Abstract
In addition to its importance in transfusion, Kell protein is a member of the M13 family of zinc endopeptidases and functions as an endothelin-3–converting enzyme. To obtain information on the structure of Kell protein we built a model based on the crystal structure of the ectodomain of neutral endopeptidase 24.11 (NEP). Similar to NEP, the Kell protein has 2 globular domains consisting mostly of α-helical segments. The domain situated closest to the membrane contains both the N- and C-terminal sequences and the enzyme-active site. The outer domain contains all of the amino acids whose substitutions lead to different Kell blood group phenotypes. In the model, the zinc peptidase inhibitor, phosphoramidon, was docked in the active site. Site-directed mutagenesis of amino acids in the active site was performed and the enzymatic activities of expressed mutant Kell proteins analyzed and compared with NEP. Our studies indicate that Kell and NEP use the same homologous amino acids in the coordination of zinc and in peptide hydrolysis. However, Kell uses different amino acids than NEP in substrate binding and appears to have more flexibility in the composition of amino acids allowed in the active site.
Introduction
The Kell blood group protein is a type II membrane glycoprotein that is important in transfusion medicine due to the immunogenicity of its many polymorphic forms. The primary structure of Kell protein and its enzyme function as an endothelin-converting enzyme, classifies it as a member of the neprilysin (M13) subfamily of zinc endopeptidases. Kell differs from other M13 members in that it is linked by a single disulfide bond to another protein, XK, which traverses the membrane 10 times (for reviews, see Lee et al1,2 ). XK has the structural characteristics of a membrane transporter, but its function has not yet been determined.3
As a group the mammalian M13 peptidases are involved in the activation of bioactive peptides by specific cleavages of larger inactive peptides and in the proteolysis of bioactive peptides.4-6 The substrate specificities of the M13 family members vary with neutral endopeptidase (NEP; EC 3.4.24.11)7 and secreted endopeptidase (SEP)8 having a wide range of substrates and endothelin-converting enzyme 1 (ECE-1; EC 3.4.24.71), endothelin-converting enzyme 2 (ECE-2),9,10 Kell,11 and phosphate-regulating protein (PHEX)12,13 with a restricted number of substrates. ECE-1 and ECE-2 preferentially cleave big endothelin-1–releasing endothelin-1 (ET-1) but can also cleave big endothelin-2 and big endothelin-3.14 ECE-1 also hydrolyzes other bioactive peptides such as bradykinin, neurotensin, and substance P.9 Kell, like ECE-1 and ECE-2, is also an endothelin-converting enzyme but, in contrast to ECE-1 and ECE-2, Kell preferentially cleaves big endothelin-3, releasing the active peptide endothelin-3 (ET-3) although it also, to a lesser degree, activates ET-1 and endothelin-2 (ET-2).11 Thus far the parathyroid hormone–related fragment 107-139 and fibroblast growth factor 23 (FGF-23), which is involved in phosphate transport in the kidney, are the only known substrates for PHEX.12,13 Substrates for XCE, which is mostly expressed in the central nervous system, have not yet been identified.15,16
Site-directed mutagenesis of conserved amino acid residues in NEP have yielded valuable information on substrate-binding sites and on the amino acid residues that are essential for hydrolytic activity.17-28 This information, together with the crystallographic structure of NEP that has been published29 and used to model ECE-1,30,31 has provided insight on the enzymatic mechanisms of these physiologically important mammalian proteases.
In this study, the ectodomains and active sites of Kell and NEP are compared. We determine the effect of selectively substituting amino acid residues by site-directed mutagenesis on the ET-3–converting activity of Kell. We also model the structure of Kell based on the crystal structure of NEP, describe differences, and map the location of polymorphic sites, which are involved in presenting different Kell antigens.
Materials and methods
Expression of wild-type and mutant ectodomains of Kell protein
The ectodomain of wild-type Kell, amino acid residues Asn68 to Trp732, was expressed, as previously described, in sf9 cells.11 The cDNA encoding the ectodomain contained a secretion signal sequence and was tagged at the 5′ end to encode 6 histidines, to facilitate isolation of the secreted protein. Also, as previously described, the ectodomain was modified to contain serine instead of cysteine at position 72 to avoid intermolecular associations because, in red cells, Cys72 is disulfide-linked to XK.11 The secreted, soluble Kell ectodomain is termed sKell. The preparation and enzymatic properties of sKell have been previously described.11 Mutant Kell proteins containing single amino acid substitutions were similarly expressed. The amino acid numbers used are as described in GenBank for Kell (accession no. M64934) and NEP (accession no. JO3779).
Construction of mutant Kell cDNAs
To create sKell mutants, polymerase chain reaction (PCR)–based site-directed mutagenesis was used. The appropriate base mutations were incorporated into PCR primers and used to copy segments of Kell cDNA that contained 2 convenient unique restriction enzyme sites. The PCR product was then used to replace the corresponding wild-type segment in sKell cDNA, which was present in pAcGp67A vector and used for expression in sf9 cells. In some cases in which the mutation was too far from the restriction sites, 2 overlapping PCR products were produced, followed by another PCR step to link the 2 products.11,32
The primer sets used, with the mutated base underlined, and the restriction enzyme sites used are listed in Table 1. The primers used to link the overlapping PCR products are marked with asterisks.
ET-3–converting enzyme analysis of wild-type and mutant sKell
Mutant and wild-type sKell secreted into the Excell 400 media (JRH Biosciences, Lenexa, KS) by infected sf9 cells were used in the enzyme assays as previously described.11 ET-3 was assayed using an ET-1 enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions. The antibody in the EIA kit reacts equally well with both ET-1 and ET-3.11
Homology modeling of the ectodomains of Kell based on crystal structure of NEP
Alignments of the ectodomains of human Kell protein and NEP were performed by the program ClustalX,33 and insertions and deletions were manually adjusted to avoid gaps in the helix regions. The Kell amino acid sequence (amino acid residues 75-732) was obtained from SWISS-PROT accession number M64934 and the sequence of NEP (amino acid residues 55-750) was from the pdb structure, 1DMT.
Homology models were built using the automated software Modeller 4.034 within Quanta 2000 (Accelrys, San Diego, CA) running on a Silicon Graphics Octane with a dual R12000 processor (sgi, Mountain View, CA). Refine 3 option in Modeller that uses conjugated gradient together with molecular dynamics by simulated annealing technique was used to optimize the models. Five models for each run were developed and the model with lowest Objective Function was selected. Zinc, glycerol, and the inhibitor phosphoramidon, which were present in the NEP structure,29 were also kept in the structure during model building. After initial modeling, Quanta Protein Health Check revealed several close contacts and the Quanta Charmm energy was high. Zinc, glycerol, and phosphoramidon were removed from the structure; polar hydrogens were added to the model and optimized by 200 steps of Steepest Descent method. The protein backbone was kept fixed during optimization. The resulting model was evaluated for its stereochemical properties by Procheck software35 at 2.0 Å and by Quanta Protein Health programs within QUANTA2000. The Charmm energy was calculated in psf mode. Zinc and phosphoramidon were docked manually into the active site using the original model that was best generated by Modeller.
Modeling of wild-type Kell was performed with 10 conserved cysteine residues forming 5 disulfide bonds as described in NEP.29 In the absence of experimental data on whether Cys596 and Cys599 also form a disulfide bond, models were built with and without this disulfide bond. In terms of energy differences between the modeled Kell proteins with and without the disulfide bond, it appeared that the disulfide bond could be formed; therefore, further studies with mutant Kell ectodomains were modeled with the Cys596 to Cys599 disulfide bond. In addition to wild-type Kell, 3 other models incorporating Glu634Asp, Leu597Pro, and Tyr588Phe were generated using the described methods to examine if these amino acid substitutions affected structure.
Results
General structural aspects of Kell protein and comparison with NEP
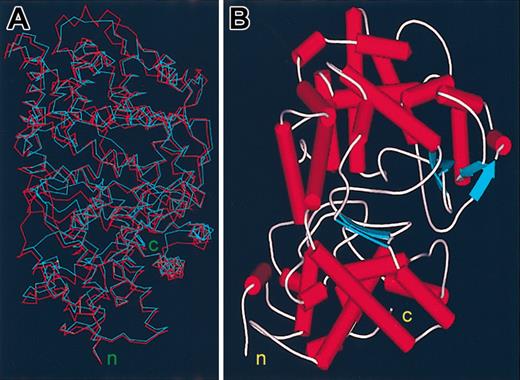
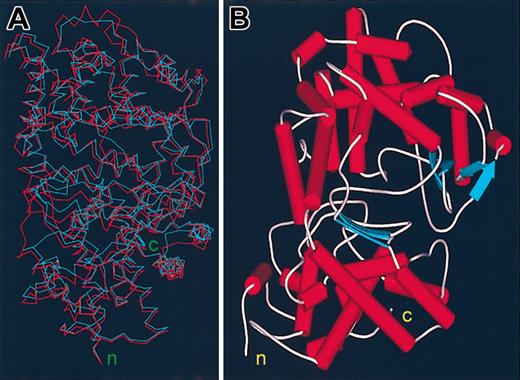
The ectodomains of human Kell and NEP share about a 30% amino acid identity including 10 conserved cysteine residues and several motifs present in all zinc endopeptidases, indicating a functional relation and a common structural fold.36 A Ramachandran plot of the modeled Kell based on the structure of NEP indicates that 88.1% of the Kell amino acid residues are in the most favored regions, 10.5% in additional allowed regions, 0.9% in generously allowed regions, and only 0.5% (3 amino acid residues) in disallowed regions. A superimposed model of Kell on the structure of NEP is shown in Figure 1A.
Models of the ectodomain of the Kell blood group protein. (A) Superimposed backbone models of NEP in blue and Kell in red. (B) Ribbon plot model of the ectodomain of the Kell blood group protein. The amino terminus (n) and the carboxy terminus (c) of the ectodomain are shown.
Models of the ectodomain of the Kell blood group protein. (A) Superimposed backbone models of NEP in blue and Kell in red. (B) Ribbon plot model of the ectodomain of the Kell blood group protein. The amino terminus (n) and the carboxy terminus (c) of the ectodomain are shown.
Kell and NEP have the same general shape consisting of 2 globular domains consisting largely of α-helical structures. One of the domains, in which both N- and C-termini reside, contains the motifs that by homology with the M13 family and with bacterial thermolysin are essential for catalytic and hydrolytic activity and also contains 4 of the 5 disulfide bonds that are conserved in the other mammalian M13 family members. Because this domain contains the N-terminus of the ectodomain, it is contiguous with the single membrane-spanning region and should be situated directly above the membrane. The other domain sits above, is more exposed to the extracellular space, and appears as a cap that may restrict the entry of large molecules into a spherical cavity that houses the substrate and enzyme-active sites (Figure 1B).
Structural positions of nonconserved cysteine residues
The ectodomain of Kell contains 15 cysteine residues, 10 of which are conserved in other mammalian M13 members.1,2,36 Our model was built with the 10 conserved cysteine residues forming disulfide bonds. The 5 Kell nonconserved cysteines are in amino acid positions 72, 319, 511, 596, and 599. Cys72 is predicted to be 5 amino acid residues past the transmembrane region into the ectodomain. The corresponding location in the ectodomain of NEP was not included in the crystal structure of NEP and, thus, Kell Cys72 was not modeled and is not shown in Figure 1. In human erythroid tissues, Kell Cys72 is linked by a disulfide bond to another protein, XK, a 10-membrane spanning protein.37
Our model predicts that cysteine residues 319 and 511 are far apart, cannot form disulfide linkage, and therefore are likely to have free sulfhydryl groups, which are positioned within the outer domain. In wild-type Kell, cysteine residues 596 and 599 are separated by leucine and alanine, and in terms of energy and distance from each other it appears that Cys596 and Cys599 can form a disulfide bond.
Alignment of catalytic active sites
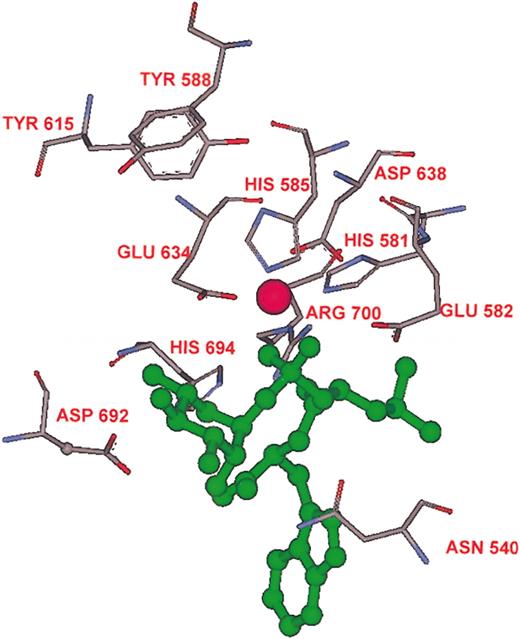
Three motifs are conserved in all zinc endopeptidases including bacterial thermolysin and are part of the active site of the M13 family (for reviews, see Roques et al5,7 ). A ClustalX sequence alignment of NEP and Kell matches the M13 consensus motifs, HExxH, ExxxD, and NAararS where “x” is any amino acid and “ar” indicates aromatic amino acids. Thus, Kell amino acids, HELLH (581-585), ENAAD (634-638), and NAYYS (540-544) align with NEP amino acids HEITH (584-588), ENIAD (647-651), and NAFYS (543-547) as shown in Figure 2. In the crystal structure of NEP the side chains of the 2 histidines in the first motif and of the glutamic acid in the second motif contact the zinc ion. In our model His581, His583, and Glu634 are in close vicinity to the zinc molecule, which is depicted as a pink sphere in Figures 4 and 5. Coordination of zinc is essential because zinc is expected to bind the oxygen in the carbonyl group of Trp21 of big ET-3 making the carbon atom in the peptide bond forming Trp21-Ile22 of big ET-3 more susceptible to nucleophilic attack by the water molecule that is polarized by Kell Glu582 and NEP Glu585.
CLUSTAL X alignment of the ectodomains of NEP and Kell. The amino acid sequences and the numbering are from GenBank: Kell accession number M64934 and NEP J03779. The ectodomain sequence of NEP was from the pdb structure, 1DMT. Signature sequences, present in mammalian M13 zinc endopeptidases, and the Ww motif, present in most M13 peptidases listed in the MEROPS database39 but absent in Kell, are boxed. The 10 conserved cysteine residues present in the M13 family are shaded. The 8 cysteines located in the domain closest to the membrane, which contains the active site, are marked by dots.
CLUSTAL X alignment of the ectodomains of NEP and Kell. The amino acid sequences and the numbering are from GenBank: Kell accession number M64934 and NEP J03779. The ectodomain sequence of NEP was from the pdb structure, 1DMT. Signature sequences, present in mammalian M13 zinc endopeptidases, and the Ww motif, present in most M13 peptidases listed in the MEROPS database39 but absent in Kell, are boxed. The 10 conserved cysteine residues present in the M13 family are shaded. The 8 cysteines located in the domain closest to the membrane, which contains the active site, are marked by dots.
The Kell active site. The amino acids in the 581H582EXXH585 and 634ENxAD638 signature sequences and their spatial relation to zinc (pink ball) and phosphoramidon (green) are shown. Also shown are the amino acids that were mutated. The enzyme activities of the mutants are given in Figure 3.
The Kell active site. The amino acids in the 581H582EXXH585 and 634ENxAD638 signature sequences and their spatial relation to zinc (pink ball) and phosphoramidon (green) are shown. Also shown are the amino acids that were mutated. The enzyme activities of the mutants are given in Figure 3.
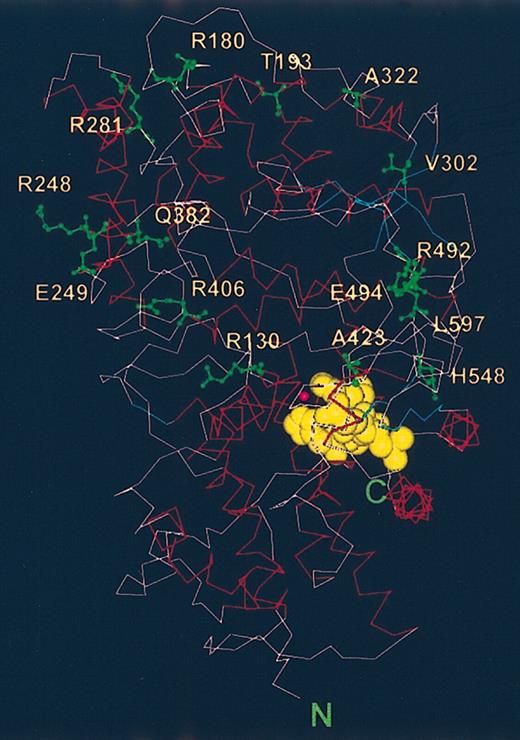
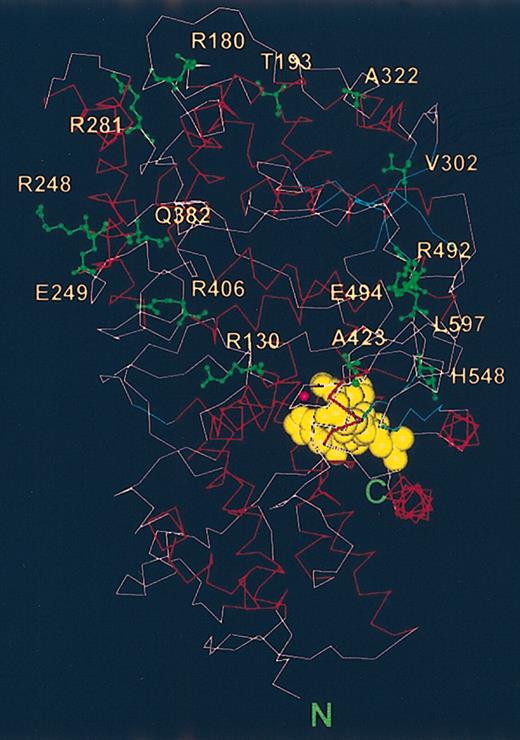
Locations of the amino acids whose substitutions led to different Kell polymorphisms. The amino acids whose mutations led to different Kell phenotypes are labeled (green) and are shown on a backbone structure of a model of the Kell ectodomain. The active and substrate binding sites are marked by the locations of zinc (pink ball) and of the inhibitor, phosphoramidon (yellow).
Locations of the amino acids whose substitutions led to different Kell polymorphisms. The amino acids whose mutations led to different Kell phenotypes are labeled (green) and are shown on a backbone structure of a model of the Kell ectodomain. The active and substrate binding sites are marked by the locations of zinc (pink ball) and of the inhibitor, phosphoramidon (yellow).
In addition to the catalytic conserved motifs described, 5 other evolutionary conserved sets have been identified in an analysis of 81 M13 endopeptidases.38 Kell contains 4 of these motifs with one exception. An M13 family alignment of 113 peptidases by the MEROPS peptidase database39 (http://merops.sanger.ac.uk/) shows that all M13 peptidases, with the exception of human and mouse Kell, 6 unassigned peptidases from Drosophila melanogaster and one from Caenorhabditis elegans, contain a Ww motif downstream from the canonical HExxH motif. In NEP, Trp606 and Trp607, which are missing in Kell, are part of a hydrophobic pocket that is a spatial neighbor of the HExxH catalytic site.29,38 Alignment of Kell and NEP sequences demonstrates a gap in the Kell sequence that corresponds to the WW motif in NEP (Figure 2). This difference differentiates Kell from most M13 family members.
The other motifs, present in 81 full-length M13 endopeptidases sequences, are also present in Kell. 99PCxxFfxfaC108 is close to the membrane-spanning region and is thought to be unique to eukaryotes. This motif is not spatially close to the catalytic active site. The other conserved motifs 459WMxxxTxxxAxxK471 and 729CxlW732 colocalize with NEP, but 481GYP483 in NEP aligns with 480GAS482 in Kell.
Site-directed mutagenesis of essential amino acid residues
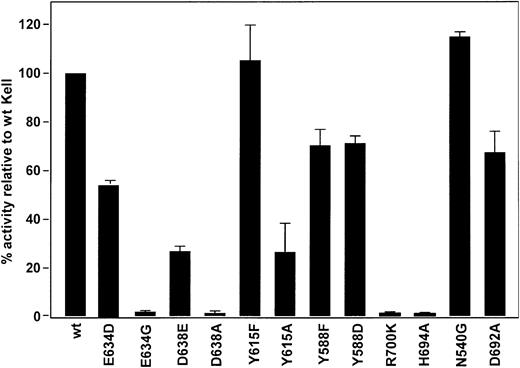
To determine if Kell and NEP use homologous amino acid residues in catalysis, selected Kell amino acids were substituted by site-directed mutagenesis and enzymatic activity of the various mutant sKells was compared to that of wild-type sKell. Enzymatic activity was measured by ability to cleave big ET-3 at Trp21-Ile22 and produce ET-3. The catalytic activities of the mutant sKells compared with wild-type sKell are shown in Figure 3 and the amino acids substituted and their spatial relation to zinc, phosphoramidon, and the amino acids that comprise the zinc endopeptidase signature sequence, HExxH, are shown in Figure 4.
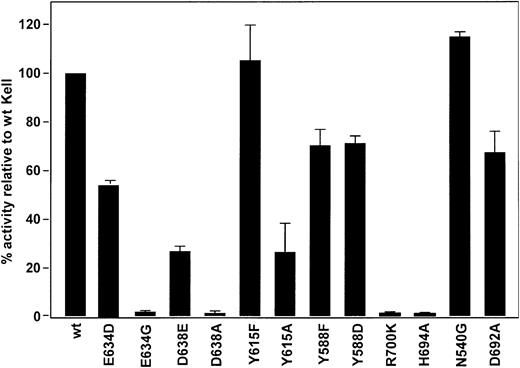
Catalytic activity of sKell mutants compared with wild-type sKell. The ET-3–converting enzyme activity of wild-type (wt) sKell and various sKell mutants were compared. For ease of comparison the catalytic activity of sKell is normalized to 100%. The SD from the mean of the various sKell mutants is shown.
Catalytic activity of sKell mutants compared with wild-type sKell. The ET-3–converting enzyme activity of wild-type (wt) sKell and various sKell mutants were compared. For ease of comparison the catalytic activity of sKell is normalized to 100%. The SD from the mean of the various sKell mutants is shown.
In all zinc endopeptidases, glutamic acid in the HExxH motif is essential for hydrolytic activity acting as a nucleophile and promoting the attack of zinc-bound water molecule to the scissile peptide. A previous study showed that in keeping with this model a Glu582Gly substitution in Kell abolished its enzymatic activity.11 In NEP, His584, His588, and Glu647 are coordinating ligands for zinc.5,7 In Kell, Glu634 is expected to play a similar role. A Kell Glu634Asp mutant, which has a shorter side chain than the wild-type, retained nearly 60% of its enzyme activity indicating structural flexibility in this region, but activity was completely lost when glutamic acid was substituted with glycine (Figure 3). This result differs from that obtained with NEP in which an Glu647Asp mutant lost most of its catalytic activity28 and indicates that Kell may have more flexibility in structure in this area compared with NEP.
In NEP, the side chains of Asp591 and Asp651 appear to form triads with the coordinating histidine residues and are involved in placing zinc in the proper position for catalysis.22 Kell differs from NEP having a tyrosine (Kell Tyr588) rather than aspartic acid (NEP Asp591) in one of these positions; however, as in NEP, an aspartic acid is conserved at the other position (Kell Asp638 and NEP Asp651). Based on its homology with NEP, Kell Asp638 is predicted to form a hydrogen bond with His581 present in the 581HELLH585 motif. Thus, Kell Asp638 should be necessary for hydrolytic activity. A Kell Asp638Ala mutant lost enzyme activity indicating that as predicted by the model Kell and NEP have a similar mechanism. However the Asp638Glu mutant, with a longer side chain, retained about 30% of its activity compared to wild-type sKell (Figure 3). To determine if Kell Tyr588, like NEP Asp591, is also involved in catalysis, we questioned if the hydroxyl group is needed because it may form a hydrogen bond with one of the zinc-binding histidines. We therefore substituted tyrosine with phenylalanine, another aromatic amino acid that lacks the hydroxyl group. The Tyr588Phe substitution resulted in only a 20% loss of catalytic activity, indicating that the hydroxyl group is not involved. Replacing tyrosine with aspartic acid, which is present in NEP, did not restore full activity indicating a difference with NEP (Figure 3).
A tyrosine residue is conserved in all members of the mammalian M13 family at NEP 626, Kell 615, ECE-1 632, and PHEX 622. In Kell, Tyr615 is spatially close to Tyr588 (Figure 4). Kell Tyr615 may form an important hydrogen bond via the hydroxyl group with other amino acid residues in the active site. To test this hypothesis, a Kell Tyr615Phe mutant, which lacks the hydroxyl group, was constructed and it retained complete catalytic activity indicating that the hydroxyl group is not necessary for catalysis. However, the tyrosine residue at this position may play a structural role because a Kell Tyr615Ala mutant displayed a significant loss of activity (Figure 3).
Another functional and structural similarity between NEP and Kell is that in NEP, Arg718 (Kell Arg700) forms a salt bridge with NEP Asp651 (Kell Asp638) and plays a role in substrate hydrolysis.26 The Kell mutant Arg700Lys has no catalytic activity (Figure 3) indicating that it is not the basic nature of the amino acid in that position that is involved in catalysis but likely the structure of the side chain, which interacts with Asp638, that is important.
In NEP the side chain of His712 (Kell His694) has been implicated in stabilization of the transition state21 and this amino acid probably plays a similar role in Kell because the His694Ala mutant has no hydrolytic activity (Figure 3).
In NEP, Asn543 forms hydrogen bonds with the P2′ residues of substrates, and NEP mutants Asn543Gly and Asn543Gln exhibit a large, 15-fold increase in Km, whereas kcat values were less affected, 2- to 3-fold, consistent with Asn543 being involved in substrate binding rather than in catalysis.23 In Kell, Asn540 also appears not to be involved in catalysis, but in contrast to NEP the Asn540Gly mutant retained full enzyme activity (Figure 3).
NEP, ECE-1, and Kell share an aspartic acid residue (Kell Asp692) upstream from histidine (Kell His694) and arginine (Kell Arg700) residues that are essential for enzyme activity. To determine the possible role of Kell Asp692, an Asp692Ala mutant was analyzed and found to retain about 70% of its activity, suggesting that Kell Asp692 is not directly involved in the activation of ET-3 (Figure 3).
Kell blood group antigens are clustered on the nonconserved domain
The Kell blood group protein is very polymorphic caused by several point mutations to the KEL gene leading to single amino acid substitutions and the development of neo-epitopes.40 The amino acids that are substituted are clustered in the domain that does not contain the motifs necessary for catalytic activity. The pertinent amino acids, which are highlighted in Figure 5, appear to be located on the rim of the outer domain and are therefore most exposed to the outside. None of the polymorphisms occur in amino acid residues that are in the domain that is conserved in the M13 family and contains the catalytic site.
The KEL6 phenotype is caused by a Leu597Pro substitution between 2 closely spaced nonconserved cysteine residues (596CPAC599) and in a linear sequence it is located close to the 581HELLH585 zinc-binding site.41 Because KEL6 has an incidence of 20% in persons of African heritage and occurs only in about 0.01% in whites, it is thought that this unequal distribution may reflect a selection pressure that influences the functional properties of Kell. However, the model does not indicate that the Leu597Pro mutation is in close spatial relation with the enzymeactive site. Neither does the Leu597Pro mutation significantly alter the ability of forming a disulfide bond between Cys596 and Cys599 because the Charmm energy, within quanta, of the wild type (–22 702 kcal/mol) and the Leu597Pro (–23 063 kcal/mol) were similar. With no disulfide bond between Cys596 and Cys599 the Charmm energies were –20 062 kcal/mol for the wild-type and –23 051 kcal/mol for the Leu597Pro mutant. A comparison of wild-type and Leu597Pro Kell mutant did not show a difference in the Km values for big ET-1, big ET-2, and big ET-3 and KEL-6 red cells, like the wild-type phenotype, preferentially activated ET-3 rather than ET-1 and ET-2 (data not shown).
Discussion
Because the ectodomains of the mammalian M13 family members share more than 30% amino acid identity and 10 conserved cysteine residues, indicating similar disulfide arrangement, it is to be expected that they also share a common fold. Based on the crystal structure of NEP, a model of ECE-1 has been presented and shown to be similar. Therefore, it is not surprising that in comparing Kell with NEP only 0.5% of the Kell amino acids, based on a Ramachandran plot, are not placed in an allowed region and Kell assumes the same general structure as NEP. There are, however, differences between Kell and NEP and also with other mammalian M13 members. A prominent difference occurs in the vicinity of the zinc-binding signature sequence HExxH. NEP, ECE-1, PHEX and more than 100 other M13 zinc peptidases listed in the MEROPS database contain a Ww motif downstream from the mandatory HexxH motif. Kell, however, lacks the Ww motif and on alignment of sequences presents an obvious gap in this region. The only other M13 peptidases that lack the Ww motif are 7 unassigned peptidases from C elegans and Drosophila. We do not know at this time, and the modeling does not allow us to predict, if lack of the Ww motif influences the catalytic site and provides functional similarity between Kell and the peptidases that also lack the Ww motif.
There is an indication that the sequence difference between Kell and NEP, which produces a gap in Kell at the Ww motif region, may influence the structure around the zinc-binding site. In NEP, Glu646 is a zinc-coordinating residue and Kell Glu634 is expected to play a similar role. An NEP Glu647Asp mutant, which conserves the negative charge but has a smaller side chain, has the same Km value as the wild-type NEP but exhibits only 3% of the catalytic activity, indicating that Glu647 is essential for catalysis but not for substrate binding.28 By contrast, Kell Glu634Asp retains 60% of its catalytic activity, indicating that the shorter side chain of aspartic acid, compared with glutamic acid, is also capable of coordinating the zinc molecule at the active center, suggesting that Kell may have more flexibility in this region than the other M13 members.
Kell protein is highly polymorphic and more than 20 different polymorphisms have been described, all of them due to point mutations that lead to amino acid substitutions.40 We do not know if the other M13 zinc endopeptidases are also polymorphic. The polymorphisms in Kell are noted because Kell is present on red cell surfaces and because the different polymorphic forms are immunogenic. Since red cells are used in transfusions, antibodies are often raised in recipients with incompatible blood. However, similar polymorphisms in NEP have not been described. Our study shows that all of the amino acids whose substitutions led to expression of different Kell antigens are situated on the outer globular domain that does not contain the conserved residues essential for catalysis. The pertinent amino acids engaged in presenting antigenic sites appear to be located on the outer rim of the Kell structure and are unlikely to affect the catalytic activity.
On red cells Kell is linked to XK by a single disulfide bond. Kell Cys72, which is close to the outer surface of the membrane, is linked to XK Cys347, which is present on the fifth extracellular loop.37 The orientation of Kell and XK remains to be determined and must await the crystal structure of the Kell/XK complex. However, the ectodomain of Kell protein is much larger than that of XK which, as a 10-membrane–spanning protein, mostly resides within the cell membrane and in the intracellular compartment with only one large extracellular loop, 42 amino acids, and 4 small extracellular loops.3 XK does not block enzymatic activity because both Kell/XK complex on red cells and recombinant sKell have similar enzymatic properties.11 We do not know, however, if the Kell ectodomain, which will tower over XK, participates or affects the presumed transport activities of XK protein.
The KEL6 phenotype, which is caused by a Leu597Pro mutation, occurs between 2 closely spaced cysteine residues (Cys596 and Cys599). Wild-type sKell and the Leu597Pro (KEL6) mutant have the same catalytic activity using the big endothelins as substrates11 and the Kell model indicates that both the Leu597Pro mutant and the wild-type can form a disulfide linkage between Cys596 and Cys599. Kell antigens are known, by serology, to be inactivated on treatment with reducing reagents and KEL6 is the most susceptible, being inactivated at the lowest concentration of reducing reagent,42 suggesting that the Leu597Pro mutation may increase the lability of the Cys596-Cys599 disulfide bond. It is interesting that the KEL6 phenotype has 20% incidence in persons of African heritage compared with about 0.01% in whites, suggesting a selective genetic advantage. Because this mutation does not affect catalytic activity for activation of the endothelins, it is possible that it may influence other not yet understood functions for Kell. A possibility is that Kell, like the other M13 members, has other substrates, in addition to the big endothelins, which have not yet been identified and that the KEL6 phenotype affects their cleavage. Also, because in Africans the greatest selective pressure is resistance to malaria, it is possible that the KEL6 phenotype may provide some resistance to malarial infection or to progress of the disease. However, the Kell model does not provide a clue to the possible different function of the KEL6 phenotype.
NEP cleaves a wide variety of biologically active peptides5,7 and although ECE-1 was initially thought to only activate the endothelins, later studies demonstrated wider substrate specificity because ECE-1 also hydrolyzes other peptide hormones such as bradykinin, substance P, and neurotensin.9 Although there are many similarities between NEP and ECE-1, in this study we note differences in the amino acids that may interact with their substrates. Kell differs from NEP and ECE-1 in that previous studies showed that wild-type sKell does not cleave a large number of synthetic peptides that are substrates for other peptidases and neither does it cleave bradykinin, which is hydrolyzed by both NEP and ECE-1.11 Our current study also shows that Kell and NEP use different amino acids for substrate binding as indicated by a comparison of the catalytic activities of the Kell mutant Asn540Gly, which retains full activity and NEP Asn542Gly, whose catalytic activity is reduced 2- to 3-fold. These results partially explain the different substrate specificities of Kell and NEP but do not explain the limited specificity of Kell. Our studies, however, do not exclude the possibility that Kell has physiologic substrates, other than the big endothelins, that have yet to be determined.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2003-05-1564.
Supported by a National Institutes of Health SCOR grant in Transfusion Biology and Medicine (HL54459).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Daniel Bur, Molecular Modeling, Actelion Pharmaceuticals Ltd (Allschwil, Switzerland) for helpful discussions during the course of the study; Aldo Mele for excellent technical assistance; and Dr James Farmar and Susan Fetics of the Laboratory of Microchemistry at the New York Blood Center for DNA sequencing.