Abstract
Lipopolysaccharide (LPS) is a major gramnegative bacterial component that stimulates innate immune response and also induces B-lymphocyte activation. Recent studies have revealed that common molecular patterns of microorganisms such as LPS are recognized by toll-like receptors (TLRs). B cells have 2 known TLRs that mediate LPS signaling, TLR4 and RP105 (CD180). While TLR4 is expressed on immune cells of various types, RP105 is preferentially expressed on mature B cells. Here we demonstrate that CD19 plays a major role in regulating signal transduction through RP105. Anti-RP105 ligation induced normal proliferation of B cells from mice deficient for MyD88, an adaptor protein that mediates most TLR pathways. By contrast, the loss of CD19 resulted in modest B-cell proliferation against anti-RP105 stimulation as well as LPS stimulation. LPS induced tyrosine phosphorylation of CD19, which was RP105-dependent but TLR4-independent. CD19 formed a complex with Lyn and Vav following RP105 ligation, and CD19 expression was required for optimal Lyn activation and Vav phosphorylation. Consistently, B cells deficient for CD19 exhibited specific defect in the activation of c-Jun N-terminal kinases following RP105 ligation and LPS stimulation. In contrast, CD19 and phosphatidylinositol 3-kinase independently regulated intracellular calcium mobilization induced by anti-RP105 stimulation. Thus, signaling through the B-cell–specific LPS receptor RP105 is uniquely regulated by the B-cell–specific signaling component, Lyn/CD19/Vav complex.
Introduction
In the mammals, 2 immune systems, innate immune response and adaptive immune response, contribute to host defense cooperatively. Adaptive immune recognition is mediated by lymphocyte antigen receptors, which have a highly diverse repertoire. In B lymphocytes, antigen receptor (BCR) engagement activates signaling pathways mediated by multiple arrays of kinases such as Lyn, Syk, Btk, phosphatidylinositol 3-kinase (PI 3-kinase), and mitogenactivated protein kinases (MAPKs); phosphatases such as SHP-1 (Src homology-2 [SH2] domain containing tyrosine phosphatase 1) and SH2-containing inositol 5-phosphatase; and other signal transducing elements such as B-cell linker protein (BLNK), Vav, Dok, B-cell adaptor for PI 3-kinase, Cbl, and Shc.1 BCR-induced signals are further modified by other cell surface molecules (eg, CD19, CD22, CD72, CD40, FcγRIIB) that inform B cells of their microenvironment and thus provide a context for BCR signal transduction.2 While adaptive immune response is highly specific and potent, primary response is inevitably delayed as it requires gene rearrangement. By contrast, innate immune system that recognizes common molecular pattern of microorganisms provides the first line of host defense and controls the initiation of the adaptive immune response. Recent studies have revealed that toll-like receptor (TLR) families play a central role in activation of the innate immune response.3,4 TLRs are characterized by an extracellular leucine-rich repeat motif and an intracellular toll/interleukin 1 (IL-1) receptor (TIR) domain.5 TLR signaling is then mediated by 2 adaptor proteins, MyD88 and TIR domain–containing adaptor protein (TIRAP).6,7 TLRs recognize conserved microbial products such as lipopolysaccharide (LPS), peptidoglycan, and bacterial DNA and RNA. LPS, a major component of gram-negative bacterial wall, is recognized by TLR4 expressed by a variety of cell types. TLR4–/– mice as well as C3H/HeJ mice, which have a point mutation in the TIR domain of TLR4, are unresponsive to LPS.8-10
B lymphocytes play an essential role in adaptive immune response but also primarily participate in innate immunity. Indeed, LPS is a well-known strong stimulant of B cells. LPS stimulation activates B cells, leading to proliferation and IgM secretion. Recent studies have revealed that LPS response in B cells is regulated not only by TLR4 but also by another TLR family receptor, RP105 (CD180).11 RP105 is a type I transmembrane protein of 105 kDa with a leucine-rich repeat motif in the extracellular domain but without TIR domain, and it has been first identified as a mouse B-cell surface molecule to protect B cells from irradiation-induced apoptosis.12,13 RP105 is preferentially expressed on mature B cells in mice and B cells and dendritic cells in humans.12,14,15 Anti-RP105 cross-linking leads to massive B-cell proliferation as well as resistance against apoptosis. RP105–/– mice exhibit a specific and profound defect in LPS response in B cells.11
While LPS has been used as a potent B-cell stimulant, the molecular mechanisms of how B cells are activated by LPS have been unclear. B cells from mice that are deficient or defective of CD19, Lyn, Btk, PI 3-kinase, BLNK, or phospholipase C γ2 exhibit impaired proliferative response to LPS by varying extents.16-24 In the case of BCR signaling, some of these molecules such as Btk, PI 3-kinase, BLNK, and phospholipase C γ2, are linearly associated in a “B cell signalosome,” regulating intracellular calcium ([Ca2+]i) responses.25 CD19 determines signaling thresholds in B cells by regulating Lyn kinase activity.26,27 Indeed, Lyn has been demonstrated to have an important role in the initiation of RP105 signaling.28 By contrast, B cells deficient for negative regulators, including CD22 or CD72, are shown to hyperrespond to LPS stimuli.29,30 CD19 also modulates CD22 functions by the CD19/CD22 regulatory loop.31 While these evidences clearly suggest that these molecules are functionally linked and play an important role in LPS signaling, how these molecules participate in this process has been poorly understood.
We analyzed the signaling pathways activated following LPS and anti-RP105 ligation in B cells. Using primary B cells and lymphoblastoid cell lines that lack CD19 expression, we have shown that Lyn/CD19/Vav is a critical functional complex for RP105 signaling, especially required for the activation of c-Jun N-terminal kinases (JNKs).
Material and methods
Animals and cells
MyD88–/– mice and CD19–/– mice were generated as described16,32 and housed in a specific pathogen-free barrier facility. Wild-type littermates generated from heterozygous matings were used appropriately as controls. C3H/HeJ and C3H/HeN mice were purchased from Clea Japan (Shizuoka, Japan). All procedures were approved by the Animal Committee of International Medical Center of Japan. Splenic B cells were purified by removing T cells with anti-Thy1.2 antibody (Ab)–coated magnetic beads (Dynal, Lake Success, NY). A20 cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum and 2-mercaptoethanol. CD19– lines were generated by repeated removal of the CD19+ population using biotinylated anti-CD19 Abs and avidin-coated magnetic beads (Dynal) as reported previously.33
Reagents and immunofluorescence analysis
Antisera used in this study included the following: anti-Lyn, anti-Vav, and anti-CD19 (Santa Cruz Biotechnology, Santa Cruz, CA); anti–phosphor–CD19-Y513 (Cell Signaling Technology, Beverly, MA); anti–extracellular signal-regulated kinase (anti-ERK), anti-JNK1, anti–PI 3-kinase (Upstate Biotechnology, Lake Placid, NY); and anti–active ERK and anti–active JNK (Promega, Madison, WI). Monoclonal Abs used in this study included the following: anti-Lyn (Wako Pure Chemicals, Osaka Japan), anti-Vav (Upstate Biotechnology), anti-B220 (RA3-6B2; BD PharMingen, San Diego, CA), anti-CD19 (MB19-1),34 anti-TLR4/MD-2 (MTS510),35 anti–MD-1 (MD113),36 and anti-RP105 (RP/14).12
Single-cell leukocyte suspensions from spleen were counted using a hemocytometer. Leukocytes (0.5 × 106 to 1 × 106) were stained for 2-color immunofluorescence analysis at 4°C using predetermined optimal concentrations of antibodies for 20 minutes. Cells with the forward and side light-scatter properties of mononuclear cells were analyzed on an Epics Altra flow cytometer (Beckman Coulter, Miami, FL) with fluorescence intensity shown on a 4-decade log scale. Fluorescence contours are shown as 50% log density plots. Positive and negative populations of cells were determined using unreactive isotype-matched antibodies (BD PharMingen) as controls for background staining.
B-cell proliferation
Spleen B cells were cultured in 0.2 mL of culture medium containing LPS (0111:B4; Sigma-Aldrich, St Louis, MO) or anti-RP105 Abs (RP/14) in triplicate wells of 96-well flat-bottom tissue culture plates. Proliferation was assessed by the incorporation of [3H]-labeled thymidine (1 μCi/well [0.037 MBq/well]) added during the last 16 hours of culture followed by scintillation counting.
B-cell activation, immunoprecipitations, and Western blot analysis
A20 cells or splenic B cells were stimulated with anti-RP105 Abs (5 μg/mL), LPS (10 μg/mL), or goat antimouse IgG Ab F(ab′)2 fragments (10 μg/mL; Zymed Laboratories, San Francisco, CA) and subsequently lysed in buffer containing 1% NP-40, 150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 1 mM Na orthovanadate, 2 mM EDTA (ethylenediaminetetraacetic acid), 50 mM NaF, and protease inhibitors. The lysates were either analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) or subjected to immunoprecipitation. Immunoprecipitations were performed by incubating the cell lysates with protein G beads plus rabbit or goat antiserum for 4 hours at 4°C after they were precleared twice by incubating with appropriate control Abs plus protein G Sepharose beads (Amersham Pharmacia Biotech, Piscataway, NJ). For CD19 immunoprecipitations, the lysates were precleared with Affigel 10 beads (Bio-Rad Laboratories, Hercules, CA) conjugated with mouse IgA Abs then incubated for 4 hours with Affigel 10 beads bearing anti-CD19 Abs (MB19.1). These membranes were incubated with horseradish peroxidase (HRP)–conjugated antiphosphotyrosine Abs (Santa Cruz Biotechnology) or were incubated with specific Abs followed by HRP-conjugated antirabbit or antigoat IgG Abs (Jackson ImmunoResearch Laboratories, West Grove, PA) and developed using an enhanced chemiluminescence kit (Pierce, Rockford, IL). Biotinylated proteins were detected using HRP-conjugated neutroavidin (Pierce). To verify equivalent amounts of protein in each lane or to detected associated proteins, the blots were stripped and reprobed with antibodies against the proteins of interest. Band intensity was quantified using Quantity One software (Bio-Rad Laboratories).
Preparation of raft fraction
After cells were surface-biotinylated (EZ-Link Sulfo-NHS-biotin; Pierce) and incubated with anti-RP105 Abs, 1 × 108 cells per sample were lysed for 30 minutes on ice in 0.05% Triton X-100 in lysis buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA; 1 mM Na orthovanadate, and protease inhibitors). Nuclei and cellular debris were pelleted by centrifugation at 900g for 10 minutes, and 1 mL of cleared supernatant was mixed with 1 mL of 85% sucrose in a Beckman Coulter 14 × 89-mm centrifuge tube. The sample was overlaid with 6 mL 35% sucrose and 3.5 mL 5% sucrose in lysis buffer. The samples were centrifuged in a SW41 rotor (Beckman Coulter) at 200 000g for 16 to 20 hours at 4°C, and 1-mL fractions were collected from the top of the gradient. Fractions 1 to 3, 4 to 6, 7 to 9, and 10 to 12 were pooled for subsequent immunoprecipitation. Fractions 1 to 3 and 10 to 12 represented the raft-containing fractions and the soluble fractions, respectively. Isolation of lipid raft membranes was confirmed by immunoblotting for GM1, Lyn, and CD45R.
Measurement of [Ca++]i
Spleen cells were isolated at room temperature, washed, and resuspended at 107/mL in RPMI-1640 medium containing 5% fetal calf serum and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and loaded with 1 μM indo-1-acetoxymethyl ester (Molecular Probes, Eugene, OR) at 37°C for 30 minutes. The cells were washed and stained with fluorescein isothiocyanate (FITC)–conjugated anti-B220 monoclonal antibodies (mAbs) for 15 minutes at room temperature, washed, and resuspended at 2 × 106 cells/mL. For analysis, the ratio of fluorescence (488:407 nm) of B220+ cells (1.5 × 106 cells in 0.75 mL samples) was determined using an EPICS Altra flow cytometer (Beckman Coulter). Baseline fluorescence ratios were collected for 1 minute before treatment with antibodies. Fluorescence ratios were collected at real time for 10 minutes following Ab addition. To inhibit PI 3-kinase activity, wortmannin (50 nM; Sigma-Aldrich), or LY294002 (15 μM; Sigma-Aldrich) was added 10 minutes prior to measuring the fluorescence ratios in some experiments. Results were plotted as the fluorescence ratio at 20-second intervals with the background subtracted. Increased fluorescence ratios indicate increased [Ca2+]i.
Statistical analysis
All data are shown as mean values ± SEM. Comparisons between groups were made using the Student t test.
Results
MyD88-deficient B cells exhibit normal, but CD19-deficient B cells exhibit modest, proliferation to RP105 ligation
Mature B cells express 2 known LPS receptors, TLR4 and RP105. TLR4-mediated proliferative response is dependent on MyD88 expression.37 Therefore, whether B-cell proliferation induced by RP105 stimulation also required MyD88 expression was first assessed. B cells from MyD88-deficient (MyD88–/–) mice showed almost identical proliferative response as those from wild-type mice at various concentrations of anti-RP105 Abs (Figure 1A). Additionally, proliferative response of B cells from TLR4-deficient mice was slightly decreased but near normal against anti-RP105 stimulation (Figure 1A), suggesting that RP105 is functionally independent of TLR4 expression. Therefore, B-cell response to RP105 engagement did not require MyD88 expression or TLR4 expression.
B-cell proliferation in response to anti-RP105 or LPS stimulation. Spleen B cells (1 × 105/well) from wild-type and MyD88–/– mice (A) and wild-type and CD19–/– mice (B-C) were cultured with the indicated concentrations of anti-RP105 Abs (A-B) or LPS (C). Proliferation was assessed by the incorporation of labeled thymidine (1 μCi/well [0.037 MBq/well]) added during the last 16 hours of 72-hour cultures. Values represent the mean counts per minute (cpm; ± SEM) obtained from triplicate cultures. Mean values significantly different from wild-type proliferation levels are indicated, **P < .01. Results represent those obtained in 3 independent experiments.
B-cell proliferation in response to anti-RP105 or LPS stimulation. Spleen B cells (1 × 105/well) from wild-type and MyD88–/– mice (A) and wild-type and CD19–/– mice (B-C) were cultured with the indicated concentrations of anti-RP105 Abs (A-B) or LPS (C). Proliferation was assessed by the incorporation of labeled thymidine (1 μCi/well [0.037 MBq/well]) added during the last 16 hours of 72-hour cultures. Values represent the mean counts per minute (cpm; ± SEM) obtained from triplicate cultures. Mean values significantly different from wild-type proliferation levels are indicated, **P < .01. Results represent those obtained in 3 independent experiments.
The above results suggested that B-cell–specific LPS receptor, RP105, was regulated not by universal TLR-signaling molecules such as MyD88 and IL-1R–activating kinase (IRAK) but by B-cell–specific proteins such as CD19. Since B cells from CD19-deficient (CD19–/–) mice show modest proliferative response to LPS, B-cell proliferation induced by anti-RP105 ligation was assessed in CD19–/– B cells. CD19–/– B cells exhibited markedly decreased proliferative response to anti-RP105 ligation (Figure 1B), which was similar to LPS-induced response in CD19–/– B cells16 (Figure 1C). These data suggest that RP105 signaling is regulated not by common TLR pathways but by CD19 and other B-cell–specific pathways.
CD19–/– B cells express normal levels of TLR-4/MD-2 and RP105
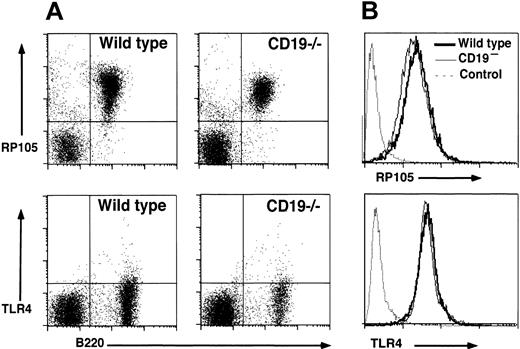
Expression level of RP105 on CD19–/– B cells was examined by flow cytometry analysis since CD19 loss might influence RP105 expression. Splenic B cells from CD19–/– mice showed wild-type levels of RP105 expression (93% ± 4%; Figure 2A). Circulating B cells also expressed wild-type levels of RP105 in CD19–/– mice (97% ± 5%, data not shown). In addition, the expression of TLR4/MD-2 complex was equivalent between wild-type and CD19–/– B cells, although the expression was rather low as shown previously11 (Figure 2A). RP105 expression was further examined in a mouse lymphoblastoma cell line, A20. CD19– cell lines, which were generated by repeatedly removing the CD19+ population,33 showed the similar expression levels of RP105 to CD19+ parental cell line (91% ± 4% of parental wild-type cells; Figure 2B). TLR4/MD-2 complex was also expressed equally in CD19– cells and parental wild-type cells (103% ± 7%; Figure 2B). These results suggest that CD19 loss did not affect RP105 expression.
RP105 and TLR4/MD-2 expression in splenocytes and A20 cell lines. (A) Splenocytes from wild-type and CD19–/– mice were examined by 2-color immunofluorescence staining with flow cytometry analysis. Quadrant gates indicate negative and positive populations of cells as determined using isotype-matched unreactive control mAbs. These results are representative of those obtained with four to seven 2-month-old mice of each genotype. (B) RP105 and TLR4/MD-2 expression by parental A20 (bold line) and CD19– A20 cells (thin line). Cell surface molecule expression was detected with flow cytometry analysis. Immunofluorescence staining with an unreactive, isotype-matched, control mAbs is also shown (dashed line). These results are representative of those obtained in 4 experiments.
RP105 and TLR4/MD-2 expression in splenocytes and A20 cell lines. (A) Splenocytes from wild-type and CD19–/– mice were examined by 2-color immunofluorescence staining with flow cytometry analysis. Quadrant gates indicate negative and positive populations of cells as determined using isotype-matched unreactive control mAbs. These results are representative of those obtained with four to seven 2-month-old mice of each genotype. (B) RP105 and TLR4/MD-2 expression by parental A20 (bold line) and CD19– A20 cells (thin line). Cell surface molecule expression was detected with flow cytometry analysis. Immunofluorescence staining with an unreactive, isotype-matched, control mAbs is also shown (dashed line). These results are representative of those obtained in 4 experiments.
LPS and anti-RP105 stimulations induce CD19 phosphorylation
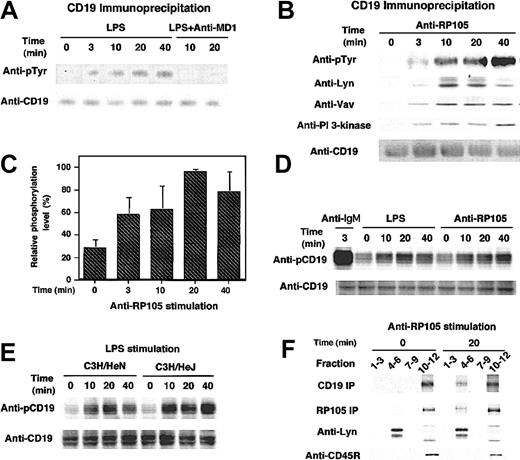
The cytolplasmic domain of CD19 contains 9 highly conserved tyrosine residues, which, when phosphorylated, serve as docking sites for SH2-containing signaling molecules such as Lyn, Vav, and PI 3-kinase. Therefore, tyrosine phosphorylation of CD19 by anti-RP105 ligation as well as LPS stimulation was further examined. Wild-type A20 cells were stimulated with LPS or anti-RP105 Abs at 37°C for various time intervals and subsequently lysed with detergent. Immunoprecipitated CD19 protein was subjected to SDS-PAGE and transferred to nitrocellulose membrane, followed by antiphosphotyrosine immunoblotting. LPS at the concentration of 10 μg/mL induced tyrosine phosphorylation of CD19 in A20 cells, although the maximum intensity of phosphorylation was approximately 10-fold lower than that in BCR ligation (Figure 3A; data not shown). However, in contrast to the strong but transient phosphorylation of CD19 following BCR ligation,27 LPS-induced CD19 phosphorylation was slow but prolonged, reaching the maximum level at 20 minutes and remaining more than 120 minutes following the ligation (Figure 3A; data not shown). Anti-RP105 ligation also induced CD19 phosphorylation with similar kinetics with LPS (Figure 3B-C). Furthermore, phosphorylated CD19 recruited Lyn, Vav, and PI 3-kinase as occurs in BCR signaling (Figure 3B). Detecting CD19 phosphorylation induced by LPS using antiphosphotyrosine Abs was difficult in primary cells, as CD19 phosphorylation is modest in primary cells even by BCR ligation. However, Abs specific for phosphorylated Tyr513, the primary phosphorylation site of CD19, revealed that LPS and anti-RP105 stimulations induced CD19 phosphorylation in primary B cells as well (Figure 3D).
CD19 tyrosine phosphorylation induced by LPS stimulation and anti-RP105 ligation. (A-B) Wild-type A20 cells were incubated with (A) LPS or (B) anti-RP105 Abs for the indicated times or anti-IgG Abs for 3 minutes. In panel A, cells pretreated with anti–MD-1 Abs were also assessed. Proteins immunoprecipitated by anti-CD19 were fractionated by SDS-PAGE and transferred onto nitrocellulose for subsequent antiphosphotyrosine (pTyr), anti-Lyn, anti-Vav, anti–PI 3-kinase, and anti-CD19 immunoblotting. (C) CD19 phosphorylation levels were quantified with background and maximum phosphorylation levels defined as 0% and 100%, respectively (mean % ± SEM). (D) Splenic B cells from a C57BL/6 mouse were incubated with LPS, anti-RP105, or anti-IgM Abs for indicated times. Cell lysates were subjected to SDS-PAGE and transferred onto a membrane for anti-phosphoCD19 immunoblotting. The membrane was reprobed with anti-CD19 Abs. (E) Splenic B cells from C3H/HeN and C3H/HeJ mice were incubated with LPS and processed as in panel D. (F) Lipid rafts were isolated by lysis of cells in 0.05% Triton X-100 at 4°C and flotation on a discontinuous sucrose gradient. Fractions were collected and subjected to CD19 or RP105 immunoprecipitation and SDS-PAGE. Fractions 4 to 6 and 10 to 12 represent the raft containing fractions and the soluble protein fractions. To detect CD19 or RP105, cell surface was biotinylated prior to anti-RP105 stimulation, and the membrane was blotted using HRP-conjugated neutroavidin. Lysates were also subjected to SDS-PAGE and immunoblotting with anti-Lyn and anti-CD45R to confirm the proper preparation of lipid rafts as well as the equivalent amounts between the samples. These results are representative of those obtained in 3 independent experiments.
CD19 tyrosine phosphorylation induced by LPS stimulation and anti-RP105 ligation. (A-B) Wild-type A20 cells were incubated with (A) LPS or (B) anti-RP105 Abs for the indicated times or anti-IgG Abs for 3 minutes. In panel A, cells pretreated with anti–MD-1 Abs were also assessed. Proteins immunoprecipitated by anti-CD19 were fractionated by SDS-PAGE and transferred onto nitrocellulose for subsequent antiphosphotyrosine (pTyr), anti-Lyn, anti-Vav, anti–PI 3-kinase, and anti-CD19 immunoblotting. (C) CD19 phosphorylation levels were quantified with background and maximum phosphorylation levels defined as 0% and 100%, respectively (mean % ± SEM). (D) Splenic B cells from a C57BL/6 mouse were incubated with LPS, anti-RP105, or anti-IgM Abs for indicated times. Cell lysates were subjected to SDS-PAGE and transferred onto a membrane for anti-phosphoCD19 immunoblotting. The membrane was reprobed with anti-CD19 Abs. (E) Splenic B cells from C3H/HeN and C3H/HeJ mice were incubated with LPS and processed as in panel D. (F) Lipid rafts were isolated by lysis of cells in 0.05% Triton X-100 at 4°C and flotation on a discontinuous sucrose gradient. Fractions were collected and subjected to CD19 or RP105 immunoprecipitation and SDS-PAGE. Fractions 4 to 6 and 10 to 12 represent the raft containing fractions and the soluble protein fractions. To detect CD19 or RP105, cell surface was biotinylated prior to anti-RP105 stimulation, and the membrane was blotted using HRP-conjugated neutroavidin. Lysates were also subjected to SDS-PAGE and immunoblotting with anti-Lyn and anti-CD45R to confirm the proper preparation of lipid rafts as well as the equivalent amounts between the samples. These results are representative of those obtained in 3 independent experiments.
To examine whether LPS induced CD19 phosphorylation through RP105 alone or in concert with TLR4, A20 cells were pretreated with anti–MD-1 Abs that block LPS binding to the RP105/MD-1 complex but do not react with MD-2.38 MD-1 interacts with RP105 but not with TLR4, while MD-2 interacts with TLR4 but not with RP105.38 Remarkably, CD19 phosphorylation by LPS was completely blocked by preincubation with anti–MD-1 Abs (Figure 3A). These data suggest that LPS-induced CD19 phosphorylation is dependent on RP105. We further assessed whether CD19 phosphorylation was completely independent of TLR4. Indeed, in B cells from C3H/HeJ mice, CD19 was phosphorylated by LPS stimulation, although the phosphorylation level was somewhat lower than wild-type C3H/HeN mice in 2 of 4 experiments (Figure 3E). C3H/HeJ and C3H/HeN B cells expressed identical levels of both CD19 and RP105 (data not shown). Collectively, while TLR4 may have a role in CD19 phosphorylation, CD19 phosphorylation induced by LPS stimulation requires RP105 expression.
RP105 ligation induces Lyn activation,28 while the cytoplasmic domain of RP105 is only 11 amino acids in length. This may suggest that CD19 associates with RP105, although this association was not clear by the immunoprecipitation of either CD19 or RP105 (data not shown). However, when the localization of CD19 and RP105 in the detergent-insoluble glycolipid-enriched membrane was assessed, both CD19 and RP105 translocated into the lipid raft fraction at least partially following RP105 ligation (Figure 3D). Thus, RP105 ligation induced the CD19 translocation into lipid rafts where CD19, RP105, and Lyn may functionally interact since Lyn is constitutively localized in lipid rafts. Consistent with this, Lyn association with CD19 increased in parallel with CD19 phosphorylation induced by RP105 ligation (Figure 3B).
CD19 regulates anti-RP105 signaling through Lyn and Vav activation
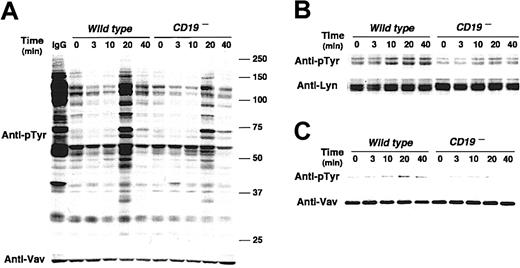
In BCR signaling, CD19 regulates Lyn kinase activity by a mechanism named processive amplification,27 which establishes signaling thresholds for B-cell activation. As a result, CD19–/– B cells exhibit markedly diminished tyrosine phosphorylation of multiple signaling molecules following BCR ligation.26 Since Lyn also regulates RP105 signaling, the influence of CD19 deficiency on tyrosine phosphorylation on cellular proteins was assessed. The loss of CD19 expression resulted in modest induction of overall tyrosine phosphorylation of B-cell proteins following RP105 ligation (Figure 4A). This result was similar to BCR signaling, and thus raised a question whether CD19 may also regulate Lyn kinase activity in RP105 signaling. Indeed, Lyn phosphorylation and kinase activity increased in CD19– cells following anti-RP105 cross-linking but at lower levels than in wild-type cells (Figure 4B; data not shown). By contrast, phospholipase C γ2 phosphorylation in CD19– cells following anti-RP105 cross-linking was comparable to that in wild-type cells (data not shown) Thus, CD19 expression positively and specifically regulated Lyn activity following anti-RP105 ligation.
CD19 regulates signal transduction mediated by RP105. (A) Protein tyrosine phosphorylation following RP105 cross-linking. Parental A20 and CD19– A20 cells (4 × 105/lane) were incubated with anti-RP105 Abs for the indicated times. Cell lysates were subjected to SDS-PAGE and transferred onto membranes for antiphosphotyrosine (pTyr) immunoblotting. Molecular weight standards (kDa) are shown on the right. The membrane was reprobed with anti-Vav Abs. (B) Lyn and (C) Vav tyrosine phosphorylation in A20 cells. Parental and CD19– A20 cells (3 × 107/lane) were incubated with anti-RP105 Abs, detergent lysed, and incubated with protein G beads and either anti-Lyn or anti-Vav Abs. Immunoprecipitated proteins were subjected to SDS-PAGE and transferred onto membranes for anti-pTyr immunoblotting. These results are representative of those obtained in 3 independent experiments.
CD19 regulates signal transduction mediated by RP105. (A) Protein tyrosine phosphorylation following RP105 cross-linking. Parental A20 and CD19– A20 cells (4 × 105/lane) were incubated with anti-RP105 Abs for the indicated times. Cell lysates were subjected to SDS-PAGE and transferred onto membranes for antiphosphotyrosine (pTyr) immunoblotting. Molecular weight standards (kDa) are shown on the right. The membrane was reprobed with anti-Vav Abs. (B) Lyn and (C) Vav tyrosine phosphorylation in A20 cells. Parental and CD19– A20 cells (3 × 107/lane) were incubated with anti-RP105 Abs, detergent lysed, and incubated with protein G beads and either anti-Lyn or anti-Vav Abs. Immunoprecipitated proteins were subjected to SDS-PAGE and transferred onto membranes for anti-pTyr immunoblotting. These results are representative of those obtained in 3 independent experiments.
Furthermore, Vav association with CD19 following RP105 ligation also increased with slower kinetics than Lyn association (Figure 3B). RP105 induced Vav phosphorylation in wild-type A20 cells, but Vav phosphorylation was diminished in CD19– A20 cells following RP105 ligation (Figure 4C). Thus, as is in BCR ligation, CD19 may have a critical role as a membrane-localized adaptor protein that recruits Lyn and Vav through SH2 bindings in RP105 signaling.
CD19 specifically regulates JNK activation induced by anti-RP105 ligation
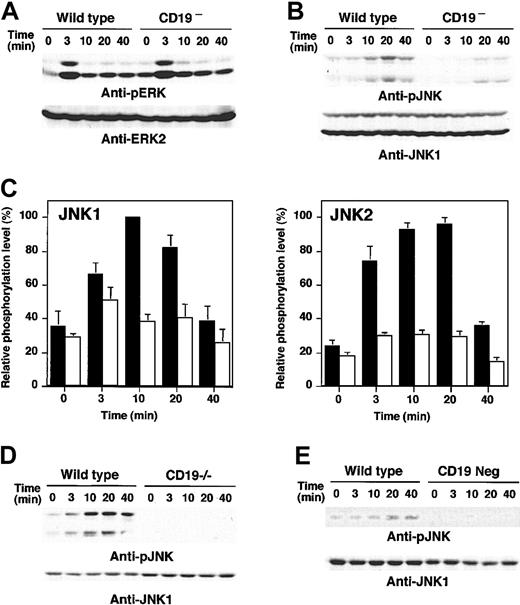
Furthermore, how CD19 loss affected downstream events activated by anti-RP105 stimulation was examined. As reported previously,28 anti-RP105 cross-linking induced both ERK and JNK phosphorylation (Figure 5A-B). ERK phosphorylation reached the maximum at 3 minutes and attenuated rapidly. ERK phosphorylation was not affected by the loss of CD19 expression (Figure 5A). JNK was fully phosphorylated at 20 minutes in response to anti-RP105 stimulation in wild-type cells. By contrast to ERK, the activation of JNK by RP105 ligation was almost completely abrogated in CD19– cells (Figure 5B). While the phosphorylation levels of both JNK1 and JNK2 increased by 3- to 4-fold from baseline after RP105 ligation in wild-type cells, the phosphorylation levels of JNK1 and JNK2 in CD19– cells were 31% and 38% of wild-type cells, respectively (Figure 5C). Impaired JNK activation by anti-RP105 ligation was also observed in purified splenic B cells from CD19–/– mice (Figure 5D). Therefore, CD19 specifically regulated JNK activation following anti-RP105 cross-linking. Furthermore, CD19– A20 cells also exhibited modest JNK phosphorylation by LPS stimulation (Figure 5E), suggesting that CD19 is indispensable for LPS-induced JNK activation in B cells.
MAPK activation in CD19-deficient cells following anti-RP105 or LPS stimulation. Wild-type and CD19– A20 cells were incubated with anti-RP105 Abs for the indicated times. Cell lysates were subjected to SDS-PAGE and transferred to nitrocellulose membranes for anti-phosphoERK (A) or anti-phosphoJNK (B) immunoblotting. (C) JNK1 (left panel) and JNK2 (right panel) phosphorylation levels were quantified and shown by mean percentages ± SEM with maximum phosphorylation levels in wild-type cells defined as 100%. ▪ indicates wild-type cells; and □, CD19– cells. (D) JNK phosphorylation following anti-RP105 stimulation (5 μg/mL) in spleen B cells from wild-type and CD19–/– mice. (E) JNK phosphorylation following LPS stimulation (10 μg/mL) in wild-type and CD19– A20 cells. These results are representative of those obtained in 3 independent experiments.
MAPK activation in CD19-deficient cells following anti-RP105 or LPS stimulation. Wild-type and CD19– A20 cells were incubated with anti-RP105 Abs for the indicated times. Cell lysates were subjected to SDS-PAGE and transferred to nitrocellulose membranes for anti-phosphoERK (A) or anti-phosphoJNK (B) immunoblotting. (C) JNK1 (left panel) and JNK2 (right panel) phosphorylation levels were quantified and shown by mean percentages ± SEM with maximum phosphorylation levels in wild-type cells defined as 100%. ▪ indicates wild-type cells; and □, CD19– cells. (D) JNK phosphorylation following anti-RP105 stimulation (5 μg/mL) in spleen B cells from wild-type and CD19–/– mice. (E) JNK phosphorylation following LPS stimulation (10 μg/mL) in wild-type and CD19– A20 cells. These results are representative of those obtained in 3 independent experiments.
CD19 and PI 3-kinase independently regulate anti-RP105–induced calcium response
By contrast to the rapid and intense [Ca2+]i increase induced by BCR ligation, RP105 engagement induces slow but sustained [Ca2+]i response.28 Since CD19 ligation also induces a similar pattern of slow increase in B cells, the effect of CD19 loss onto RP105-induced [Ca2+]i responses was assessed using B cells from CD19–/– mice. B cells from CD19–/– mice generates near-normal [Ca2+]i responses following IgM ligation,17,39 with a delayed peak during the acute phase and a more prolonged late-phase response. RP105 cross-linking also induced near-normal [Ca2+]i responses with a delayed increase in CD19–/– B cells (Figure 6A). In 3 of 5 experiments, CD19–/– B cells exhibited a more sustained response than wild-type B cells (Figure 6A; data not shown). Therefore, while delayed responses indicated that CD19 participates in RP105-induced [Ca2+]i mobilization, CD19 expression was not essential for RP105-induced [Ca2+]i mobilization.
Ca2+ responses following RP105 ligation. (A) Splenocytes from wild-type or CD19–/– mice were loaded with 1 μM indo-1-am ester and stained with FITC-labeled anti-B220 mAbs. B cells were examined for relative [Ca2+]i levels by flow cytometry after gating on the B220+ population of cells. (B-C) Wild-type B cells were pretreated with 1, 10, or 25 nM of Wortmannin for 10 minutes (B), and wild-type and CD19–/– B cells were pretreated with 15 μM of LY294002 for 10 minutes (C). Cells were stimulated by anti-RP105 Abs (5 μg/mL) as indicated by arrows. An increase in [Ca2+]i over time is shown as an increase in the ratio of indo-1 fluorescence. Results represent those obtained in at least 3 experiments.
Ca2+ responses following RP105 ligation. (A) Splenocytes from wild-type or CD19–/– mice were loaded with 1 μM indo-1-am ester and stained with FITC-labeled anti-B220 mAbs. B cells were examined for relative [Ca2+]i levels by flow cytometry after gating on the B220+ population of cells. (B-C) Wild-type B cells were pretreated with 1, 10, or 25 nM of Wortmannin for 10 minutes (B), and wild-type and CD19–/– B cells were pretreated with 15 μM of LY294002 for 10 minutes (C). Cells were stimulated by anti-RP105 Abs (5 μg/mL) as indicated by arrows. An increase in [Ca2+]i over time is shown as an increase in the ratio of indo-1 fluorescence. Results represent those obtained in at least 3 experiments.
Since CD19 associated with PI 3-kinase after anti-RP105 ligation, whether CD19 regulated [Ca2+]i mobilization through PI 3-kinase activation was then examined. To assess how PI 3-kinase regulated cytosolic [Ca2+]i concentration in RP105 signaling, wild-type B cells were incubated with PI 3-kinase inhibitors, wortmannin, or LY294002, prior to RP105 engagement. Notably, both PI 3-kinase inhibitors abrogated RP105-induced [Ca2+]i flux in a dose-dependent way (Figure 6B-C). This effect was also observed in CD19–/– B cells (Figure 6C). Therefore, RP105-induced [Ca2+]i mobilization required PI 3-kinase, and this regulation was independent of CD19 expression. By contrast to [Ca2+]i responses, JNK was normally activated in wortmannin- or LY294002-treated cells (data not shown). Collectively, PI 3-kinase and CD19 may regulate different downstream pathways independently.
Discussion
The current study demonstrates the molecular mechanism of how CD19 regulates signal transduction mediated by LPS stimulation. Of 2 known LPS receptors expressed on B cells, RP105 and TLR4, CD19 regulated RP105-mediated pathway and not TLR4-mediated pathway (Figure 7). Intriguingly, RP105 pathway was independent of MyD88 expression, which is essential to all the other known mammalian TLRs. Instead, RP105 ligation induced Lyn activation (Chan et al28 ; Figure 4), recruited CD19 into lipid rafts, and likely induced Lyn phosphorylation of CD19 (Figure 3). In turn, CD19 amplified Lyn kinase activity (Figure 4). Furthermore, phosphorylated CD19 recruited Vav, which appeared to act upstream of JNK pathway (Figures 4, 5). Thus, LPS signaling in B cells consists of 2 independent pathways, RP105-mediated pathway and TLR4-mediated pathway, and the B-cell–specific LPS receptor RP105 shares signaling molecules with BCR to induce cellular activation (Figure 7). This is a novel concept of how B cells respond to LPS stimuli and explains why LPS-induced activation is impaired in B cells lacking various signaling molecules that are apparently not involved in the common TLR signaling pathway.
Potential models for how LPS signaling is mediated in B lymphocytes. LPS activates B cells through 2 known receptors: RP105 and TLR4. RP105 is B-cell specific while TLR4 is universally expressed, and each used distinct pathways. LPS binding to RP105 induces Lyn activation and CD19 phosphorylation. Phosphorylated CD19 augments Lyn kinase activity and also mediates the Lyn and Vav interaction, which may be crucial for JNK activation. PI 3-kinase and Btk are activated independent of CD19 and regulate [Ca2+]i responses. LPS binding to TLR4 activates MyD88/IRAK and MyD88-independent TIRAP pathways to activate JNK and NFκB.
Potential models for how LPS signaling is mediated in B lymphocytes. LPS activates B cells through 2 known receptors: RP105 and TLR4. RP105 is B-cell specific while TLR4 is universally expressed, and each used distinct pathways. LPS binding to RP105 induces Lyn activation and CD19 phosphorylation. Phosphorylated CD19 augments Lyn kinase activity and also mediates the Lyn and Vav interaction, which may be crucial for JNK activation. PI 3-kinase and Btk are activated independent of CD19 and regulate [Ca2+]i responses. LPS binding to TLR4 activates MyD88/IRAK and MyD88-independent TIRAP pathways to activate JNK and NFκB.
CD19–/– B cells showed severely diminished proliferative response not only to LPS but also to anti-RP105 (Figure 1). Intriguingly, MyD88, an adaptor protein that plays a critical role for all the other TLR signaling, was dispensable for RP105-induced B-cell proliferation (Figure 1). This is consistent with the evidence that RP105 does not have TIR domain that can recruit MyD88.40 Instead, RP105 uses the Lyn and CD19 complex that specifically regulates B-cell signaling thresholds.27 RP105 ligation induced Lyn activation and CD19 phosphorylation (Figure 3). LPS stimulation also induced CD19 phosphorylation, although this was blocked by the pretreatment of Abs against MD-1, which complexes with RP105 and is important for the LPS binding to this receptor. Also, LPS stimulation induced CD19 phosphorylation in B cells from C3H/HeJ mice that have a point mutation in the TIR domain of TLR4 and thus cannot transduce signals through the MyD-88–mediated pathway (Figure 3). Thus, RP105 and TLR4 require different molecules for the signal initiation. TLR4 requires MyD88 and is independent of CD19 expression like other cell types that do not express CD19. By contrast, RP105 ligation induces Lyn activation, which was diminished in CD19– cells (Figure 4). Although physical association between CD19 and RP105 was not detected (data not shown), CD19 was recruited into lipid rafts upon RP105 engagement (Figure 3). Therefore, it is likely that phosphorylated CD19 augments Lyn kinase activity presumably in lipid rafts.
Vav phosphorylation induced by RP105 ligation was modest when compared with BCR ligation but was distinct (Figure 4C). Lyn may phosphorylate Vav directly since Syk was not activated by RP105 cross-linking (data not shown and Chan et al28 ). Thus, as in BCR signaling,26 CD19 may also mediate the interaction between Lyn and Vav in RP105 signaling. Consistent with this, Vav phosphorylation was markedly diminished in CD19– cells following RP105 ligation (Figure 4C). RP105 ligation has been demonstrated to induce activation of ERK, JNK, and p38.28 In addition, these activations have been shown to require Lyn expression. While CD19 loss did not influence ERK activation, JNK was markedly reduced both in CD19– A20 cells and in splenic B cells from CD19–/– mice (Figure 5). These findings demonstrate that CD19 is essential for JNK activation and that Vav lies upstream of JNK in RP105-mediated signaling pathways. Furthermore, although TLR4-MyD88 pathway is intact, the loss of CD19 abrogated LPS-induced JNK activation. Thus, CD19 may contribute mainly to JNK activation in LPS signaling.
RP105 engagement on wild-type B cells resulted in a slow rise in [Ca2+]i, which lacked the initial rapid phase and reached a maximum at 7 minutes (Figure 6A). This pattern resembles anti-CD19–induced [Ca2+]i flux and may be characteristic of [Ca2+]i mobilization without Syk activation. While delayed response in CD19–/– B cells suggested that CD19 facilitates [Ca2+]i increase, that CD19 loss did not abrogate [Ca2+]i mobilization induced by RP105 ligation suggests that this response is not totally dependent on CD19 expression. Rather, PI 3-kinase inhibitors, wortmannin and LY294002, abrogated [Ca2+]i response completely in wild-type cells as well as CD19–/– B cells, suggesting that RP105-induced [Ca2+]i flux is dependent on PI 3-kinase. PI 3-kinase regulates [Ca2+]i mobilization by recruiting Btk to the membrane.41 Consistently, B cells that lack CD19 expression and Btk function (CD19–/– Xid B cells) had more profound defect to respond to LPS when compared with CD19–/– B cells and Xid B cells (data not shown). By contrast, CD19– cells exhibited near-normal Akt activation (data not shown), while PI 3-kinase inhibitors, wortmannin and LY294002, did not block JNK activation (data not shown). Recent studies have demonstrated that the PI 3-kinase and Btk pathway contribute to nuclear factor κB (NFκB) activation in B cells.42-44 Collectively, CD19 and PI 3-kinase are not linear components but independently play crucial roles in RP105 signaling pathways.
The mechanism of how RP105 and TLR4 cooperatively regulate LPS response is still unclear. RP105 was constitutively expressed at high levels, while TLR4/MD-2 expression was low when compared with B-lymphoblastoid cell line A20 (Figure 1) or macrophages.45 However, TLR4 is essential for LPS-induced B-cell proliferation.8-10 By contrast, LPS can still induce weak proliferation in the absence of RP105 expression.11 This discrepancy must be clarified, although TLR4 expression may be required for LPS-induced B-cell proliferation and RP105 may augment this response.
In summary, B cells use 2 known receptor-signaling systems for LPS stimuli. The universal pathway by TLR4 and MyD88, which are ubiquitously expressed, is required for B cells as well. In addition, B cells express B-cell–specific RP105 receptor, which activates the similar pathways to BCR signaling, such as the Lyn/CD19/Vav complex and the PI 3-kinase and “signalosome” molecules. RP105 and TLR4 pathways may lead to NFκB and JNK activation synergistically. This unique signaling system may be characteristic of B cells, which link innate immunity and adaptive immunity. Furthermore, since a recent study has shown that TLRs can drive autoAb production,46,47 the disruption of these pathways may also contribute to the development of autoimmunity.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-11-3573.
Supported by grants from the Ministry of Education, Science, and Culture of Japan (M.F., S.S.); the Japan Health Sciences Foundation (M.F.); the Uehara Memorial Foundation (M.F.); and National Institutes of Health grants CA81776 and CA54464 (T.F.T.).
T.F.T. and K.T. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. B-cell proliferation in response to anti-RP105 or LPS stimulation. Spleen B cells (1 × 105/well) from wild-type and MyD88–/– mice (A) and wild-type and CD19–/– mice (B-C) were cultured with the indicated concentrations of anti-RP105 Abs (A-B) or LPS (C). Proliferation was assessed by the incorporation of labeled thymidine (1 μCi/well [0.037 MBq/well]) added during the last 16 hours of 72-hour cultures. Values represent the mean counts per minute (cpm; ± SEM) obtained from triplicate cultures. Mean values significantly different from wild-type proliferation levels are indicated, **P < .01. Results represent those obtained in 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2002-11-3573/6/m_h81634769001.jpeg?Expires=1763510222&Signature=3I6~Fk1DYfgMxrNaAtXyku~tcxWuh37IDsIK~jGq~aA5~XXbrmStP3Txu~HgAh6Qy5ZCzUDuFvGA1vXTfEZD2M1mXSdL6~RYagUgLpxOTbrmdHK1wCbx~NsA977Ko2AtoaB0z4wvOcF5FNUbk95-vxs1NmV18lhM9XQ1EEZWD4xblIjgY8GYZnx~H526n3GTY4YvounteZUfMkHhw3joHqHxG-zWJfPHMXNppOczF~ka~n9ypyYMYZXpfD540zjPYVq1WdlHRpXknD~ONzuZznRDhdWu1o3YnXg1nPMniyfvvJSux5tdX8DZ0B895y0OWm7CKBgnm-z6UNG78cNoyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Ca2+ responses following RP105 ligation. (A) Splenocytes from wild-type or CD19–/– mice were loaded with 1 μM indo-1-am ester and stained with FITC-labeled anti-B220 mAbs. B cells were examined for relative [Ca2+]i levels by flow cytometry after gating on the B220+ population of cells. (B-C) Wild-type B cells were pretreated with 1, 10, or 25 nM of Wortmannin for 10 minutes (B), and wild-type and CD19–/– B cells were pretreated with 15 μM of LY294002 for 10 minutes (C). Cells were stimulated by anti-RP105 Abs (5 μg/mL) as indicated by arrows. An increase in [Ca2+]i over time is shown as an increase in the ratio of indo-1 fluorescence. Results represent those obtained in at least 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2002-11-3573/6/m_h81634769006.jpeg?Expires=1763510222&Signature=PWBNB-Wi1s-z6kk8p7wqB~o5sTwGRQ2stz3PDfh~gAqntwKjXtZBYp1GIEH76iXBtxp~~yVZcJV9xEULNKVEcGAt6iC~huk82JopW1Njigj5ywablXmOJylFmPTSxqVfeYhA8JdDWNaSmFwp3y~J8Oymjujf-ZYOrRr6zmAl5-aDM7j9FTB-KvQhRXQ3zvp6b4~UTvPOrhU7eq-ECS~Iq8sD61g1fqDTngHCNvPZHxClv7jL9R85LwqzTdEaa~SHnOrNMiAYmksbleYvMBkY1X3zXlah1ZaG5uJWevuvgKdYrnRrBVFGDah~i~q09XIi4CrcOs3LNmsBAG~-oksyWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Potential models for how LPS signaling is mediated in B lymphocytes. LPS activates B cells through 2 known receptors: RP105 and TLR4. RP105 is B-cell specific while TLR4 is universally expressed, and each used distinct pathways. LPS binding to RP105 induces Lyn activation and CD19 phosphorylation. Phosphorylated CD19 augments Lyn kinase activity and also mediates the Lyn and Vav interaction, which may be crucial for JNK activation. PI 3-kinase and Btk are activated independent of CD19 and regulate [Ca2+]i responses. LPS binding to TLR4 activates MyD88/IRAK and MyD88-independent TIRAP pathways to activate JNK and NFκB.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2002-11-3573/6/m_h81634769007.jpeg?Expires=1763510222&Signature=C4TD4sMX8kIvy~BnBy4L4unLsE9rtoIMbTtYxqog-y43WUmRyVvlY966uFW-jssvhcGDolq53eVcE2ohKh5mdzp~FoKkxoWnhnLuJA0yILvSkpMuGMiYzElh5MvhU3nKrPAgBtDfEdTbMVarc6x~w9SvNaVn2RsGlkzfg5HpQSMs8i2gUggA-6hXSZW-cXM9KarYR4LCAGqWsnOcYG8i5YVAkbEgYe1Bzi522ms5iZbtiqVJ3TVrC~YyUydyAei4FTRtT~~d-UVSfBxsB3ofUv0PJrlDEUWY8AllH0JudndpVSz4eTF3YT-rm3Z--m9rMnwsHjaoLVfOcOWo7MD92g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
