Abstract
It is widely accepted that the platelet release reaction is mediated by heterotrimeric complexes of integral membrane proteins known as SNAREs (SNAP receptors). In an effort to define the precise molecular machinery required for platelet exocytosis, we have analyzed platelets from cellubrevin/VAMP-3 knockout mice. Cellubrevin/VAMP-3 has been proposed to be a critical v-SNARE for human platelet exocytosis; however, data reported here suggest that it is not required for platelet function. Upon stimulation with increasing concentrations of thrombin, collagen, or with thrombin for increasing time there were no differences in secretion of [3H]-5HT (dense core granules), platelet factor IV (alpha granules), or hexosaminidase (lysosomes) between null and wild-type platelets. There were no gross differences in bleeding times nor in agonist-induced aggregation measured in platelet-rich plasma or with washed platelets. Western blotting of wild-type, heterozygous, and null platelets confirmed the lack of cellubrevin/VAMP-3 in nulls and showed that most elements of the secretion machinery are expressed at similar levels. While the secretory machinery in mice was similar to humans, mice did express apparently higher levels of synaptobrevin/VAMP-2. These data show that the v-SNARE, cellubrevin/VAMP-3 is not a requirement for the platelet release reaction in mice.
Introduction
Upon detecting vascular lesions, platelets become activated and release components from 3 intracellular stores (dense core, alpha granule, and lysosome; reviewed in Reed1 ). The platelet release reaction is a critical step in hemostasis since the secreted components are important for activation, recruitment, and hemostatic plug formation. This regulated-exocytosis process is mediated by a complex of integral membrane proteins known as SNAREs (SNAP receptors, reviewed in Reed et al2 ). One class of SNAREs called v-SNAREs is found predominantly on granule membranes,3 whereas the other class, the t-SNAREs, are found on both granules and membranes of the open canalicular system (OCS3,4 ). The v- and t-SNAREs form a transmembrane, heterotrimeric complex that mediates bilayer mixing5 and is required for granule-OCS membrane fusion and granular cargo release.3,4,6-8 Platelets contain several members of the t-SNARE class, for instance, SNAP-23, and syntaxins 2, 4, 7, and 12.4,6,7,9,10 Syntaxin 2 and SNAP-23 are important for release from each of the 3 granules.7 Syntaxin 4 is important for release from alpha granules and lysosomes but apparently plays no role in dense core granule release.4,6,11
Human platelets contain the v-SNAREs cellubrevin/VAMP-3 and endobrevin/VAMP-8.3,6,8,9,12 The role for v-SNAREs in alpha granule release was first shown by Flaumenhaft et al using tetanus toxin, a v-SNARE–specific protease.6 This toxin specifically inactivates synaptobrevin/VAMP-1,-2, and cellubrevin/VAMP-3 (reviewed in Montecucco et al13 and Schiavo et al14 ) and was shown to block alpha granule release.6 Since neither synaptobrevin/ VAMP-1 nor -2 were found in human platelets,3,6,8,9,12 it was assumed that cellubrevin/VAMP-3 is required for exocytosis from alpha granules. Subsequent studies, using specific antibodies3 and v-SNARE cytosolic domains as inhibitors, indicate that cellubrevin/VAMP-3 and endobrevin/VAMP-8 may play a role in release from alpha granules and dense core granules.8 When the cytoplasmic domain of synaptobrevin/VAMP-2 was added, there was no inhibitory effect. This apparent role for cellubrevin/VAMP-3 in regulated platelet exocytosis is different than its proposed roles in nucleated cells. In COS-7 cells, cellubrevin/VAMP-3 cycles between the plasma membrane and a perinulcear region identified as the recycling endosome.15 These data suggested that cellubrevin/VAMP-3 is important for receptor recycling (eg, transferrin receptor) by facilitating the transit of receptors from the recycling endosome to the plasma membrane. In accordance with this model, tetanus toxin treatment of permeabilized cells inhibits the re-exposure of endocytosed transferrin and its receptor.16 Other studies with cellubrevin/VAMP-3 knockout animals have not confirmed such a general role.17 These studies suggested that cellubrevin/VAMP-3 may be important for endocytosis, but of a specific subset of ligand-bound receptors.18 From studies of regulated recycling of the Glut4 transporter, cellubrevin/VAMP-3 is not required for mobilization of the transporter from its intracellular compartment to the plasma membrane.17
To further clarify the role of this v-SNARE, we used a knockout mouse system17 to determine the requirement for cellubrevin/ VAMP-3 in platelet exocytosis. No overt bleeding-time phenotype was detected in the cellubrevin/VAMP-3 null or heterozygous mice, and there was no apparent difference in the levels of thrombin- and collagen-induced secretion from any of the 3 platelet granular stores. There also were no significant structural defects detected. As expected, platelets from the null mice contained no detectible cellubrevin/VAMP-3. Other elements of the platelet exocytosis machinery showed no significant differences in expression. One surprise was the presence of the v-SNARE, synaptobrevin-2/VAMP-2, since it had been undetected in human platelets.3,6,8,9,12 We report here that this lack of detection is apparently due to the protease sensitivity of synaptobrevin/VAMP-2 and its low level of expression. Upon inclusion of an extensive cocktail of protease inhibitors to the preparation of extracts from freshly prepared platelets, it is now possible to detect this v-SNARE in human platelets.
Materials and methods
Antibodies
Polyclonal anti–SNAP-23, anti-RabGDIα and anti–syntaxin 2 and 4 antibodies were generated in our laboratory by immunization of rabbits with the appropriate recombinant proteins.4,7,19,20 Anti–syntaptobrevin/VAMP-2 monoclonal antibody (mAb; C1 69.1) was a generous gift from Dr Reinhard Jahn.21 Anti–cellubrevin/VAMP-3 polyclonal antibody was prepared as previously reported.17 Anti–endobrevin/VAMP-8 polyclonal antibody was obtained from Synaptic Systems (Gottingen, Germany). Anti–Rab11 mAb was obtained from Transduction Laboratories (Lexington, KY). Anti-Rab5a polyclonal antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–murine fibrinogen polyclonal was obtained from Innovative Research (Southfield, MI), and the anti–α-SNAP mAb was obtained from Gamma One Laboratories (Lexington, KY). Anti–N-ethylmaleimide Sensitive Factor (NSF) monoclonal, 2E5, was prepared and characterized as described in Tagaya et al22 and Whiteheart et al.23 Appropriate anti-immunoglobulin secondary reagents (antimouse, antisheep, and antirabbit) coupled to horseradish peroxidase were obtained from Sigma (St Louis, MO).
Bleeding time measurements
Tail-bleeding measurements were performed concurrently with removal of tail portions for genotyping (see “Genotyping”). Mice, at least 4 weeks of age, were first sedated with ketamine/HCl 150 mg/kg, intraperitoneally, and their tails were transected 3 mm from the tip with a fresh scalpel blade. The tail was placed in phosphate buffered saline at 37°C, and the time required for blood flow to stop was measured. The clotting time was not considered complete until the tail had stopped bleeding for 1 minute. When required, measurements were terminated at 600 seconds and bleeding was stopped.
Genotyping
The genotype of each mouse was determined by polymerase chain reaction (PCR) using DNA prepared from the distal portion of the tail. A 3-mm piece from the tip of the tail was transected with a scalpel and digested in 50 mM Tris (tris(hydroxymethyl)aminomethane)/HCl, pH 7.5, 100 mM EDTA (ethylenediaminetetraacetic acid), 100 mM NaCl, 1% sodium dodecyl sulfate (SDS) with 0.5 mg/mL proteinase K (Promega, Madison, WI) for 18 hours at 55°C. Samples were brought up to 2 M NaCl final, mixed with a vortex mixer for 2 minutes and insoluble material was removed by centrifugation for 30 minutes in a microfuge (13 800 × g). The supernatants were harvested, and the genomic DNA was recovered by ethanol precipitation. PCR analysis was carried out using the H and J primers described in Yang et al,17 as well as the Neo primer (5′-GAGCAGCCGATTGTCTGTTG).
Blood collection
Mice were killed by carbon dioxide narcosis, and blood was collected by first exposing the heart via an incision in the ventral thoracic area. A 1-mL syringe containing 1.8% sodium citrate, pH 7.4 (70-100 μL) was used to aspirate the blood (0.7-1 mL) directly from the right ventricle. The blood was mixed with an additional 1.8% sodium citrate (pH 7.4) to a final concentration of 0.18%.
Platelet preparation
Citrated blood from several mice of identical genotype was pooled and diluted with an equal volume of saline (pH 7.4) or HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)/Tyrode buffer (10 mM HEPES/NaOH, pH 7.4, 5.56 mM glucose, 137 mM NaCl, 12 mM NaHCO3, 2.7 mM KCl, 0.36 mM NaH2PO4, 1 mM MgCl2). Platelet-rich plasma (PRP) was prepared by centrifugation at 100 × g for 10 minutes and then used for aggregation experiments (see “Genotyping”). Care was taken to harvest only the platelet-containing upper layer to avoid the buffy coat layer of nucleated cells above the red blood cells. Washed platelets were prepared from PRP by centrifugation at 5000 × g for 2 minutes. The platelet pellets were resuspended in HEPES/Tyrode buffer in the presence of 3 μg/mL apyrase and 10 ng/mL prostaglandin I2 (PGI2). Platelets were washed twice.
Platelet counts
PRP was collected as above and diluted 1/100 in HEPES/Tyrode buffer. The platelets were counted with a hemocytometer at 60 × magnification. In no case was any contamination of nucleated cells detected in the platelet preparations. Platelet counts were consistently approximately 5.0 × 105/μL for all of the genotypes.
Platelet aggregation
Platelet aggregation was measured using an optical aggregometer (Chrono-Log, Havertown, PA). PRP was adjusted to 2.5 × 105/μL with HEPES/Tyrode buffer, pH 7.4, and platelets were recalcified by addition of 1 mM CaCl2 final. Washed platelets from wild-type (+/+) and cellubrevin/VAMP-3 knockout mice (–/–) were collected from citrated PRP by centrifugation and resuspended in HEPES/Tyrode buffer to a concentration of 3.5 × 105 platelets/μL. This higher concentration is required to affectively measure aggregation in the absence of plasma. Platelets were incubated at 37°C for 5 minutes prior to addition of agonist. For all aggregation experiments, platelet suspensions (250 or 500 μL) were placed in siliconized aggregation cuvettes, warmed to 37°C with stirring for 5 minutes, and aggregation was initiated by the addition of the indicated amounts of thrombin (Chrono-Log), collagen (Chrono-Log), and adenosine diphosphate (ADP; Chrono-Log).
Measurement of thrombin-induced secretion
Platelets were assayed for dense core release of [3H]-5HT, lysosomal release of β-hexosaminidase, and alpha granule release of platelet factor IV (PF4), as described previously,4,7,11 with the following modifications. Prior to isolation of platelets, the PRP was incubated with 0.4 μCi (0.0148 MBq)/mL [3H]-5HT for 45 minutes at 37°C with gentle shaking. Platelets were then washed as described above and resuspended in HEPES/Tyrode buffer to a concentration of 5.0 × 105/μL. A titration of CaCl2 (0-1 mM) was conducted to determine an optimal calcium concentration (0.7 mM) that would not spontaneously activate the washed platelets under the conditions used for the assay. Thrombin and collagen were then titrated into the release assay using the optimal calcium concentration, and the cells were incubated for the indicated times at 25°C. The platelets were then pelleted at 13 800 × g for 1 minute in a microfuge, and the reaction supernatants were retained and flash frozen. Pellets were resuspended in a volume equal to the original reaction in Buffer A4,7,11 and disrupted by 5 successive freeze-thaw cycles. Equal volumes of both supernatant and pellet fractions were then assayed for all granule cargo markers. Secretion of platelet factor IV was measured from supernatants and pellets using the anti–mouse PF4/CXCL4 ELISA kit from R & D Systems (Minneapolis, MN) following the manufacturer's instructions. The data were tabulated as the percent release compared to the total present in each reaction.
Electron microscopy
Resting platelets were treated with apyrase and PGI2 as above and prepared for electron microscopy as described previously,11 with the following modifications. An equal volume of 6% glutaraldehyde (Electron Microscopy Sciences, Ft Washington, PA), 80 mM lysine (Sigma) in 0.1 M Sorenson phosphate buffer (8.1 mg/mL KH2PO4, 1.88 mg/mL Na2HPO4) was added to the reactions, and the platelets were incubated at 4°C for 12 hours. The platelets were washed 4 times for 15 minutes with 0.1 M Sorenson phosphate buffer and osmicated with 1% OsO4 in 0.1 M Sorenson for 30 minutes on ice. Following 2 brief washes in ice-cold H2O, the platelets were dehydrated in a series of ethyl alcohol solutions (50%, 70%, 80%, 90%, and 100%) for 5 minutes each step. The platelets were rinsed with 3 changes of propylene oxide and infiltrated overnight in a 1:1 mixture of propylene oxide and Spurr resin. Samples were embedded in Spurr resin at 50°C for 48 hours. Polymerized blocks were sectioned, mounted on copper grids, and examined using a Philips Tecnai 12 transmission electron microscope (FEI, Hillsboro, OR) with AMT v54208 software (AMT, Boston, MA) for photography and measurement.
Western blotting
Platelets were collected from PRP and washed twice in HEPES/Tyrode with 0.18% sodium citrate. During the final wash, platelets were counted, collected, and resuspended to a concentration of 1.0 × 107/μL. Platelets were solubilized with 2 × SDS buffer, 1 mM EDTA, 2 × complete EDTA-free protease inhibitor (Roche Diagnostics, Indianapolis, IN), 300 μM calpeptin (Calbiochem, San Diego, CA), 100 μM caspase 3 inhibitor (Calbiochem), and 100 [um]M cathepsin inhibitor (Calbiochem). Samples (5.0 × 107 platelets) were boiled for 10 minutes and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. For detection of synaptobrevin/VAMP-2 in human platelets, cells were prepared from freshly drawn blood from healthy volunteers or from banked units and solubilized with 1 × SDS-PAGE buffer containing 2 × complete EDTA-free protease inhibitor (Roche Diagnostics), 2 mM EDTA, 2 mM ethylene glycol tetraacetic acid (EGTA), 300 μM calpeptin (Calbiochem), 100 μM caspase 3 inhibitor (Calbiochem), and 100 μM cathepsin inhibitor (Calbiochem). Samples were then heated to 42°C for 20 minutes prior to electrophoresis and Western blotting.
Results
Cellubrevin/VAMP-3 knockout mice have no bleeding time defect
As previously reported in Yang et al,17 the cellubrevin/VAMP-3 knockout mice showed no defect in activity or fecundity and showed no gross morphological defects. To initially assess the role of cellubrevin/VAMP-3 in platelet function, bleeding times for wild-type, heterozygous, and null mice were measured using the transected-tail method. Despite the variability of the bleeding times measured, there was no statistically significant (P ≤ .05, 2-tailed Student t test) difference between the average clotting times measured for wild-type (318 seconds ± 211, n = 34), heterozygous (271 seconds ± 190, n = 51), or null (246 seconds ± 181, n = 28) animals (data not shown). When the sex of the animal was taken into account, there was still no statistically significant difference in the average bleeding times (data not shown). Taken as a whole, these data indicate that the loss of cellubrevin/VAMP-3 does not lead to a gross hemostatic defect.
Platelets from cellubrevin/VAMP-3 knockout mice contain normal levels of secretory machinery proteins
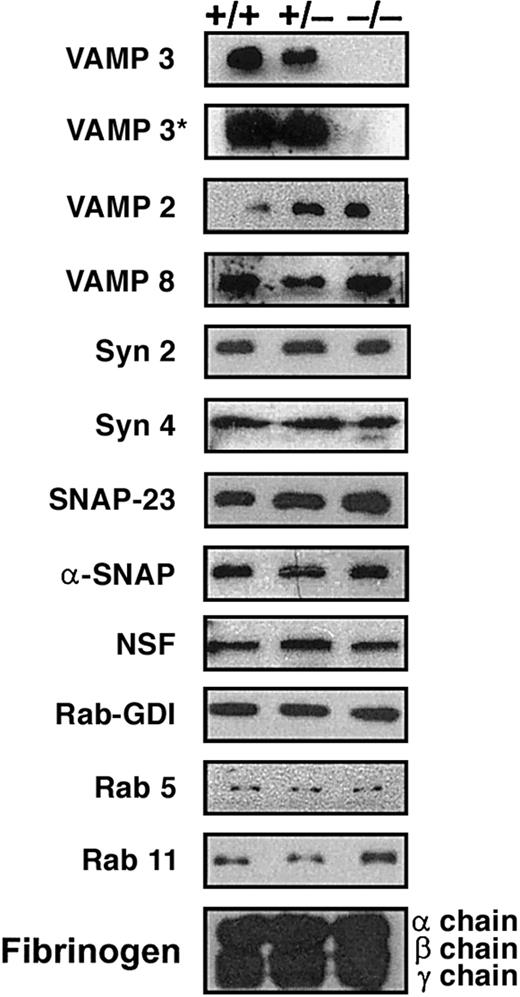
Platelets were prepared from animals of each genotype and were tested by Western blotting to determine, first, if the null platelets truly lacked cellubrevin/VAMP-3, and then to determine if other known secretory machinery components were affected by its loss. The platelet preparations were free of contaminating nucleated cells (as judged by microscopy), and all 3 genotypes yielded approximately equal numbers of platelets per μL (∼ 5.0 × 105/μL). As shown in the first 2 panels of Figure 1, cellubrevin/VAMP-3 was not detectible in platelets from null animals (–/–) even after extended exposures (5 hours, VAMP-3*) of the Western blot. SNAREs, known to be present in human platelets (ie, endobrevin/VAMP-8, syntaxin 2, 4, and SNAP-23) were present in the murine platelets. Their levels did not significantly differ when equal numbers of platelets from the 3 genotypes were compared. This was true also for the general elements of the secretory machinery, α-SNAP, and NSF, as well as some of the potential regulatory proteins such as Rab-GDI, Rab 5, and 11. The levels of an alpha granule cargo protein, fibrinogen (α, β, and γ chain), also were similar. Based on the secretion experiments discussed below, the platelet levels of hexosaminidase and platelet factor IV (PF4) also were similar between the 3 genotypes. These data confirm the lack of cellubrevin/VAMP-3 and show that most of the known elements of platelet exocytosis machinery and some of the granule cargo proteins were not affected by its absence. This analysis did however demonstrate that synaptobrevin/VAMP-2 was present in mouse platelets. Equally surprising, synaptobrevin/VAMP-2 levels were affected by the loss of cellubrevin/VAMP-3. When platelet equivalents were compared, there was a consistent increase in the synaptobrevin/VAMP-2 in both null and heterozygous platelets relative to wild type. This increase was seen in 3 preparations of platelets from 3 distinct sets of animals. Such an increase was not seen in any of the 8 tissues examined by Yang et al,17 nor was it seen in our independent tests of liver, kidney, and spleen (data not shown).
Secretory machinery in mouse platelets. Platelets were collected from platelet-rich plasma (PRP) and washed twice in Tyrode buffer with 0.18% sodium citrate. During the final wash, platelets were counted and resuspended to a concentration of 1.0 × 107/μL in 2 × SDS-PAGE sample buffer containing 1 mM EDTA, 2 × complete EDTA-free protease inhibitor, 300 μM calpeptin, 100 μM caspase 3 inhibitor, and 100 μM cathepsin inhibitor. Platelet equivalents (5.0 × 107 per lane) were loaded and analyzed by Western blotting with antibodies to the indicated proteins. The blots labeled VAMP 3 and VAMP 3* were exposed for 5 minutes and 5 hours, respectively.
Secretory machinery in mouse platelets. Platelets were collected from platelet-rich plasma (PRP) and washed twice in Tyrode buffer with 0.18% sodium citrate. During the final wash, platelets were counted and resuspended to a concentration of 1.0 × 107/μL in 2 × SDS-PAGE sample buffer containing 1 mM EDTA, 2 × complete EDTA-free protease inhibitor, 300 μM calpeptin, 100 μM caspase 3 inhibitor, and 100 μM cathepsin inhibitor. Platelet equivalents (5.0 × 107 per lane) were loaded and analyzed by Western blotting with antibodies to the indicated proteins. The blots labeled VAMP 3 and VAMP 3* were exposed for 5 minutes and 5 hours, respectively.
Morphology of platelets from cellubrevin/VAMP-3 knockout mice
Platelets from wild-type and null animals were prepared and analyzed by transmission electron microscopy. As shown in Figure 2, platelets from null (panel A) and wild-type (panel B) animals had essentially normal morphology, containing alpha granules, dense core granules, and mitochondria. There was no statistically relevant difference between the number of granules per platelets between wild-type and null platelets.
Electron microscopy analysis of cellubrevin/VAMP-3 null platelets. Platelets were prepared, maintained as resting, and fixed for electron microscopy as described in “Materials and methods.” (A) Null (–/–) platelets. (B) Wild-type (+/+) platelets. Scale bars equal 0.5 μm.
Electron microscopy analysis of cellubrevin/VAMP-3 null platelets. Platelets were prepared, maintained as resting, and fixed for electron microscopy as described in “Materials and methods.” (A) Null (–/–) platelets. (B) Wild-type (+/+) platelets. Scale bars equal 0.5 μm.
Platelets from cellubrevin/VAMP-3 knockout mice do not have a defect in secretion
Based on the literature discussed above, it was expected that cellubrevin/VAMP-3 would be required for secretion, yet given the lack of a bleeding time defect it was important to determine if the loss of cellubrevin/VAMP-3 affected the secretory process. Intact platelets were first loaded with [3H]-5HT, washed with HEPES/Tyrode buffer, and then stimulated with increasing concentrations of thrombin or collagen (Figure 3). The platelets were then pelleted, and the supernatants were assayed for the presence of granule cargo markers: [3H]-5HT from dense core granules, PF4 from alpha granules, and hexosaminidase from lysosomes. As expected, agonist-induced release of dense core and alpha granule cargo from wild-type platelets (open symbols, Figures 3, 4) was greater than the release from lysosomes. Comparison of platelets from wild-type and null mice (closed symbols) showed that each responded equally to both thrombin and collagen and released granular cargo to relatively similar extents (Figure 3). To assess the efficiency of release, the time course of granule release was analyzed (Figure 4). Thrombin stimulation initiated dense core granule release (•,○) rapidly and appeared to be complete by 30 seconds. This same response also was seen for alpha granule release (▵,▴), although the extent of release was not as great. Lysosome release proceeded more slowly (□,▪). Comparison of release kinetics between wild-type and null platelets showed no significant difference in the time course of their granule release. Based on these assays, it appears that cellubrevin/VAMP-3 is not required for agonist-stimulated exocytosis from murine platelets.
Thrombin- and collagen-induced granule secretion is normal in cellubrevin/VAMP-3 platelets. Platelets from null (–/–; filled symbols) and wild-type (+/+; open symbols) mice were prepared as described in “Materials and methods.” Aliquots of intact washed platelets (2.5 × 108) were incubated with 0.7 mM CaCl2 at room temperature for 5 minutes and then incubated with the indicated thrombin or collagen concentrations at room temperature for 2 minutes (thrombin) or 4 minutes (collagen). Release of [3H]-5HT from dense core granules, PF4 from alpha granule release, and hexosaminidase from lysosomes were measured as described in “Materials and methods.” Release is calculated as the percent of total platelet marker present in reaction supernatant. The points represent the average of triplicate measurements and the standard deviation is indicated.
Thrombin- and collagen-induced granule secretion is normal in cellubrevin/VAMP-3 platelets. Platelets from null (–/–; filled symbols) and wild-type (+/+; open symbols) mice were prepared as described in “Materials and methods.” Aliquots of intact washed platelets (2.5 × 108) were incubated with 0.7 mM CaCl2 at room temperature for 5 minutes and then incubated with the indicated thrombin or collagen concentrations at room temperature for 2 minutes (thrombin) or 4 minutes (collagen). Release of [3H]-5HT from dense core granules, PF4 from alpha granule release, and hexosaminidase from lysosomes were measured as described in “Materials and methods.” Release is calculated as the percent of total platelet marker present in reaction supernatant. The points represent the average of triplicate measurements and the standard deviation is indicated.
The rate of thrombin-induced secretion of granule cargo is normal in cellubrevin/VAMP-3 platelets. Platelets from null (–/–; filled symbols) and wild-type (+/+; open symbols) mice were prepared as described in “Materials and methods.” Aliquots of intact washed platelets (2.5 × 108 cells) were incubated with 0.7 mM CaCl2 at room temperature for 5 minutes and then incubated with thrombin (0.5 U/mL) at room temperature for the indicated times. Release of [3H]-5HT from dense core granules (○,•), of PF4 from alpha granule release (▵,▴), and of hexosaminidase from lysosomes (▪,□) were measured as described in “Materials and methods.” Release is calculated as the percent of total platelet marker present in reaction supernatant. The points represent the average of triplicate measurements, and the standard deviation is indicated.
The rate of thrombin-induced secretion of granule cargo is normal in cellubrevin/VAMP-3 platelets. Platelets from null (–/–; filled symbols) and wild-type (+/+; open symbols) mice were prepared as described in “Materials and methods.” Aliquots of intact washed platelets (2.5 × 108 cells) were incubated with 0.7 mM CaCl2 at room temperature for 5 minutes and then incubated with thrombin (0.5 U/mL) at room temperature for the indicated times. Release of [3H]-5HT from dense core granules (○,•), of PF4 from alpha granule release (▵,▴), and of hexosaminidase from lysosomes (▪,□) were measured as described in “Materials and methods.” Release is calculated as the percent of total platelet marker present in reaction supernatant. The points represent the average of triplicate measurements, and the standard deviation is indicated.
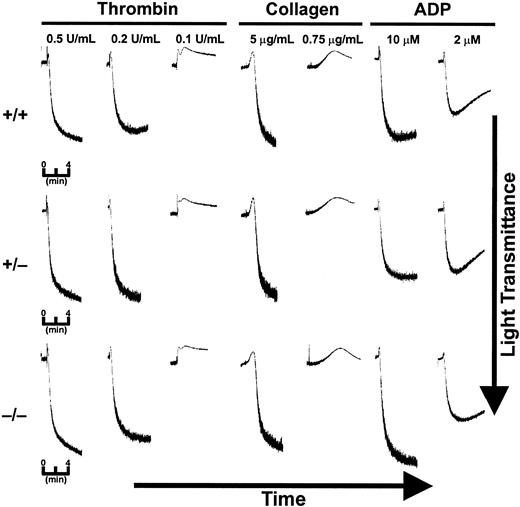
Platelets from cellubrevin/VAMP-3 knockout mice can aggregate in response to stimuli
Platelet aggregation was tested for each of the 3 genotypes. PRP, containing citrate as an anticoagulant, was diluted to a constant platelet number (2.5 × 105/μL) and a final citrate concentration of 0.09% (3 mM). The PRP was recalcified to 1 mM CaCl2. Under these conditions platelets from all 3 of the genotypes were responsive to thrombin, collagen, and ADP (Figure 5). The platelets showed an initial, agonist-induced shape-change response followed by aggregation as indicated by the increase in light transmission. The kinetics and extent of aggregation to agonist at high (0.5 U/mL thrombin, 10 μg/mL collagen, 10 μM ADP) and intermediate levels (0.2 U/mL thrombin, Figure 5; 1.5 μg/mL collagen, data not shown; 5 μM ADP, data not shown) were essentially identical between the 3 groups. Washed platelets (3.5 × 105/μL) also were tested for their ability to aggregate in response to agonists (Figure 6). Higher concentrations of washed platelets are required to effectively measure aggregation in the absence of plasma, but the cells tend to be more responsive due to the removal of citrate. Under these conditions, the platelets did aggregate in response to thrombin and collagen. When wild-type and null platelets were compared, there was no difference in the extent or in the rate of aggregation in response to the levels of thrombin or collagen used. These aggregometer measurements are consistent with the normal bleeding times and the normal secretion (Figures 3, 4) in the cellubrevin/VAMP-3 null platelets. Thus, the absence of this v-SNARE does not result in any gross defect in platelet function.
Aggregation is normal in platelets from cellubrevin/VAMP-3 knockout mice. PRP was prepared from wild-type (+/+), heterozygous (+/–), and cellubrevin/VAMP-3 knockout mice (–/–) and diluted to a final concentration of 0.09% sodium citrate as described in “Materials and methods.” PRP was preincubated with 1 mM CaCl2 at 37°C for 5 minutes prior to incubation with agonist as indicated.
Aggregation is normal in platelets from cellubrevin/VAMP-3 knockout mice. PRP was prepared from wild-type (+/+), heterozygous (+/–), and cellubrevin/VAMP-3 knockout mice (–/–) and diluted to a final concentration of 0.09% sodium citrate as described in “Materials and methods.” PRP was preincubated with 1 mM CaCl2 at 37°C for 5 minutes prior to incubation with agonist as indicated.
Washed platelets from cellubrevin/VAMP-3 knockout mice show normal aggregation. Washed platelets from wild-type (+/+) and cellubrevin/VAMP-3 knockout mice (–/–) were collected from citrated PRP by centrifugation and resuspended in HEPES/Tyrode buffer to a concentration of 3.5 × 105 platelets/μL. Platelets were incubated at 37°C for 5 minutes prior to addition of agonist. Aggregation was measured as an increase in light transmission versus time.
Washed platelets from cellubrevin/VAMP-3 knockout mice show normal aggregation. Washed platelets from wild-type (+/+) and cellubrevin/VAMP-3 knockout mice (–/–) were collected from citrated PRP by centrifugation and resuspended in HEPES/Tyrode buffer to a concentration of 3.5 × 105 platelets/μL. Platelets were incubated at 37°C for 5 minutes prior to addition of agonist. Aggregation was measured as an increase in light transmission versus time.
Synaptobrevin/VAMP-2 in human platelets
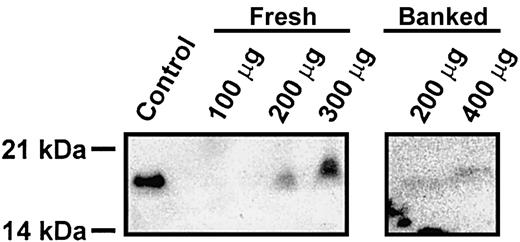
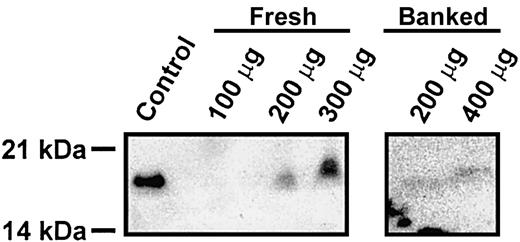
From the data presented here, it is clear that cellubrevin/VAMP-3 is not required for rodent platelet exocytosis. Given the presence of synaptobrevin/VAMP-2 and its known association with other regulated secretory events,24-26 it seems highly probable that it is the critical v-SNARE for platelet exocytosis in rodents. However, it is not clear why mouse and human platelets would have such divergent secretory machinery. Given this conundrum, we reexamined whether human platelets contained synaptobrevin/VAMP-2. In Figure 7, specific anti–syntaptobrevin/VAMP-2 antibodies did detect a protein of the correct molecular weight in extracts prepared from fresh platelets in the presence of a large number of protease inhibitors. In some preparations, even in the presence of protease inhibitors, degradation of synaptobrevin/VAMP-2 was evident (data not shown). Also, when the source of extracts was banked platelets, the level of synaptobrevin/VAMP-2 was significantly lower (Figure 7). These data clearly demonstrate that synaptobrevin/VAMP-2 is present in human platelets. It was not detected in earlier experiments from our laboratory12 and others,3,8 due to its low level of expression and its apparent susceptibility to proteolysis.
Synaptobrevin/VAMP-2 is present in human platelets. Human platelets were prepared from freshly drawn blood or from banked units and solubilized with 1 × SDS-PAGE buffer containing 2 × Roche Complete EDTA-free protease inhibitor, 2 mM EDTA, 2 mM EGTA, 300 μM calpeptin, 100 μM caspase 3 inhibitor, and 100 μM cathepsin inhibitor. Samples were then heated to 42°C for 20 minutes, and the indicated amounts of total proteins were analyzed by electrophoresis and Western blotting. As a blotting control, 1 μg mouse cerebrum was used.
Synaptobrevin/VAMP-2 is present in human platelets. Human platelets were prepared from freshly drawn blood or from banked units and solubilized with 1 × SDS-PAGE buffer containing 2 × Roche Complete EDTA-free protease inhibitor, 2 mM EDTA, 2 mM EGTA, 300 μM calpeptin, 100 μM caspase 3 inhibitor, and 100 μM cathepsin inhibitor. Samples were then heated to 42°C for 20 minutes, and the indicated amounts of total proteins were analyzed by electrophoresis and Western blotting. As a blotting control, 1 μg mouse cerebrum was used.
Discussion
In this manuscript we report the characterization of platelets from mice lacking the cellubrevin/VAMP-3 protein. As previously reported,17 these knockout mice showed no defect in breeding, activity, or any gross morphological differences from wild-type littermates. Platelet levels were comparable between wild-type, heterozygous, and null animals with no statistically significant differences in average bleeding times. Thrombin-induced release from each of the 3 intraplatelet stores (dense core, alpha granules, and lysosomes) was normal in the null mouse platelets (Figures 3, 4), suggesting that cellubrevin/VAMP-3 is not required for secretion. Consistently, when testing either PRP or washed platelets in HEPES/Tyrode buffer, there was no defect in aggregation in response to different agonists (Figures 5, 6). Western blotting analysis showed that most of the elements of the secretory machinery were present in mice and equally expressed in both wild-type and null platelets (Figure 1). The one notable difference between human and mouse platelets was the presence of the v-SNARE synaptobrevin/VAMP-2. Surprisingly, this protein's level appeared greater in the heterozygous and null platelets relative to wild type. The increase was not seen in other tissues (Yang et al17 and data not shown) and therefore appears to be platelet specific.
Based on studies of human platelets, the lack of a secretion defect in the cellubrevin/VAMP-3 null platelets is surprising. Two separate studies have implicated cellubrevin/VAMP-3 as a major component of human platelet exocytosis.3,8 In one study, the cytosolic domain of cellubrevin/VAMP-3 was shown to inhibit P-selectin exposure (secretion from alpha granules) by 100% and to block serotonin release by ∼ 80%. A similar domain of endobrevin/VAMP-8 had an intermediate effect, blocking only serotonin release (by ∼ 25%). The cytosolic domain of synaptobrevin/VAMP-2 had no effect.8 This inhibition by v-SNARE cytoplasmic domains could be due to spurious pairing of SNARE coiled-coil domains as has been reported,27,28 but it is consistent with the report of Feng et al,3 which shows that antibodies directed to the unique amino terminus of cellubrevin/VAMP-3 inhibited exposure of P-selectin (by ∼ 50%-60%). Unfortunately, Fab fragments of this antibody were not used, so it is difficult to assure that the inhibition seen was not due to nonspecific steric effects that could block the close association of membranes required for fusion. By comparison, a slightly higher degree of inhibition (60%-70%) was seen when permeabilized platelets were pretreated with the v-SNARE cleaving tetanus toxin.6 This toxin cleaves 3 different v-SNAREs: synaptobrevin/VAMP-1, -2, and cellubrevin/VAMP-3.13,14 Despite potential technical caveats, these data consistently point to an important role for cellubrevin/VAMP-3 in human platelets; however, the data presented in this manuscript suggest that this is not necessarily the case for release from mouse platelets.
In past experiments, human platelets were exhaustively probed for the presence of synaptobrevin/VAMP-2 using Western blotting,3,6,8,9,12 mass-spectrometry,8 and molecular biology methods,12 and it was not detected. There are several reasons for this series of negative results, but the most likely explanation is that synaptobrevin/VAMP-2 is expressed at low levels and is degraded during sample preparation. Consistently, Lai and Flaumenhaft29 have shown that cellubrevin/VAMP-3 can be degraded in activated platelets by an unknown protease, therefore degradation appears to be a key problem when examining platelet v-SNAREs. Taking this into consideration, we now demonstrate that synaptobrevin/VAMP-2 is present in human platelets. This is most convincingly shown in fresh platelets prepared in the presence of an extensive cocktail of protease inhibitors (Figure 7).
Two possibilities could explain the differences in the requirement for cellubrevin/VAMP-3 in human versus mouse. First, given the apparent increase in the levels of synaptobrevin/VAMP-2, it is possible that this v-SNARE could have a compensatory effect, thus masking the lack of cellubrevin/VAMP-3. Both v-SNAREs can interact with the same sets of t-SNAREs.30-33 This is consistent with other reports of functional promiscuity between SNARE–SNARE complexes (for a recent example, see Bhattacharya34 ) and would suggest that the pairing of the SNARE machinery in platelets may not be as restricted as was originally proposed.11 As a second possibility, this could represent differences between mouse and human platelet function and physiology. Examples of such differences have been reported in signaling systems such as the thrombin receptor.35 Murine and human platelets clearly contain both synaptobrevin/VAMP-2 and cellubrevin/VAMP-3 (Figures 1, 7); however, it is possible that only synaptobrevin/VAMP-2 is required in mice, and either cellubrevin/VAMP-3 or syntaptobrevin/VAMP-2 is required for release in humans.
The data in this manuscript have several implications and raise several questions about the mechanisms of platelet exocytosis. The lack of a secretion phenotype in the cellubrevin/VAMP-3 null mouse platelets is clearly different from what would be expected, based on previous studies of human platelets.3,6,8 However, given that syntaptobrevin/VAMP-2 is present in human platelets, it is clear that the original reports need to be re-examined since they were based on the premise that synaptobrevin/VAMP-2 was absent. The data reported in this manuscript serve to focus several specific questions that must be resolved regarding the importance of synaptobrevin/VAMP-2 in human platelets as we seek to gain a more defined picture of the molecular mechanism involved in platelet function.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2003-01-0331.
Supported by grants from the Ohio Valley Affiliate of the American Heart Association (0215130B and 0150841B) (T.D.S. and S.W.W.) and the National Institutes of Health (HL56652-06) (S.W.W.).
T.D.S. and T.W.R. contributed equally to this study.
The authors declare that they have no competing financial interests.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to acknowledge Dr William Dean and his invaluable assistance and discussions, and the staff members of the University of Kentucky, Department of Laboratory Animal Research for their assistance. We would specifically like to thank Dr Elena Matveeva for her careful reading of this manuscript.


![Figure 3. Thrombin- and collagen-induced granule secretion is normal in cellubrevin/VAMP-3 platelets. Platelets from null (–/–; filled symbols) and wild-type (+/+; open symbols) mice were prepared as described in “Materials and methods.” Aliquots of intact washed platelets (2.5 × 108) were incubated with 0.7 mM CaCl2 at room temperature for 5 minutes and then incubated with the indicated thrombin or collagen concentrations at room temperature for 2 minutes (thrombin) or 4 minutes (collagen). Release of [3H]-5HT from dense core granules, PF4 from alpha granule release, and hexosaminidase from lysosomes were measured as described in “Materials and methods.” Release is calculated as the percent of total platelet marker present in reaction supernatant. The points represent the average of triplicate measurements and the standard deviation is indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2003-01-0331/6/m_h81734869003.jpeg?Expires=1767767199&Signature=4fkKl5fuYe5A2tp2f86ftvvwiG~s6NQo5BS4KLzxibRrqrWCuJbcqmuqimekplxmvGz4s0xoFny3MZmf8emDS6PBJmqIGGBGxcxMZc16kfrUaYNchJXLtQa5eC8xVhohr-VVQIKRcUNKoQelfES1R63FH~lMHQRwlF7g5HDsKpiZ7-PBlHJixXy0QsKkltCBbG44vERpqCP88duGSYh96Dths3g4DfyD0W6DX4uK2LD8eJnY8KDGtcGX8VMtnfntUMEUT7kE2HQWWhWOgmhIoq8-523xzwPGpg7at3vmNKw69tQieePN3bKuTw~c-9aoFVDA9rgnFCr4eC3BKEyUdw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. The rate of thrombin-induced secretion of granule cargo is normal in cellubrevin/VAMP-3 platelets. Platelets from null (–/–; filled symbols) and wild-type (+/+; open symbols) mice were prepared as described in “Materials and methods.” Aliquots of intact washed platelets (2.5 × 108 cells) were incubated with 0.7 mM CaCl2 at room temperature for 5 minutes and then incubated with thrombin (0.5 U/mL) at room temperature for the indicated times. Release of [3H]-5HT from dense core granules (○,•), of PF4 from alpha granule release (▵,▴), and of hexosaminidase from lysosomes (▪,□) were measured as described in “Materials and methods.” Release is calculated as the percent of total platelet marker present in reaction supernatant. The points represent the average of triplicate measurements, and the standard deviation is indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2003-01-0331/6/m_h81734869004.jpeg?Expires=1767767199&Signature=EgqtkLiOmQxtNo80NKK2z-WClN7aATwYfF00rWW7gVXcRY2HODKg6l~WiF8FH~-7EVvEV~6vdbvGMHkwlrkzJkAgu87sniClUdN0qoOI12QYvsgFI6fKG-emB47dC5ustlKibP9HJNBI695qI7Bi1j78P-img-nGuKqXOFOCWvy47qU0wbGQIRZwTjkPHNdBM2TDQZ4KYZ8lQVvJzaI0tnL4LHBSCER92LBl529lJcNOnGU5gxYhZnTOR01AN6z3TnLXhXgJpf5I7hmvoYa~lQLj1y-MQKPOjkDeFyycr73-GZOBFxjYGI73qq8b6yM2hmfGBE09pbQrXs99WhZ-xg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)