface expression of CD95 and CD28, known to most consistently delineate naive and memory T-cell subsets in both human and the RM14,17 : naive cells were identified by intermediate to high expression of CD28 and a lack of CD95; memory phenotype T cells acquire surface expression of CD95, and can further be divided into CD95hiCD28high central-memory cells, and CD95hiCD28– effector-memory cells, hypothesized to be terminally differentiated.
Typical FCM profiles for the 3 RM age groups (Figure 1) illustrate that, although the neonatal spleen predictably contained mostly naive, CD95– cells, such cells were rapidly lost in the postnatal period, with a shift toward the dominance of CD95high memory cells (Figures 1 and 2A-B). At the population level, a sharp increase in the proportion of CD95high T cells was seen during the first 5 years of life, followed by further accumulation during aging, eventually taking up more than 90% of both the splenic CD4 and CD8 T-cell compartments (Figure 2A-B) (differences were most marked between neonates and juvenile animals and between juveniles and old [Figure 1]; less marked accumulation between adulthood and old age is shown for the whole cohort in Figure 2). This parallel acquisition of CD95 in CD4 and CD8 T cells could be due to similar kinetics of major histocompatibility complex (MHC) class I and class II antigenic exposure, or to other, presently unknown factors. The above data agree with previously published results obtained on RM peripheral blood lymphocytes (PBLs),14 underscoring an extremely early transition toward a memory phenotype in RMs, imposing a question of the functional potential of the above subsets.
Rapid postnatal development of T-cell memory and loss of naive T-cell phenotype during aging differs between CD8 and CD4 T cells in the rhesus macaque. Representative FCM dot plots of naive (CD95lowCD28int./high; N), centralmemory (CD95highCD28high; CM), and effector-memory (CD95highCD28low; EM) T-cell phenotype distribution. Spleen cells were stained with FITC-conjugated CD3, PE-conjugated CD8β, or PerCP-conjugated CD4, PE- or CyC-conjugated CD28, and APC-conjugated CD95 mAbs. Gates were set on CD3+CD8+ or CD3+CD4+ cells as indicated. The percentage values represent the proportions of total CD8 and CD4 T-cells, respectively. In both CD4 and CD8 T-cell subsets, the percentage of naive cells declines profoundly during the early postnatal period, as demonstrated for a 2-year-old animal. Naive cells are almost completely depleted during advanced aging in both T-cell subsets, as illustrated by a representative profile from a 23-year-old animal. It is important to note that within the total CD95high memory population, CD28high CM cells predominate in CD4, whereas CD28– EM cells predominate in CD8 T cells.
Rapid postnatal development of T-cell memory and loss of naive T-cell phenotype during aging differs between CD8 and CD4 T cells in the rhesus macaque. Representative FCM dot plots of naive (CD95lowCD28int./high; N), centralmemory (CD95highCD28high; CM), and effector-memory (CD95highCD28low; EM) T-cell phenotype distribution. Spleen cells were stained with FITC-conjugated CD3, PE-conjugated CD8β, or PerCP-conjugated CD4, PE- or CyC-conjugated CD28, and APC-conjugated CD95 mAbs. Gates were set on CD3+CD8+ or CD3+CD4+ cells as indicated. The percentage values represent the proportions of total CD8 and CD4 T-cells, respectively. In both CD4 and CD8 T-cell subsets, the percentage of naive cells declines profoundly during the early postnatal period, as demonstrated for a 2-year-old animal. Naive cells are almost completely depleted during advanced aging in both T-cell subsets, as illustrated by a representative profile from a 23-year-old animal. It is important to note that within the total CD95high memory population, CD28high CM cells predominate in CD4, whereas CD28– EM cells predominate in CD8 T cells.
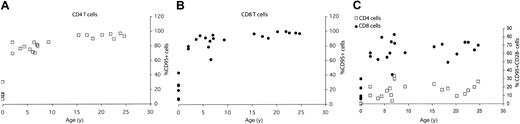
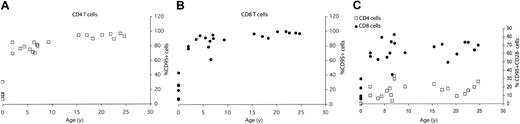
Age-related acquisition of CD95high (memory T cell) phenotype is similar in both CD4 and CD8 cells, but CD95highCD28– subpopulation accumulates with age only in the CD8 T-cell subset. The percentage of total splenic T cells within CD4 (A) and CD8 (B) T-cell subset expressing CD95high memory phenotype or CD95highCD28– effector-memory phenotype (C) was determined from FCM staining profiles as shown in Figure 1, and values were plotted against the age for each animal.
Age-related acquisition of CD95high (memory T cell) phenotype is similar in both CD4 and CD8 cells, but CD95highCD28– subpopulation accumulates with age only in the CD8 T-cell subset. The percentage of total splenic T cells within CD4 (A) and CD8 (B) T-cell subset expressing CD95high memory phenotype or CD95highCD28– effector-memory phenotype (C) was determined from FCM staining profiles as shown in Figure 1, and values were plotted against the age for each animal.
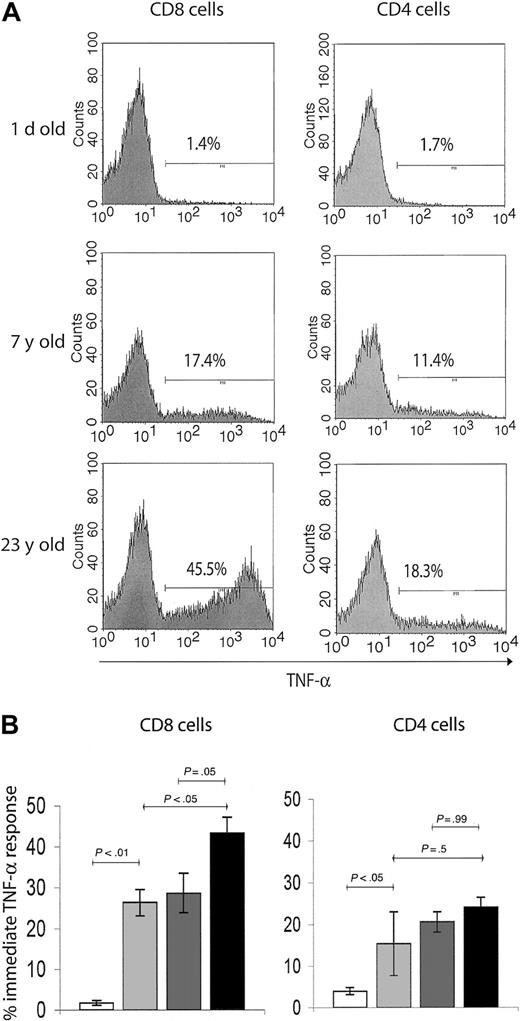
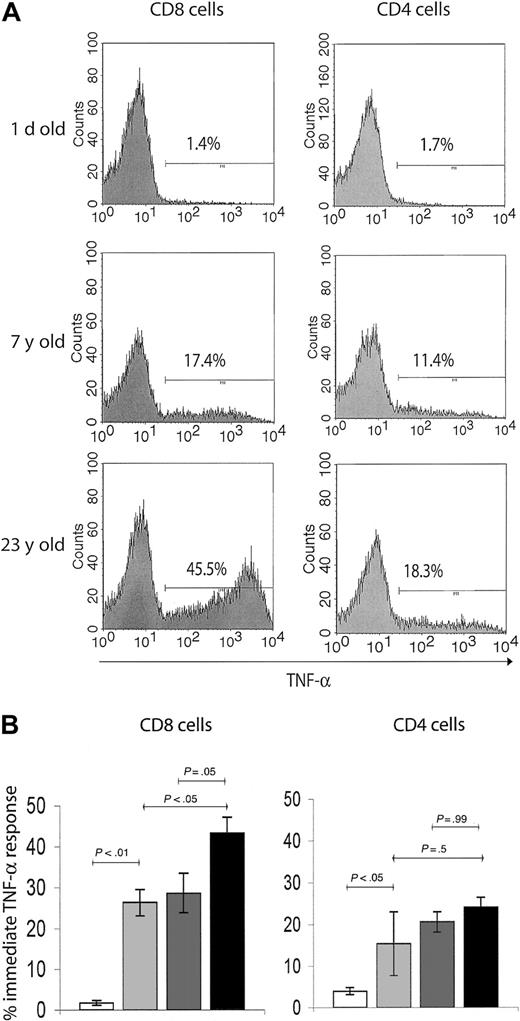
We, therefore, investigated functional hallmarks of T-cell memory by evaluating immediate effector responses of the above subsets to TCR-mediated activation in a costimulation-independent manner. Although the immediate effectors are known to entirely belong to the CD95high T-cell subset8 (not shown), they represent only a fraction of this population. To determine the age-related accumulation of immediate effector T cells within the total memory pool, we used brief ex vivo anti-CD3–mediated stimulation, which provides a strong activating signal to all memory/effector T cells. The capacity of T cells to produce intracellular TNF-α (the most stable and reliable correlate of effector immunity in the RM14 ) on such activation was used as an assay of immediate effector function. Again, neonatal spleens predictably had very few cells capable of immediate effector function (Figure 3A-B). The percentage of TNF-α–secreting cells increased more than 10-fold in the juvenile, compared with the neonate RMs, reaching somewhat higher levels in CD8 than in CD4 cells (Figure 3). Still, less than 33% of all CD95+ cells were immediate effectors in juvenile animals, and that percentage did not change between juveniles and adults. Therefore, although the initial increase in cells capable of immediate effector function coincided with the initial acquisition of the memory phenotype after postnatal exposure to pathogens, most memory-phenotype cells had no immediate effector function. But marked differences were found between the adulthood and the old age cells and also between CD8 and CD4 cells (Figure 3). In the CD4 T-cell subset, immediate effectors, which accumulated early in the postnatal period, did not appreciably increase in representation during advanced aging (Figure 3). Meanwhile, the proportion of TNF-α+ CD8 T cells gradually accumulated, reaching significantly higher levels in the old (> 15 years) compared with juvenile or adult RMs (Figure 3). Therefore, the representation of CD8 immediate effectors T cells within the memory T-cell population specifically increased with aging.
T cells with effector-memory function selectively accumulate in the CD8 T-cell subset during advanced aging. (A) Representative FCM histograms of TNF-α ICS in splenic T cells on 6-hour in vitro anti-CD3 stimulation. Cells were surface-stained with CD8β and CD4 mAbs, and gates were set on CD4+ or CD8+ cells as indicated. Background levels of TNF-α–producing cells in nonstimulated control cultures were below detection. A neonate (1 day old), young adult (7 years old), and an aging (23 years old) animal were chosen to illustrate significant age-related changes in TNF-α response, which served as a readout of effector-memory T-cell function. Percentage of TNF-α+ cells was ∼10-fold higher in the adult compared with the neonate animal. Although both the adult and the old subject had comparable levels of TNF-α+ cells in the CD4 subset, there was an evidently higher percentage of TNF-α+ CD8 T cells in the aging animal compared with the young adult animal. (B) Bar graphs represent mean ± SEM values for percentage of TNF-α+ cells from animals pooled in 4 age groups, in CD8 (left panel) and CD4 (right panel) subsets as indicated. Statistical significance (P value) was determined by using the ANOVA test. White bar indicates neonate (1 day old); light gray, juvenile (5 years old); dark gray, adult (5-10 years old); and black, old (aged 15 years or more).
T cells with effector-memory function selectively accumulate in the CD8 T-cell subset during advanced aging. (A) Representative FCM histograms of TNF-α ICS in splenic T cells on 6-hour in vitro anti-CD3 stimulation. Cells were surface-stained with CD8β and CD4 mAbs, and gates were set on CD4+ or CD8+ cells as indicated. Background levels of TNF-α–producing cells in nonstimulated control cultures were below detection. A neonate (1 day old), young adult (7 years old), and an aging (23 years old) animal were chosen to illustrate significant age-related changes in TNF-α response, which served as a readout of effector-memory T-cell function. Percentage of TNF-α+ cells was ∼10-fold higher in the adult compared with the neonate animal. Although both the adult and the old subject had comparable levels of TNF-α+ cells in the CD4 subset, there was an evidently higher percentage of TNF-α+ CD8 T cells in the aging animal compared with the young adult animal. (B) Bar graphs represent mean ± SEM values for percentage of TNF-α+ cells from animals pooled in 4 age groups, in CD8 (left panel) and CD4 (right panel) subsets as indicated. Statistical significance (P value) was determined by using the ANOVA test. White bar indicates neonate (1 day old); light gray, juvenile (5 years old); dark gray, adult (5-10 years old); and black, old (aged 15 years or more).
Emergence of the CD28– T-cell phenotype and of the immediate TNF-α function differs sharply between RM CD4 and CD8 T cells
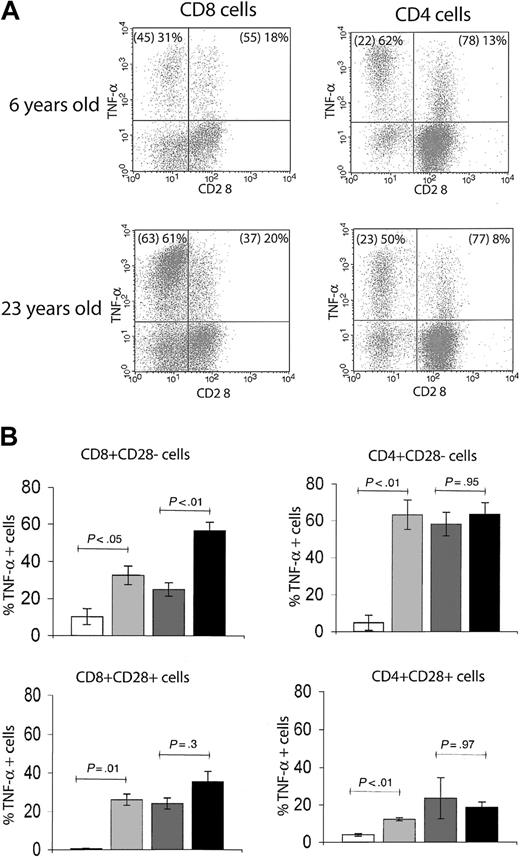
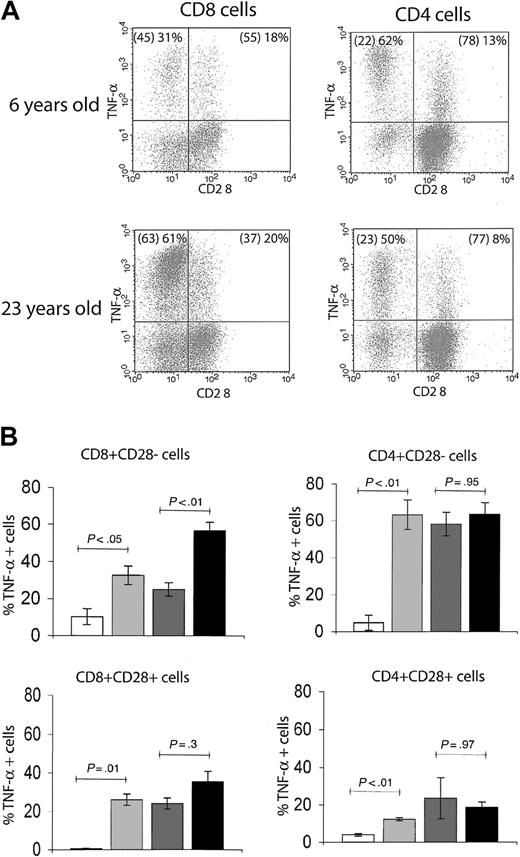
During aging, the emergence of cells within the memory T-cell pool that have lost surface CD28 expression has been extensively documented in humans18-21 and recently reported in RMs.14 In our cohort, we detected an age-related increase in the percentage of CD8+CD95hiCD28– effector-memory T cells, reaching a plateau of between 50% and more than 80% total CD8 T cells in early adulthood. Although CD4+CD95hiCD28– cells also increased in the first couple of years14 (Figure 2C), their plateau was far lower (10%-30% of total CD4 T cells) (Figure 2C). Moreover, immediate effector function within the CD28+ and CD28– phenotypic subsets differed with age between CD8 and CD4 cells (Figure 4). Although relatively infrequent, the CD4+ CD28– cells exhibited a high level of immediate effector function irrespective of age: in both young adult and old RMs, nearly 60% of this population scored as TNF-α+ on TCR ligation at all chronologic ages (Figure 4). By contrast, association of the CD8+CD28– phenotype with the TNF-α effector response increased with age: the proportion of immediate effectors rose from around 30% in juvenile and adult to more than 60% in old animals (Figure 4A-B). We also noted that among both the CD4 and CD8 cells, a fraction of CD28+ lymphocytes produced TNF-α in response to stimulation; however, that fraction did not significantly change with age (Figure 4B). At the present, the relationship between these cells and the CD28– fraction is unclear. Several reports have associated the loss of CD28 surface expression with an armed effector/memory T-cell phenotype, responsible primarily for rapid recall response.14,19,22 Our findings suggest that this connection of surface phenotype and TNF-α–secreting function as a measure of armed effector/memory status is not uniform in CD4 and CD8 T cells, and that in CD8 cells it becomes significantly more pronounced during aging, at least under the conditions examined here.
The association of CD28– T-cell phenotype with in vitro effector-memory functional response differs between CD4 and CD8 subsets. (A) Representative FCM dot plots of ICS for TNF-α in CD28– versus CD28+ populations. The percentages of total CD4 or CD8 T cells with CD28– and CD28+ phenotype are given in parentheses, followed by the proportion of the respective population that is positive for TNF-α. In both young adult (6 years old) and old (23 years old) animals, there was a larger proportion of TNF-α+ effector-memory cells within CD28– than CD28+ population. Within the CD4+CD28– T cells, the distribution of TNF-α+ cells was comparable between the adult and the old animal; by contrast, in CD8+CD28– T cells, the proportion of TNF-α+ cells was significantly higher in the old animal. This observation held for the entire cohort, as shown in panel B. (B) Bar graphs represent mean ± SEM values for percentages of TNF-α+ T cells with CD28– or CD28+ phenotype from animals pooled in 4 age groups. Statistical significance (P value) was determined as in Figure 3. Shading key is the same as in Figure 3.
The association of CD28– T-cell phenotype with in vitro effector-memory functional response differs between CD4 and CD8 subsets. (A) Representative FCM dot plots of ICS for TNF-α in CD28– versus CD28+ populations. The percentages of total CD4 or CD8 T cells with CD28– and CD28+ phenotype are given in parentheses, followed by the proportion of the respective population that is positive for TNF-α. In both young adult (6 years old) and old (23 years old) animals, there was a larger proportion of TNF-α+ effector-memory cells within CD28– than CD28+ population. Within the CD4+CD28– T cells, the distribution of TNF-α+ cells was comparable between the adult and the old animal; by contrast, in CD8+CD28– T cells, the proportion of TNF-α+ cells was significantly higher in the old animal. This observation held for the entire cohort, as shown in panel B. (B) Bar graphs represent mean ± SEM values for percentages of TNF-α+ T cells with CD28– or CD28+ phenotype from animals pooled in 4 age groups. Statistical significance (P value) was determined as in Figure 3. Shading key is the same as in Figure 3.
Proliferative response of RM T cells reveals fundamental differences between cycling properties of the CD4 and CD8 subsets
During aging, T cells undergo repeated antigen exposures, which lead to multiple rounds of clonal expansions. This process is thought to largely contribute to the functional senescence of T cells through gradual exhaustion of the T-cell replicative potential, a phenomenon equated by some researchers to replicative senescence of cultured T cells in vitro.23 We wondered whether the possible differences in the dynamics of cell division between CD4 and CD8 cells would correlate to the dominance of phenotypic and functional changes in the CD8 compartment. One possibility was that there are distinct proliferative and/or surface phenotype changes in response to TCR-mediated stimulation between the 2 lineages. To test this hypothesis we followed division patterns of the CD4 and CD8 T cells in anti-CD3–stimulated splenocyte cultures, using progressive dilution of fluorescent label to directly visualize the number of cell divisions and thereby track population dynamics on TCR stimulation in vitro.
As expected, in neonate cell cultures, containing predominantly naive T cells, there was very little proliferation in response to anti-CD3 stimulation in the absence of costimulatory signals (Figure 5). A swift increase in memory T-cell numbers in adult RMs (Figure 2) was predictably followed by a marked increase in the extent of cell division in response of adult T cells to anti-CD3. Interestingly, we consistently detected a higher number of divisions in CD8 than in CD4 cells among the splenocyte cultures from adult animals (Figure 5), and that difference remained in old (not shown) animals. Therefore, CD8 T cells appear to be programmed for more extensive clonal expansion in response to TCR stimulation than CD4 T cells. In addition, when anti-CD28 costimulatory antibody was included, naive neonate CD4 and CD8 T cells readily proliferated, with no difference between them (Figure 5, second panel from the left), indicating that differential programming of proliferative responses occurs postnatally, perhaps in conjunction with the acquisition of memory phenotype and/or with repeated stimulation. Meanwhile, adding anti-CD28 to TCR-stimulated adult T cells shifted the relative height distribution of CFSE division peaks to the left (indicating that more cells were leaving G0) but did not affect the difference in the extent of division between CD4 and CD8 subsets (Figure 5A). Therefore, the difference in number of cell divisions is determined by the response to TCR stimulation and is not substantially influenced by specific costimulatory requirements of CD4 and CD8 T cells.
In adult rhesus macaques with predominant memory T-cell phenotype, CD8 T cells divide more extensively than CD4 T cells in response to in vitro anti-CD3 stimulation. FCM histogram overlays represent CD4 (dotted line) and CD8 (solid line) T cell division profiles detected by dilution of the CFSE label. Splenocytes were cultured for 96 hours with immobilized anti-CD3 mAb alone or in the presence of soluble costimulatory anti-CD28 mAb. Cells were stained with CD4 and CD8β mAbs, and gates were set on CD4+ or CD8+ populations as indicated. Each of the distinct CFSE peaks represents cells that underwent a discrete number of divisions; height of individual peaks represents the relative numbers of cells that underwent that number of divisions. Of note, cell division mostly depended on CD28 costimulation in the neonate (1 day old) but not in the adult (6 years old) animal. Also, there was a higher number of cell divisions in CD8 cells compared with CD4 cells in the adult animal, irrespective of the presence of CD28 costimulation. These results are representative of 6 neonate and 8 adult (from 5 to 23 years old) animals analyzed in 3 independent experiments.
In adult rhesus macaques with predominant memory T-cell phenotype, CD8 T cells divide more extensively than CD4 T cells in response to in vitro anti-CD3 stimulation. FCM histogram overlays represent CD4 (dotted line) and CD8 (solid line) T cell division profiles detected by dilution of the CFSE label. Splenocytes were cultured for 96 hours with immobilized anti-CD3 mAb alone or in the presence of soluble costimulatory anti-CD28 mAb. Cells were stained with CD4 and CD8β mAbs, and gates were set on CD4+ or CD8+ populations as indicated. Each of the distinct CFSE peaks represents cells that underwent a discrete number of divisions; height of individual peaks represents the relative numbers of cells that underwent that number of divisions. Of note, cell division mostly depended on CD28 costimulation in the neonate (1 day old) but not in the adult (6 years old) animal. Also, there was a higher number of cell divisions in CD8 cells compared with CD4 cells in the adult animal, irrespective of the presence of CD28 costimulation. These results are representative of 6 neonate and 8 adult (from 5 to 23 years old) animals analyzed in 3 independent experiments.
Our complementary investigation of cell-cycle programs and homeostasis of CD4 and CD8 T-cell subsets in vivo, using 5-bromo-2-deoxy uridine, suggested that similar differences may exist in vivo, and that CD28– cells label and turn over at least as vigorously, if not faster, than their CD28+ counterparts, confirming the results of Pitcher et al14 and questioning the definition of replicative senescence of CD28– cells (I.M. et al, manuscript in preparation).
Selective cell division–linked down-modulation of CD28 surface expression in CD8 cells
The earlier described data show that the relation of functional aging to the CD28– surface phenotype is likely quite different for CD4 and CD8 T cells. We wanted to test whether distinct patterns of CD28 expression in the 2 subsets could be explained by differential regulation of CD28 expression in response to TCR-mediated activation. Several groups reported conflicting results in that regard: moderate increases or decreases in CD28 expression were observed on T-cell stimulation, depending on the type and source of human T cells.22,24,25 When we analyzed total splenocytes, we saw a broad distribution of CD28 intensities: moderate up-regulation of CD28 on some, and also a down-regulation on a smaller subset of cells (Figure 6A). To precisely delineate changes in CD28 surface levels on CD4 and CD8 cells and to correlate them to cell division over a 96-hour in vitro anti-CD3 stimulation, we used CFSE to label purified CD28+ splenocytes prior to stimulation. Over 4 days of culture, the changes were progressive and gradual, and we are, therefore, showing only the final time point, which contains cumulative changes in stimulated cells. Interestingly, although proliferating CD4 cells maintained and up-regulated CD28 surface expression, CD8 cells showed marked CD28 down-regulation directly proportional to the number of cell divisions (Figure 6B). This finding was not due to differential survival of CD28–CD8 T cells, as we saw no evidence for significant apoptosis over the period of experiment (annexin V staining < 5% at any time point). This result correlates well with preferential loss of CD28 expression on CD8 T cells, which could be mediated by the elucidated events at the CD28 promoter.20,26 Moreover, our data for the first time demonstrate significant differences in CD28 regulation in response to short-term TCR-mediated activation in primary CD8 versus CD4 T cells.
Surface expression of CD28 is progressively down-modulated in a cell division–dependent manner in dividing CD8 but not CD4 T cells. (A) FCM histogram overlay of CD28 staining in total CD28+-purified splenocytes stimulated for 96 hours with immobilized anti-CD3 (solid line) or parallel nonstimulated control cultures (dashed line). Activation leads to the broadening of CD28 staining intensities, suggesting simultaneous maintenance, down-regulation, and moderate up-regulation of CD28 surface levels in different subpopulations of stimulated cells. The result is representative of 4 adult (5-7 years old) animals analyzed in 2 independent experiments. (B) Representative FCM dot plots of CD28 surface levels in different generations of dividing cells after 96 hours of anti-CD3 stimulation of CD28+-purified splenocytes, gated on CD4 and CD8 cells as indicated. There is an evident down-regulation of CD28 surface levels in CD8 cells, directly proportional to the number of divisions, whereas CD4 cells maintain or slightly up-regulate CD28 expression. (C) Relative CD28 staining intensity (relative fluorescence index, RFI) in different generations of dividing CD4 and CD8 cells, compared with the undivided population (RFI = 1). The data were obtained from 4 young adult animals (5-7 years old) and are representative of 2 independent experiments.
Surface expression of CD28 is progressively down-modulated in a cell division–dependent manner in dividing CD8 but not CD4 T cells. (A) FCM histogram overlay of CD28 staining in total CD28+-purified splenocytes stimulated for 96 hours with immobilized anti-CD3 (solid line) or parallel nonstimulated control cultures (dashed line). Activation leads to the broadening of CD28 staining intensities, suggesting simultaneous maintenance, down-regulation, and moderate up-regulation of CD28 surface levels in different subpopulations of stimulated cells. The result is representative of 4 adult (5-7 years old) animals analyzed in 2 independent experiments. (B) Representative FCM dot plots of CD28 surface levels in different generations of dividing cells after 96 hours of anti-CD3 stimulation of CD28+-purified splenocytes, gated on CD4 and CD8 cells as indicated. There is an evident down-regulation of CD28 surface levels in CD8 cells, directly proportional to the number of divisions, whereas CD4 cells maintain or slightly up-regulate CD28 expression. (C) Relative CD28 staining intensity (relative fluorescence index, RFI) in different generations of dividing CD4 and CD8 cells, compared with the undivided population (RFI = 1). The data were obtained from 4 young adult animals (5-7 years old) and are representative of 2 independent experiments.
Discussion
Numerous clinical observations point to defective responses to intracellular pathogens as well as to inefficient tumor surveillance in the elderly,1,27 of which both processes depend on proper function of class I–restricted CD8 T cells. The phenotypic signature of an aging T-cell compartment, based on shortened telomeres, loss of CD28 surface expression, as well as reduced in vitro proliferative response, immediate effector function (cytotoxicity and cytokine secretion), and reduced TCR diversity,28 is much more evident in CD8 than in CD4 T cells. That fact is also reflected in dominant, if not exclusive, appearance of the age-related T-cell clonal expansions (TCEs) in the CD8, and not the CD4, compartment in both mice and humans.5,28-30 The above characteristics seem to segregate with CD28– cells (believed to be the end-stage memory cells), which originate from CD28+ counterparts that have lost CD28 expression through initial or repeated activation.19,21,31,32 As CD28– cells accumulate not only in the elderly but also in patients with chronic viral infections (HIV, cytomegalovirus [CMV]) or with prolonged immune activation,33-36 these findings led to the current concept that lifelong cumulative effect of repeated antigenic stimulation, via the exhaustion of immune resources, may be one of the major contributors to immune senescence. However, at the present, the precise functional relevance of CD28– T cells37 in primate immune senescence remains poorly understood.
Here, we report on the fundamental differences between the NHP CD8 and CD4 T-cell programs that control proliferation, turnover, and surface phenotype, providing explanation for some of the earlier observations. First, the percentage of cells capable of immediate TNF-α secretion on TCR stimulation increased with age among RM CD8 T cells. By contrast, the percentage of CD4 cells with such effector function does not increase with age. Excessive inflammatory cytokine responses have been implicated in the immunopathology of aging,38-40 and a subset of CD8 memory T cells in old mice that escapes control of homeostatic proliferation was recently reported to exhibit an enhanced interferon γ (IFN-γ) response to polyclonal stimulation.41 In that regard, the observed increase in the number of TNF-α–secreting CD8 cells might be both a sign of a functional dysregulation specifically affecting CD8 T cells during senescence and a cause for cytokine dysbalance.
In a previous study using CD95 and CD28 to track memory T cells in elderly humans, Fagnoni et al18 have found a more profound loss of CD8 than CD4 naive cells, arguing that this loss of naive CD8 T cells could be the determining factor in immune senescence. We found that age-related loss of CD95– naive cells in RMs did not differ between CD4 and CD8 cells. Therefore, at least among the RM CD8 T cells, functional changes within the CD8 memory population appear to correlate with the aging process better than acquisition of CD95+CD28– phenotype. Indeed, in both humans and RMs, putatively senescent CD28– T cells predominantly accumulate in the CD8 subset and are far less frequent among CD4 cells. Although these data could be interpreted as accelerated functional aging of CD8 compared with CD4 T cells, our data show that loss of CD28 has distinct functional significance in the 2 T-cell lineages. Irrespective of age, CD4+CD28– cells contained a high and steady proportion of immediate Cytokine-Producing effectors. By contrast, CD8+CD28– showed a significant age-related increase in immediate cytokine-producing effectors. Moreover, CD8 T cells readily lost CD28 as they proliferated, and this loss became more pronounced with every division, whereas dividing CD4 cells maintained CD28 expression. In parallel, in vitro–stimulated CD8 T cells underwent more divisions than CD4 T cells and proliferated more vigorously in the absence of overt stimulation in vivo. On the basis of these results, we conclude that the CD28– phenotype does not define a functionally homogenous population of T cells and that the loss of this marker cannot be taken as a reliable predictor of the replicative state or of aging. In light of our data, the possibility that dominance of the CD8+CD28– phenotype in aging may in part be due to the execution of different programs in CD8 (pronounced and protracted proliferation with CD28 loss) and CD4 (lower proliferation with CD28 maintenance) T-cell lineages must be considered. Therefore, inherent programs that determine immune responses of different T-cell subsets might well influence the age-related changes of these same subsets. Similar differences in CD8 and CD4 lineage programs were recently described in a mouse model42 and were suggested on the basis of the studies of acute and chronic viral diseases in humans.43-45 Experiments are in progress to ascertain the long-term status of CD28 on in vitro–stimulated CD8 cells and to investigate this regulation in vivo.
These findings pose important questions about the link between CD28 down-regulation and functional aging of T cells. Previous reports have demonstrated an impaired in vitro proliferative response of CD28– T cells,19,22 and a recent study has correlated loss of CD28 expression with deficient telomerase inducibility during replicative senescence in vitro, with both being more prominent in the CD8 as compared with the CD4 T cells.46 If this analogy holds true in vivo and between the species of the primate genus, one would expect CD8+CD28– cells to be close to or at the replicative senescence state, particularly in older individuals. In a recent study, Pitcher et al14 showed that CD28– cells actively cycle in vivo. Our data (I.M. et al, manuscript in preparation) confirm these results; we found that CD28– cells vigorously turned over, at rates similar to or higher than their CD28+ counterparts, even in the oldest animals in our cohort. These results appear incompatible with uniform replicative senescence of CD28– cells, even in very old RM animals. Several explanations could reconcile these discrepant observations: (1) differences between human and RM models (unlikely), (2) differences between in vitro and in vivo tests, and (3) differences because of the heterogeneity within the CD28– cells (whereby only a subset would turn over continuously, perhaps stimulated by persistent pathogens). Of these explanations, we favor the second. In support of that view, a study demonstrated that the mechanism of CD28 transcriptional inactivation in ex vivo isolated aging CD28– T cells resembles the effects of TCR-mediated activation but not those of in vitro replicative senescence.24 Further studies comparing the in vivo behavior of CD4+CD28– and CD8+CD28– cells in the primate model, particularly combining better phenotypic definition, longer BrdU labeling, and controlled stimulation/pathogen load, will be crucial for resolving this issue. Our results indicate that more reliable phenotypic markers of T-cell senescence in the RM model are needed, particularly for the CD8 subset. Recently, CD57 was proposed as a reliable marker for replicative senescence in HIV-infected humans.47 It will be of interest to validate its association with the same phenotype in aging and to study its distribution in NHP once a cross-reactive or NHP-specific antibody becomes available.
On the basis of these results, we propose that changes in surface T-cell phenotype are regulated by the mechanisms linked to T-cell activation, but at least partly independent of the gradual exhaustion of replicative potential. Thus, although even an acute (and certainly also chronic and/or repeated) T-cell activation plays a role in modulation of CD28 expression coordinately with cell division, replicative senescence, if it exists in vivo, would require additional, possibly quite different, signals and events that could nevertheless be regulated by the overlapping programs that govern cell cycling in activated T cells. On the basis of the observed differences between the CD4 and CD8 T cells and their differential manifestations of the age-related dysfunction, optimal T-cell vaccination strategies in the elderly might have to differ for class I–versus class II–restricted responses.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-03-0927.
Supported by the U.S. Public Health Service awards (NIA AG 21384) (J.N.-z.) and by Core National Primate Center Support Grant (NCRR P51 RR 00163) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Heather Sharpless, Bree Fisher, and Jessica Warner for help with specimen collection and processing; Ms Dragana Nikolich-Zugich for flow cytofluorometry; Dr Motomi Mori for expert statistical analysis; and Drs Louis Picker, Mark Slifka, and Michael Axthelm for helpful advice, sharing of samples, and critical perusing of the manuscript.