Abstract
Activation of protein C by thrombin bound to thrombomodulin is enhanced by endothelial protein C receptor. This pathway may inhibit inflammation. We investigated effects of protein C and activated protein C on neutrophils as well as whether an endothelial protein C receptor is involved in mediating protein C effects. Neutrophils were from venous blood of healthy donors. Cell migration, respiratory burst, phagocytic activity, and apoptosis were studied by micropore filter assays and fluorometry. Receptor expression was investigated by reverse transcriptase–polymerase chain reaction (PCR) for mRNA, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography of immunoprecipitated receptor protein, and fluorescence-activated cell-sorter scanner (FACS) analysis using the anti–endothelial protein C receptor antibody RCR-252. Neither protein C nor activated protein C induced migration, yet both of them inhibited neutrophil chemotaxis triggered by interleukin-8, formyl-Met-Leu-Phe, antithrombin, or C5a. A protein C activation–blocking antibody against endothelial protein C receptor diminished inhibitory effects of protein C or activated protein C on migration. No effect of either protein C preparation was seen in neutrophil's respiratory burst, bacterial phagocytosis, or apoptosis assays. Endothelial protein C receptor immunoreactivity was confirmed on neutrophils by FACS. De novo synthesis is suggested by endothelial protein C receptor mRNA expression as demonstrated by reverse transcriptase PCR and immunoprecipitation SDS-PAGE analyses. Data suggest that an endothelial protein C receptor is expressed by human neutrophils whose active site ligation with either protein C or activated protein C arrests directed cell migration. Inhibitory effects of these components of the protein C pathway on neutrophil function may play a role in the protein C–based treatment of severe sepsis.
Introduction
The natural anticoagulant activated protein C (APC) plays an important role in coagulation homeostasis by inactivating the procoagulant factors factor Va and VIIIa.1 Protein C (PC) is activated by thrombin bound to thrombomodulin, and this effect is enhanced in the presence of the endothelial protein C receptor (EPCR).2 Patients with homozygous PC deficiency usually have life-threatening thrombotic complications in infancy3 that can be corrected by administration of PC.4 In vivo and in vitro studies have revealed that components of this pathway also may inhibit inflammatory responses. APC is able to inhibit leukocyte adhesion to vascular endothelial cells and to reduce neutrophil accumulation in rat lungs.5 It also inhibits proinflammatory cytokine release in monocytes6 that were shown to express EPCR.7 Soluble EPCR binds to proteinase-3 and CD11b/CD18 on activated neutrophils,8 which were previously shown to synthesize thrombomodulin but not to promote thrombin-dependent PC activation.9
Because of its anticoagulatory and anti-inflammatory effects, the PC pathway may play an important role in host defense. Previous studies have shown that infusion of APC protects baboons from a lethal response to Escherichia coli (E coli) and that inhibition of PC activation exacerbates the response to a sublethal number of E coli.10 The EPCR has been shown to aid in host defense against E coli sepsis,11 and treatment with APC significantly reduces mortality in patients with severe sepsis.12 Whether protein C directly affects neutrophil functions has not yet been conclusively demonstrated. In this study we examined the effects of PC and APC on neutrophils and show that neutrophils express an EPCR that may be involved in inhibitory effects of PC and APC on cell migration.
Materials and methods
Reagents
Lymphoprep was from Nycomed Pharma AS (Oslo, Norway) and dextran was from Sigma Chemical (St Louis, MO). The MACS separation columns and microbeads were from Miltenyi Biotech (Auburn, CA).
RPMI 1640 was purchased from Biological Industries (Kibbutz Beit Haemek, Israel). Bovine serum albumin (BSA) was from Dade Behring (Marburg, Germany), interleukin-8 (IL-8), formyl-Met-Leu-Phe, C5a, phorbol myristate acetate (PMA), and activated plasma-derived human protein C (AphPC) were from Sigma Chemical. Plasma-derived human protein C (phPC, Ceprotin) was from Baxter (Deerfield, IL), and activated recombinant human protein C (ArhPC, Xigris) was from Lilly (Indianapolis, IN). Antithrombin (AT, Kybernin P) was from Aventis Behring (Vienna, Austria), the thrombin receptor activator peptide SFLLRNPNDKYEP, hirudin, and prothrombin were from Sigma Chemical. The thrombin receptor antibody ATAP2 and the PAR2 antibody (C-17) were from Santa Cruz Biotechnology (Santa Cruz, CA), and the PAR2 agonist was from Neosystem (Strasbourg, France). Protein S was from Enzyme Research Laboratories (South Bend, IN), and the Gla antibody was from American Diagnistica (Greenwich, CT). Hanks balanced salt solution (HBSS) without phenol red was from Invitrogen (Carlsbad, CA). The microchemotaxis chambers were from Neuroprobe (Bethesda, MD) and nitrocellulose filters were from Sartorius (Goettingen, Germany).
Staurosporine was from Sigma Chemical, granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leucomax) was from Novartis (Vienna, Austria), and 2′,7′-dichlorofluorescin diacetate (H2DCFDA) was from Molecular Probes (Eugene, OR).
RNA-Bee was from Tel-Test (Friendswood, TX), reverse transcriptase was from Invitrogen (Carlsbad, CA), RNAsin was from Promega (Madison, WI), and dNTPs were from Amersham (Buckinghamshire, United Kingdom). Hot Star Taq polymerase was purchased from Qiagen (Valencia, CA), and primers were from MWG Biotech (Ebersdorf, Germany). Certified PCR Agarose was from Bio-Rad (Hercules, CA).
The biotinylated mouse anti–rat antibody and the IgG isotype control were from eBioscience (San Diego, CA), streptavidin-PE was from Beckton-Dickinson (San Jose, CA), and human IgG was from Sigma.
Dulbecco phosphate-buffered saline (PBS) and l-glutamine were from PAA Laboratories (Linz, Austria), RPMI without l-methionine and l-glutamine were from Biochrom (Berlin, Germany), EasyTag EXPRESS Protein Labeling Mix [35S] was from PerkinElmer (Wellesley, MA), Nonidet P40 was from Roche (Mannheim, Germany), the protein A sepharose was from Amersham Biosciences (Buckinghamshire, England), and the enhancer solution was from DuPont (Wilmington, DE).
Preparation of leukocytes
Peripheral blood mononuclear cells (PBMCs) were prepared from forearm venous blood of healthy volunteers (anticoagulated with EDTA [ethylenediaminetetraacetic acid]). After Lymphoprep density gradient centrifugation, peripheral blood mononuclear cells were collected and washed 3 times with normal saline. Positive selection of monocytes was performed by adding MACS colloidal superparamagnetic microbeads conjugated with monoclonal anti–human CD14 antibodies to cooled, freshly prepared peripheral blood mononuclear cell preparations in MACS buffer (PBS with 5 mM EDTA and 0.5% bovine serum albumin) according to the manufacturer's instructions. Cells and microbeads were incubated for 15 minutes at 4-6°C. In the meantime, the separation column was positioned in the MACS magnetic field and washed with MACS buffer at room temperature. Cells were washed with MACS buffer, resuspended, and loaded onto the separation column. The eluent containing CD14– cells was withdrawn and after removal of the column from the magnet, trapped monocytes (CD14+) were eluted with the 6-fold amount of cold MACS buffer, centrifuged, and resuspended in medium containing 0.5% BSA. Preparations yielded a purity of more than 97%.
Neutrophils were obtained from peripheral EDTA-anticoagulated blood of healthy volunteers by Lymphoprep density gradient centrifugation, followed by dextran sedimentation and hypotonic lysis of contaminating erythrocytes. Cell preparations yielded more than 95% neutrophils (by morphology in GIEMSA stains) and more than 99% viability (by trypan dye exclusion). For mRNA detection and immunoprecipitation gel electrophoresis experiments, neutrophils were further purified using MACS superparamagnetic microbeads conjugated with monoclonal anti–human CD14 antibodies to maximally eliminate contaminating monocytes, and in another set of experiments, conjugated with anti–human CD16 antibodies to eliminate eosinophils.
Preparation of monoclonal anti-EPCR antibodies
Wistar rats were immunized from the tail base with human EPCR-positive RE-1 cells (5 × 107 cells per rat) using a Freund complete adjuvant under anesthesia. After one week, cells were isolated from the superficial inguinal lymph nodes and fused with murine SP2/0 myeloma cells (CRL1581, American Type Culture Collection, Manassas, VA) with polyethylene glycol, and hybridomas were selected as described.13 Supernatants were screened by cell surface staining of EPCR-positive SE-1 cells and monitoring on a flow cytometer as described.14 Clones were essentially isolated by 2 rounds of the limiting dilution method.15 Subclasses of these monoclonal antibodies (mAbs) were determined with a monoclonal antibody typing kit (The Binding Site, Birmingham, England). All mAbs were purified from ascites of SCID mice as previously described.16
Leukocyte chemotaxis assay
Migration of neutrophils or monocytes into cellulose nitrate filters to gradients of soluble attractants was measured using a 48-well microchemotaxis chamber in which a 5 μm pore–sized filter separated the upper from the lower chamber, as described previously.17 In various experiments, neutrophil migration was tested toward phPC, ArhPC, AphPC (0.1 pg/mL to 10 μg/mL), interleukin-8 (IL-8) (1 nM), formyl-Met-Leu-Phe (fMLP), C5a (both 10 nM), or AT (1 U/mL). For deactivation, neutrophils were incubated for 20 minutes at various concentrations of PC or APC (0.1 pg/mL to 10 μg/mL). After they were washed twice in HBSS, neutrophil migration toward IL-8, fMLP, C5a, or AT in the lower chambers was assessed for 30 minutes at 37°C in a humidified atmosphere (5% CO2). In some experiments, cells were incubated with either function-blocking (RCR-252) or nonblocking (RCR-92) antibodies (20 μg/mL) against the EPCR or with other substances in combination with the PC preparations followed by testing migration toward chemoattractants as described.
Respiratory burst activity of neutrophils
Neutrophil respiratory burst activity was detected by an assay with the fluorochrome 2′, 7′-dichlorodihydrofluorescein diacetate (H2DCFDA). This assay is based on the oxidation of nonfluorescent H2DCFDA to highly fluorescent 2′, 7′-dichlorofluorescein (DCF) both intracellularly and extracellularly.18 Neutrophils were incubated with various concentrations of PC or the APCs for 20 minutes at 37°C (5% CO2 atmosphere), washed twice, and resuspended in HBSS. Then 20 μL/well (96-well plate) of 8 × 106 neutrophils were immersed at 37°C in a 10-μM solution of H2DCFDA in phenol red–free HBSS containing 4 μM fMLP or 1 μM PMA, as triggering agents, or medium. The plates were covered with lids and placed in a humidified incubator for various time intervals. Fluorescence activity was determined at 485 ± 20 nm excitation and 530 ± 25 nm emission wavelengths using the CytoFluor 4000 fluorescence measurement system (Millipore, Bedford, MA). Since cells in the plates were not disturbed by the measuring procedure, the plates could be incubated for various time periods and reread as desired. Readings were taken every 10 minutes for 30 minutes.
Neutrophil phagocytic activity
Phagocytic activity of neutrophils was tested using the Phagotest kit (Orpegen, Heidelberg, Germany) for whole blood samples. To investigate if phagocytosis is affected by PC/APC, 100 μL whole heparinized blood was incubated with exogenous phPC, AphPC, or ArhPC added for 20 minutes at 37°C in a humidified atmosphere. After incubation, samples were prepared for analysis in the Phagotest kit according to the manufacturer's instructions. In brief, samples were incubated (10 minutes, 37°C) with 20 μL opsonized and fluorescein isothiocyanate (FITC)–labeled E coli (2 × 108/mL). Negative controls were kept on ice. After addition of 100 μL quenching solution and 3 mL washing solution, the samples were centrifuged (5 minutes, 250g, 4°C). Then 2 mL lysis solution was added and the mixture was incubated for a further 20 minutes at room temperature. The samples were then washed and stained by adding 200 μL DNA staining solution. Finally, cells were analyzed by FACS (Becton-Dickinson FACScan with Cellquest software, San Jose, CA).
Apoptosis of neutrophils
Effects of PC/APC on early apoptosis (5 hours) of neutrophils were tested using the Annexin-V/FITC kit (Bender MedSystems, Vienna, Austria). Freshly prepared neutrophils were incubated in medium or phPC, ArhPC (10 μg/mL), or AphPC (1 μg/mL) for 5 hours at 37°C in a humidified atmosphere. Staurosporine (1 μg/mL; proapoptotic) and GM-CSF (granulocyte-macrophage colony-stimulating factor, 40 ng/mL; antiapoptotic) served as controls and were used with or without PC/APC. For quantification, neutrophils were washed twice in PBS and resuspended in binding buffer (1 × 106 cells/mL); 195 μL cells were incubated with 5 μL Annexin V–FITC for 10 minutes at room temperature, washed, and analyzed on a FACS (Becton-Dickinson FACScan with Cellquest software).
RT-PCR detection of EPCR mRNA in leukocytes
Total RNA was isolated from 107 cells by an acid guanidinium thiocyanate-phenol-chloroform mixture. A reverse transcriptase reaction was performed on 1 μg RNA using random hexamers reverse transcriptase. Then 1 μg of the resulting cDNA was subjected to 33 cycles of PCR in a 50-μL reaction mixture containing 1 pmol of sense and antisense primer pairs in a Biometra (Goettingen, Germany) thermocycler: 94°C for 60 seconds (denaturing), 54°C for 60 seconds (annealing), and 74°C for 60 seconds (extension). Primers were designed to amplify a 409-bp coding sequence of human EPCR. The sense primer sequence was GGC AGT TTC ATC ATT GCT GG. The antisense primer sequence was TTG AAC GCC TCA GGT GAT TC. The PCR products were subjected to agarose gel analysis.
FACS analysis of EPCR expression on neutrophils
A total of 5 × 105 cells were washed twice in Dulbecco phosphate-buffered saline, containing 0.5% bovine serum albumin and incubated with 150 μg/mL human IgG for 20 minutes at 4°C. After pelleting, cells were incubated with 10 μg/mL anti-EPCR antibody RCR-252 or the respective isotype-matched control IgG (eBioscience, San Diego, CA) for 30 minutes at 4°C. After washing, 10 μg/mL biotinylated mouse anti–rat IgG (eBio-science) was incubated for another 30 minutes. Cells were washed twice, and the neutrophils were subsequently incubated with a 1:25 dilution of streptavidin-PE, washed twice, then immediately analyzed on a FACS with Cellquest software.
SDS-PAGE analysis of EPCR in leukocytes
Cells (5 × 106) were washed and incubated for 10 minutes in 2 mL met–/cys– RPMI in a humidified atmosphere at 37°C. EasyTag EXPRESS Protein Labeling Mix [35S] was added to the pelleted cells for an incubation period of 40 minutes at 37°C. Then cells were washed in cold PBS with Ca2+/Mg2+, and 500 μL Nonidet P-40 lysis buffer was added. After 10 minutes of incubation on ice, samples were centrifuged at 4°C. To the 500 μL supernatant, 1 μg anti-EPCR antibody RCR-2 was added for an overnight incubation on a turnover table at 4°C. Then 1 μg of a secondary anti–rat IgG antibody was added for an additional 4 hours at 4°C. Immune complexes were captured with protein A sepharose beads for 1 hour at 4°C. Beads were then washed in PBS containing decreasing amounts of Triton X (1%, 0.1%, and 0.05%). The eluted proteins were applied to 7.5% SDS-PAGE and analyzed by autoradiography after treatment with enhancer solution (DuPont, Wilmington, DE).
Statistical methods
Data are expressed as mean ± standard error of the mean (SEM). The chemotaxis index is the ratio between the distances of directed and undirected migration of neutrophils into the nitrocellulose filters. Means were compared by Mann-Whitney test, paired t test, and Kruskal-Wallis analysis of variance. P < .05 was considered significant.
Results
Effects of protein C and activated protein C on leukocyte migration
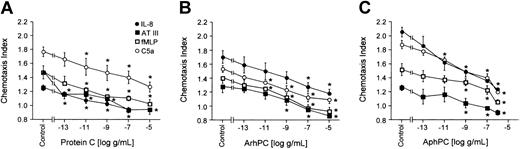
Arrest of inflammatory cells at sites of coagulation is an important component of the response to injury. Effects of PC/APC on leukocyte motility were therefore tested. Neutrophils were pretreated at 37°C for 20 minutes with various concentrations of phPC, AphPC, or ArhPC in order to investigate the effects on their migration toward different chemoattractants. Both phPC as well as the 2 APC preparations inhibited migration of neutrophils toward IL-8 (1 nM), fMLP (10 nM), C5a (10 nM), and AT (1 U/mL) in a dose-dependent manner; the plasma zymogen and the activated proteins were similarly active (Figure 1).
Effects of protein C on neutrophil migration. Deactivation of directional migration of neutrophils by protein C (A), activated recombinant human protein C (ArhPC) (B), and activated plasmatic human protein C (AphPC) (C). Experiments were performed in modified Boyden chambers using nitrocellulose micropore filters. Cells were preincubated with varying PC/APC concentrations for 20 minutes, followed by washing. Then chemotaxis toward IL-8 (1 nM), AT (1 U/mL), fMLP (10 nM), or C5a (10 nM) was monitored. Results are given as the mean ± SEM of the migration index, which is the ratio of the distance of migration (μm) toward attractant and that toward medium. Random migration in the absence of an attractant gradient was 50.780 ± 1.588 μm(n = 42). *P < .05, Mann-Whitney U test versus medium incubation after multiple group comparison by Kruskal-Wallis test. n = 3 (except for protein C-C5a, ArhPC-IL8, fMLP; n = 5).
Effects of protein C on neutrophil migration. Deactivation of directional migration of neutrophils by protein C (A), activated recombinant human protein C (ArhPC) (B), and activated plasmatic human protein C (AphPC) (C). Experiments were performed in modified Boyden chambers using nitrocellulose micropore filters. Cells were preincubated with varying PC/APC concentrations for 20 minutes, followed by washing. Then chemotaxis toward IL-8 (1 nM), AT (1 U/mL), fMLP (10 nM), or C5a (10 nM) was monitored. Results are given as the mean ± SEM of the migration index, which is the ratio of the distance of migration (μm) toward attractant and that toward medium. Random migration in the absence of an attractant gradient was 50.780 ± 1.588 μm(n = 42). *P < .05, Mann-Whitney U test versus medium incubation after multiple group comparison by Kruskal-Wallis test. n = 3 (except for protein C-C5a, ArhPC-IL8, fMLP; n = 5).
To explore for chemotactic properties of PC/APC in the absence of chemoattractants, freshly prepared neutrophils were allowed to migrate toward different concentrations of phPC, AphPC (10 μg/mL to 1 pg/mL), or ArhPC (1 μg/mL to 1 pg/mL); IL-8 (10–9 M) was used as a positive control. Neither phPC nor AphPC nor ArhPC induced a significant migratory response of neutrophils (data not shown).
As it was previously reported that monocytes express EPCR and that APC reduces stimulated cytokine release from monocytes,7,19 effects of PC/APC on fMLP-induced chemotaxis of monocytes also were tested. Both phPC and AphPC or ArhPC were able to decrease directed migration of monocytes toward fMLP (Table 1). Experiments were performed in modified Boyden chambers using nitrocellulose micropore filters. Cells were preincubated with PC, ArhPC (both 10 μg/mL), or AphPC (1 μg/mL) for 20 minutes followed by washing. Then chemotaxis toward fMLP (10 nM) was monitored. Results are given as the mean ± SEM of the migration index, which is the ratio of the distance of migration (μm) toward attractant and that toward medium. Random migration in the absence of an attractant gradient was 36.73 ± 4.330 SEM μm (n = 3).
Receptor mechanisms in the deactivation of leukocyte chemotaxis by PC/APC
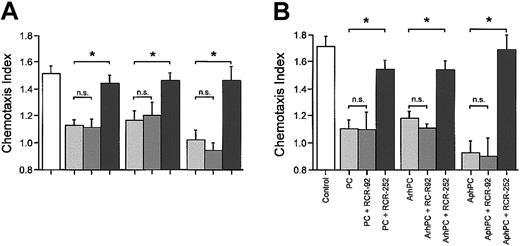
Inhibitory effects of PC and APC on migration may be mediated via EPCR on leukocytes. Therefore, effects of a function-blocking anti-EPCR antibody on leukocyte chemotaxis were compared with those of a nonblocking anti-EPCR antibody. In the presence of the function-blocking anti-EPCR antibody, RCR-252 (20 μg/mL), phPC, AphPC, and ArhPC failed to inhibit chemotaxis toward various chemoattractants. Coincubation of either PC or APC with the anti-EPCR antibody RCR-92 (20 μg/mL), which does not block its PC activator function, had no significant effect on the inhibition of chemotaxis (Figure 2A).
Effects of EPCR in monocyte and neutrophil migration. Effects of anti-EPCR antibodies on the inhibition by protein C, ArhPC, and AphPC of chemoat tractant-induced migration of neutrophils (A) and monocytes (B). Experiments were performed in modified Boyden chambers using nitrocellulose micropore filters. Cells were pretreated either with protein C (PC), activated recombinant human protein C (ArhPC), or activated plasmatic human protein C (AphPC) alone or with protein C/APC and the function blocking anti-EPCR antibody RCR-252 or the nonblocking anti-EPCR antibody RCR-92 for 20 minutes, followed by washing. Then chemotaxis toward IL-8 (1 nM) (neutrophils) or fMLP (10 nM) (monocytes) was monitored. Results are given as the mean ± SEM of the migration index, which is the ratio of the distance of migration (μm) toward attractant and that toward medium. Random migration in the absence of attractant gradients was 38 556 ± 2.072 SEM μm(n = 9) and 36.726 ± 4.330 SEM μm (n = 3) for neutrophils and monocytes, respectively. Statistical analysis was performed using the Mann-Whitney U test after the Kruskal-Wallis test. *P < .05. n = 3 and n = 4 (for neutrophils-RCR-252). n.s. indicates not significant.
Effects of EPCR in monocyte and neutrophil migration. Effects of anti-EPCR antibodies on the inhibition by protein C, ArhPC, and AphPC of chemoat tractant-induced migration of neutrophils (A) and monocytes (B). Experiments were performed in modified Boyden chambers using nitrocellulose micropore filters. Cells were pretreated either with protein C (PC), activated recombinant human protein C (ArhPC), or activated plasmatic human protein C (AphPC) alone or with protein C/APC and the function blocking anti-EPCR antibody RCR-252 or the nonblocking anti-EPCR antibody RCR-92 for 20 minutes, followed by washing. Then chemotaxis toward IL-8 (1 nM) (neutrophils) or fMLP (10 nM) (monocytes) was monitored. Results are given as the mean ± SEM of the migration index, which is the ratio of the distance of migration (μm) toward attractant and that toward medium. Random migration in the absence of attractant gradients was 38 556 ± 2.072 SEM μm(n = 9) and 36.726 ± 4.330 SEM μm (n = 3) for neutrophils and monocytes, respectively. Statistical analysis was performed using the Mann-Whitney U test after the Kruskal-Wallis test. *P < .05. n = 3 and n = 4 (for neutrophils-RCR-252). n.s. indicates not significant.
Since it has previously been described that monocytes7 express EPCR, effects of the antibodies to EPCR were confirmed in assays of monocytes with results similar to those obtained for neutrophils. The function-blocking anti-EPCR antibody RCR-252 reversed the inhibitory effects of phPC, AphPC, or ArhPC on fMLP-triggered migration, whereas the anti-EPCR antibody RCR-92 did not. (Figure 2B).
It was previously described that protease-activated receptor (PAR) signaling may play a role in mediating effects of protein C in endothelial cells.20 Therefore, we tested if PAR-agonists/antagonists are able to influence PC/APC-mediated effects in neutrophils.
Treatment of neutrophils with PC or APC in the presence of a PAR1 agonist (SFLLRNPNDKYEPF, 1 μM) did not significantly affect the inhibitory actions of phPC, AphPC, or ArhPC on directed migration of neutrophils toward IL-8 (1 nM). Also, no effect was observed after coincubation with an antibody against PAR1 (ATAP2, 10 μg/mL) or a PAR2 agonist (SLIGKV, 100 μM). A PAR2 antibody (C-17, 10 μg/mL) failed to affect the inhibitory actions of phPC, ArhPC, or AphPC as well. To exclude effects of thrombin, which may be present in contaminating doses, and complex with PC, thrombomodulin and EPCR, coincubation of PC/APC and hirudin (1 U/mL) also was performed. Again, no effect on the inhibitory properties of PC/APC on neutrophil migration of interfering with a thrombin-dependent pathway could be detected. Coincubation of PC/APC and protein S (10 μg/mL-10 pg/mL) also had no significant influence on the migration-inhibitory effects of PC/APC. As the Gla domain of protein C has been shown to be important in the protein C-EPCR interaction,21 we tested if blocking the Gla-domain can influence PC/APC effects. Coincubation with a Gla antibody (1 μg/mL) was able to diminish the migration-inhibitory effects of PC, ArhPC, or AphPC (Table 2). Because of these results, we also tested if the Gla-containing clotting factors prothrombin and protein S are able to influence neutrophil chemotaxis. Neither prothrombin (100 mU/mL to 10 pU) nor protein S (10 μg/mL to 10 pg/mL) was able to inhibit neutrophil migration toward IL-8 (1 nM) (data not shown). Experiments were performed in modified Boyden chambers using nitrocellulose micropore filters. Cells were coincubated with PC, ArhPC, or AphPC (1 μg/mL), and the substances shown in the table for 20 minutes followed by washing. Then chemotaxis toward IL-8 (1 nM) was monitored. Results are given as the mean ± SEM of the migration index, which is the ratio of the distance of migration (μm) of cells incubated with PC, ArhPC, or AphPC alone and cells coincubated with PC, ArhPC, or AphPC and the other shown substances toward IL-8.
Effects of PC and APC on phagocytosis, respiratory burst and apoptosis of neutrophils
In addition to their ability to migrate, neutrophils ingest bacteria, release oxygen free radicals, and undergo apoptosis, which are all important functional properties that are activated during an inflammatory response. Neutrophil phagocytosis of E coli was therefore quantified after a 20-minute pretreatment with phPC, ArhPC (10 μg/mL), or AphPC (1 μg/mL) in a whole blood test system. Both PC and APC failed to affect the amount of ingested FITC-labeled E coli by neutrophils (data not shown).
Respiratory burst activity of neutrophils was tested fluorometrically on isolated cells. After a 20-minute incubation period at various concentrations of protein C and APC, neutrophil oxygen free radical release was triggered by fMLP (4 μM) or PMA (1 μM). At all concentrations tested (10 μg/mL to 0.1 pg/mL for phPC and ArhPC, and 1 μg/mL to 0.1 pg/mL for AphPC), phPC, ArhPC, and AphPC lacked significant effects on neutrophils' basal or triggered oxygen free radical release (Table 3). Data are shown for an optimal migration-inhibitory dose of PC/APC (10 μg/mL for phPC and ArhPC and 1 μg/mL for AphPC) and are given as mean ± SEM of percentage of control. Fluorescence arbitrary units of spontaneous respiratory burst in the absence of PC/APC were 1524 266 ± 169 827 SEM (n = 16). Cells were preincubated for 20 minutes with PC, ArhPC, or AphPC at 37°C in a humidified atmosphere. After washing, the respiratory burst was triggered by medium, fMLP (4 μM), or PMA (1 μM) and measured fluorimetrically after 30 minutes.
Effects of PC/APC on spontaneous apoptosis of isolated neutrophils were studied using the Annexin V/FITC labeling kit. The number of apoptotic cells was quantified by FACS analysis after 5 hours of incubation with serum-free medium, PC, ArhPC (10 μg/mL), or AphPC (1 μg/mL). Staurosporine (1 μg/mL; proapoptotic) and GM-CSF (40 ng/mL; antiapoptotic) were used as controls. After 5 hours 59.3% ± 8.5% (n = 3) of neutrophils underwent apoptosis. Incubation with GM-CSF reduced this number to 47.3% ± 6.6% (P < .05 vs medium) and with staurosporine 77.1% ± 7.9% (P < .05 vs medium) of the cells were apoptotic. Neither protein C (65.3% ± 12.0%) nor ArhPC (56.9% ± 9.2%) or AphPC (59.0% ± 10.1%) altered the rate of neutrophil apoptosis (P > .1 vs medium).
Expression of EPCR in neutrophils
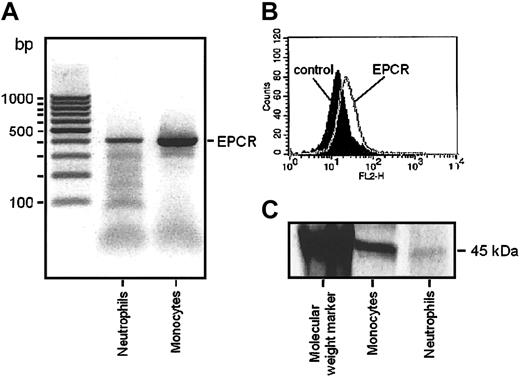
EPCR has been suggested to mediate anti-inflammatory effects of APC22 and its expression in monocytes has been described.7 To determine whether EPCR gene is expressed in neutrophils, PCR was performed. Data confirm that EPCR mRNA is expressed in human neutrophils as well as in monocytes that were used as control cell type (Figure 3A).
RT-PCR, FACS, and immunoprecipitation analysis of EPCR in neutrophils. (A) EPCR mRNA in neutrophils and monocytes. 1 μg total RNA from each sample was reverse transcribed into cDNA and amplified for the EPCR gene using PCR. EPCR is represented by the 409–base pair product. (B) FACS analysis of anti–EPCR mAb binding to neutrophils. Fluorescence analysis used a FACScan Flow cytometer, and a histogram of PE fluorescence is shown. Cells were either incubated with isotype-matched control IgG (black curve) or anti–EPCR mAb (gray line) and stained with PE-conjugated streptavidin. (C) Synthesis of EPCR in neutrophils. Cells were radiolabeled for 40 minutes, followed by lysis and immunoprecipitation with anti-EPCR antibody RCR-2, SDS-PAGE, and autoradiography.
RT-PCR, FACS, and immunoprecipitation analysis of EPCR in neutrophils. (A) EPCR mRNA in neutrophils and monocytes. 1 μg total RNA from each sample was reverse transcribed into cDNA and amplified for the EPCR gene using PCR. EPCR is represented by the 409–base pair product. (B) FACS analysis of anti–EPCR mAb binding to neutrophils. Fluorescence analysis used a FACScan Flow cytometer, and a histogram of PE fluorescence is shown. Cells were either incubated with isotype-matched control IgG (black curve) or anti–EPCR mAb (gray line) and stained with PE-conjugated streptavidin. (C) Synthesis of EPCR in neutrophils. Cells were radiolabeled for 40 minutes, followed by lysis and immunoprecipitation with anti-EPCR antibody RCR-2, SDS-PAGE, and autoradiography.
Presence of EPCR-like immunoreactivity on the surface of neutrophils was tested by FACS analysis. Inconsistently, a slight but significant shift of fluorescence was observed by the anti-EPCR antibody RCR-252, which is indicative of low grade cell surface presence of EPCR (Figure 3B). To confirm that this shift in immunofluorescence is due to de novo synthesis of EPCR receptor protein, analysis of metabolic radiolabeled immunoprecipitation and SDS-PAGE experiments were performed. Neutrophils and purified CD14+ PBMCs (monocytes) were metabolically radiolabeled with EXPRESS [35S], and EPCR was recovered from cell lysates by immunoprecipitation using anti-EPCR antibody RCR-2. Newly synthesized EPCR was clearly discernible on SDS-PAGE autoradiographs as a 45-kDa band for both neutrophils and monocytes (Figure 3C).
Discussion
The central aim of this study was to examine the influence of PC and APC on neutrophil motility. Previous studies reported that APC did not affect fMLP-triggered release of neutrophil elastase and oxygen free radicals or neutrophil aggregation23 but inhibited selectin-mediated adhesion of neutrophils to vascular endothelial cells24 and reduced endotoxin-dependent tissue accumulation of neutrophils in animal models.5 Our working hypothesis was that PC and APC might modulate neutrophil chemotaxis directly and independently of other components of the coagulation cascade. The present study implicates that EPCR of leukocytes may be involved in the effects of PC on cell migration. PC and APC blocked the chemotaxis of neutrophils and monocytes toward chemokines and other attractants. This effect could be reversed by an anti-EPCR antibody directed against a PC/APC binding site of EPCR. Expression of EPCR in neutrophils was confirmed by identification of expression of mRNA for EPCR and newly synthesized protein.
In endothelial cells, APC was shown to modulate patterns of gene expression clustering into anti-inflammatory and cell survival pathways independent of its anti-inflammatory action via inhibition of thrombin generation. APC blocks expression of NFκB-regulated genes inhibiting expression of adhesion molecules, modulates several genes in the endothelial apoptosis pathway, and inhibits the induction of apoptosis by the potent inducer staurosporine.25 Endothelial cells express EPCR, a cell surface glycoprotein that binds directly to PC and APC with high affinity.26 Whether this anti-inflammatory effect in endothelial cells is mediated by ligation of EPCR with APC is unknown.
Similar observations have been previously reported for monocytes. APC inhibits the endotoxin-induced elaboration of proinflammatory cytokines from monocytes. APC was shown to inhibit lipopolysaccharide-induced production of tumor necrosis factor-α and activation of NF-κB by monocytes.27 APC also inhibits the release of macrophage migration inhibitory factor from lipopolysaccharide-stimulated human monocytes.19 APC inhibits host cytokine production but maintains mononuclear phagocyte responses associated with adhesion, phagocytosis, and killing of gram-negative bacteria.6 Monocytes express EPCR on their surface,7 but whether EPCR mediates the effects of APC on monocyte function has not yet been tested.
In the present study, it was observed that PC and APC inhibit directed migration of monocytes in an EPCR-dependent fashion since the inhibitory effect could be reversed with an anti-EPCR antibody (Figure 2). This is the first observation suggesting that EPCR of monocytes is functional in monocytes. EPCR gene expression in primary human monocytes as described previously7 was confirmed in the present study by PCR (Figure 3A). De novo synthesis of EPCR protein in monocytes is further suggested by pulse-labeling experiments followed by immunoprecipitation and SDS-PAGE (Figure 3C). These findings in monocytes build an important background for the exploration of a role of the PC-dependent pathway in neutrophils. Data on neutrophils can help mechanistic understanding not only in elucidating the mechanism of action and of the physiologic role of PC and APC, but also of their potential therapeutic role in neutrophil-mediated diseases such as adult respiratory distress syndrome, systemic inflammatory response syndrome, or sepsis.
Migration of neutrophils was tested toward various chemoattractants that elicit the migratory response via different signaling pathways. PC and APC were able to inhibit migration toward each of these substances in a dose-dependent manner (Figure 1). Near-complete abrogation of stimulated migration was seen at concentrations in the range of 1 to 10 μg/mL, which is the amount of PC circulating in animals and humans with normally functioning endothelium.28 One may speculate that circulating PC contributes to the prevention of premature activation of migration of circulating neutrophils.
PC and APC inhibited migration of neutrophils toward attractants of different classes that differ in their receptor signaling cascades. This observation may indicate that the PC pathway of neutrophils affects mechanisms central to the process of cell migration rather than chemoattractant receptor or postreceptor signaling. Neither PC nor APC exerted a significant effect on respiratory burst or phagocytic activity of neutrophils which, on the one hand, partly confirms previously reported data20 and, on the other, may indicate that inhibition of leukocyte functions is related to mechanisms of cell adhesion5,21 and motility.
In the present study, PC, ArhPC, and AphPC failed to affect neutrophil apoptosis. The persistence of a neutrophil-mediated inflammatory response is due in part to a delay in their spontaneous rates of apoptosis or cell death. Stimulation of apoptosis has important implications for the resolution of inflammatory disorders.29 Inhibition of apoptosis with PC or APC such as that reported in endothelial cells23 was not observed with neutrophils, which is compatible with an anti-inflammatory action of APC.
The ability of APC to inhibit thrombin generation has the potential to reduce the proinflammatory activities of thrombin, which are mediated by protease-activated receptor (PAR)-1, PAR-3, and PAR-4.30 PARs mediate thrombin-induced inflammation by generating signals that provide the intracellular, functional link between inflammation at sites of vascular injury, modulating platelet, and endothelial cell activation.31-33 Therefore, reduced thrombin-mediated PAR cleavage is likely to be an important component of APC's anti-inflammatory activity. Recently, Riewald et al20 reported that PAR-1, the prototypical thrombin receptor, is a target for EPCR-dependent APC signaling and that PAR-1 signaling could account for all APC-induced protective genes against sepsis. As thrombin is a potential contamination of PC preparations, control experiments in which thrombin was directly inactivated by hirudin also were performed. PC and APC effects appeared independently of thrombin. Neutrophils were shown to express PAR-2.34 PAR-1 expression by neutrophils was described only in pregnant women but not by neutrophils of healthy subjects under normal conditions.35,36 Nevertheless, in the present study potential involvement of PARs was tested. Neither a PAR-1 agonist nor an antibody against PAR-1 affected PC- or APC-induced inhibition of neutrophil chemotaxis. Also, a PAR-2 agonist left the effects on directed neutrophil migration of PC or APC unchanged. According to these data, involvement of PAR-1 or PAR-2 in the effects of PC or APC on neutrophils is unlikely.
As EPCRs are expressed by monocytes,7 and effects of PC and APC on monocyte function are anti-EPCR antibody reversible (Figure 2), EPCR also may be involved in the neutrophil's response to PC and APC. A blocking antibody against EPCR abolished effects of PC or APC on neutrophil migration similar to that observed in monocytes. A control antibody that binds to EPCR but does not affect EPCR in the activation of PC37 also failed to affect PC- or APC-dependent inhibition of migration (Figure 2). This may indicate that ligation of the high-affinity binding site of EPCR26 for PC or APC is required for the functional response to occur. Data show that neutrophils express EPCR mRNA (Figure 3A). Theoretically, EPCR-like immunoreactivity identified on the surface of neutrophils (Figure 3B) may be due to binding of soluble EPCR from plasma38 to neutrophils.8 For this reason, neutrophils were metabolically radiolabeled, and newly synthesized EPCR could be identified in cell lysate by immunoprecipitation (Figure 3C). Taken together, biochemical and functional data strongly suggest that neutrophils express EPCR. Further studies will have to focus not only on the regulation of EPCR gene expression in neutrophils but also on the effect of neutrophil activation on the shedding of EPCR,39,40 as neutrophils are a rich source of proteases.
The potential physiological significance of the similar effects of PC and APC on neutrophil and monocyte chemotaxis remains uncertain. Recently, EPCR and APC have been demonstrated to be important in survival in the baboon sepsis model9 and in patients with severe sepsis,12 respectively. It will be important to determine how in neutrophils and monocytes as well as in endothelial cells this receptor and its high-affinity ligands are involved in cell signaling.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-12-3880.
Supported by the Verein zur Förderung von Forschung und Fortbildung in klinischer Kardiologie und Intensivmedizin–Innsbruck.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.