Abstract
Survivin, a member of the inhibitor of apoptosis protein family, is expressed in almost all types of malignancies, making this protein a useful tool for the development of broadly applicable vaccination therapies. We used a recently identified HLA-A2 binding peptide and dendritic cells (DCs) from healthy donors to induce survivin-specific cytotoxic T lymphocytes (CTLs) in vitro. These T cells efficiently lysed target cells pulsed with the cognate peptide. Furthermore, survivin-specific CTLs recognized HLA-A2–matched tumor cell lines and primary malignant cells from patients with leukemia in an antigen-specific and HLA-restricted manner as demonstrated with the use of cold target inhibition assays and blocking antibodies. To validate the immunogenicity of survivin we performed the experiments in an autologous setting and used monocyte-derived DCs as targets. Interestingly, we found that DCs up-regulate survivin expression on stimulation with tumor necrosis factor α (TNF-α). However, these mature DCs were not recognized by survivin-specific CTLs, whereas they lysed autologous mature DCs pulsed with the antigenic peptide or transfected with whole tumor RNA purified from a survivin-expressing cell line. To further analyze the possible use of survivin-specific CTLs in cancer therapies, we induced survivin-specific CTLs using peripheral blood mononuclear cells (PBMNCs) and DCs from a patient with chronic lymphocytic leukemia (CLL). The in vitro–generated T cells efficiently recognized autologous malignant CLL cells, whereas they spared autologous-purified nonmalignant B cells or DCs. Our results demonstrate that survivin epitopes are presented on a broad variety of malignancies and can be applied in vaccination therapies.
Introduction
Survivin is a newly identified member of the inhibitor of apoptosis (IAP) gene family that has been implicated in suppression of apoptotic cell death and regulation of cell division.1,2 Survivin is present during normal fetal development but undetectable in differentiated adult tissues with the exception of thymus, testis, placenta, and growth hormone-stimulated hematopoietic progenitor and endothelial cells. In contrast to other members of the IAP family, which are widely expressed in human tissues, survivin expression is aberrantly elevated in most human cancers of epithelial and hematopoietic origin.3-5 This expression correlates with poor prognosis in these patients.6-8 The fact that survivin overexpression may provide a survival benefit for tumor cells and that its enhanced expression is almost completely restricted to malignant tissues makes survivin an interesting target for the development of immunotherapeutic strategies.
It was shown that survivin-reactive T cells can be found in the peripheral blood of patients with malignant melanoma or chronic lymphocytic leukemia and that these cytotoxic T lymphocytes (CTLs), when isolated by magnetic beads, can lyse allogeneic HLA-matched breast cancer and melanoma cells.9-15 In other studies, survivin-specific CTL responses in vitro could be induced using peptide-pulsed dendritic cells (DCs), and these CTLs were able to recognize Epstein-Barr virus (EBV)–immortalized B cells transfected with survivin cDNA or allogeneic lung tumor cells.11 However, the survivin-specific lysis of autologous targets or tumors remains to be demonstrated.
We used a previously identified HLA-A2 binding peptide derived from the survivin protein for CTL induction in vitro to determine the presentation of survivin-specific T-cell epitopes in malignant cells. We show here that the CTLs generated from several healthy donors and a patient with chronic lymphatic leukemia elicited an antigen-specific and HLA-A2–restricted cytolytic activity against tumor cells endogenously expressing the survivin protein, including renal cell carcinomas, breast cancer, colon cancer, melanoma, and multiple myeloma cell lines, as well as primary malignant cells from patients with AML (acute myelogenous leukemia), ALL (acute lymphoblastic leukemia), or CLL (chronic lymphocytic leukemia). Furthermore, they lysed autologous DCs transfected with RNA from a survivin-expressing tumor, indicating that this peptide is also expressed on transfection of DCs. Interestingly, we found that survivin expression can be up-regulated in activated B and T lymphocytes and DCs on stimulation with tumor necrosis factor α (TNF-α). These mature DCs and activated lymphocytes were not recognized by survivin-specific CTLs, whereas these cells were susceptible to lysis after pulsing with the cognate peptide.
In addition, we show that survivin-specific CTLs can be induced in a patient with CLL that are able to lyse primary autologous malignant CLL cells while sparing nonmalignant B cells.
Materials and methods
Tumor cell lines
The following tumor cell lines were used in experiments: MCF-7 (breast cancer, survivin+, HLA-A2+; purchased from ATCC [American Type Culture Collection], Manassas, VA), A 498, MZ 1774, MZ 1257 (renal cell carcinoma cell lines, survivin+, HLA-A2+; kindly provided by Prof A. Knuth, Frankfurt, Germany), U 266 (multiple myeloma, survivin+, HLA-A2+), HCT 116 (colon cancer, survivin+, HLA-A2+), Mel 1479 (malignant melanoma, survivin+, HLA-A2+; kindly provided by Prof G. Pawelec, Tübingen, Germany), T2 (survivin+, HLA-A2+, TAP-deficient), Croft (EBV-immortalized B-cell line, survivin+, HLA-A2+, kindly donated by O. J. Finn, Pittsburgh, PA), SK-OV-3 (ovarian cell line, survivin+, HLA-A3+; kindly provided by O. J. Finn). Tumor cell lines were grown in RP10 medium (RPMI 1640 with glutamax-I, supplemented with 10% heat inactivated fetal calf serum [FCS], 50 μM 2-mercaptoethanol, and antibiotics [Invitrogen, Karlsruhe, Germany]). B cells from patients with CLL were grown in RP10 medium for 24 hours before they were used as target cells in a standard 51Cr-release assay. K 562 cells (CML cell line) were used to determine the natural killer (NK) cell activity.
Cell isolation and generation of DCs from adherent peripheral blood mononuclear cells
Generation of DCs from peripheral blood monocytes was performed as described previously.16-18 In brief, peripheral blood mononuclear cells (PBMNCs) were isolated by Ficoll/Paque (Biochrom, Berlin, Germany) density gradient centrifugation of heparinized blood obtained from buffy coat preparations of healthy volunteers from the blood bank of the University of Tübingen. Cells were seeded (1 × 107 cells/3 mL per well) into 6-well plates (BD Falcon, Heidelberg, Germany) in RP10 medium. After 2 hours of incubation at 37°C and 5% CO2, nonadherent cells were removed, and the adherent blood monocytes were cultured in RP10 medium supplemented with the following cytokines: human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; Leukomax; Novartis, Basel, Switzerland; 100 ng/mL), interleukin 4 (IL-4; R&D, Wiesbaden, Germany; 10 μg/mL), and TNF-α (R&D; 10 ng/mL). The phenotype of DCs was analyzed by flow cytometry after 7 days of culture. Isolation of B and T lymphocytes from peripheral blood was performed using magnetically activated cell sorter (MACS) technology, as recommended by the manufacturer. For activation, T cells were incubated overnight with phorbol 12-myristate 13-acetate (Sigma, Deisenhofen, Germany; 25 ng/mL) and ionomycin (Sigma; 1 μg/mL). Purified B lymphocytes were activated through CD40 engagement by treating the cells with sCD40L (Biozol, Eching, Germany; 1 μg/mL) and IL-4 (R&D; 10 μg/mL).
RT-PCR
Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed with some modifications as previously described.19 Total RNA was isolated from cell lysates using QIAGEN RNeasy Mini anion-exchange spin columns (QIAGEN, Hilden, Germany) according to the instructions of the manufacturer. Up to 5 μg total RNA was subjected to a 20-μL cDNA synthesis reaction (SuperScript First-Strand Synthesis System for RT-PCR; Invitrogen). Oligo(dT) was used as primer. cDNA (1 μL) was used in a 15-μL PCR amplification reaction. To control the integrity of the RNA and the efficiency of the cDNA synthesis, 1 μL cDNA was amplified by an intron-spanning primer pair for the β2-microglobulin gene. For the survivin and the β2-microglobulin cDNA the PCR temperature profiles were as follows: 2 minutes pretreatment at 94°C and 30 cycles at 94°C for 30 seconds, annealing at 59°C for 30 seconds and 72°C for 60 seconds with a final extension at 72°C for 7 minutes. Primer sequences were deduced from published cDNA sequences (accession no. AF077350); β2-microglobulin, 5′-GGGTTTCATCCATCCGACAT-3′ and 5′-GATGCTGCTTACATGTCTCGA-3′; survivin, 5′-CGACCCCATAGAGGAACATAAA-3′ and 5′-GGAATAAACCCTGGAAGTGGTG-3′. RT-PCR reactions (10 μL) were electrophoresed through a 2% agarose gel and stained with ethidium bromide for visualization under UV light.
Induction of antigen-specific CTL response using HLA-A2–restricted synthetic peptides
The HLA-A2 binding peptides derived from survivin (ELTLGEFLKL11 ), adipophilin (SVASTITGV,20 used as an irrelevant control), and HIV (pol HIV-1 reverse transcriptase peptide, amino acids 476-484, ILKEPVHGV, used as an irrelevant control) were synthesized using standard F-moc chemistry on a peptide synthesizer (432A; Applied Biosystems, Weiterstadt, Germany) and analyzed by reversed-phase high-performance liquid chromatography (HPLC) and mass spectrometry. For CTL induction, 5 × 105 DCs were pulsed with 50 μg/mL synthetic peptide for 2 hours, washed, and incubated with 3 × 106 autologous PBMNCs in RP10 medium. After 7 days of culture, cells were restimulated with autologous peptide-pulsed PBMNCs, and 1 ng/mL human recombinant IL-2 (R&D Systems) was added on days 1, 3, and 5. The cytolytic activity of induced CTLs was analyzed on day 5 after the last restimulation in a standard 51Cr-release assay.16-18
CTL assay
The standard 51Cr-release assay was performed as described previously.16-18 Target cells were pulsed with 50 μg/mL peptide for 2 hours and labeled with [51Cr]-sodium chromate in RP10 for 1 hour at 37°C and 5% CO2. Cells (104) were transferred to a well of a round-bottomed 96-well plate. Varying numbers of CTLs were added to give a final volume of 200 μL and incubated for 4 hours at 37°C. At the end of the assay supernatants (50 μL/well) were harvested and counted in a beta-plate counter. The percentage of specific lysis was calculated as 100 × (experimental release - spontaneous release/maximal release - spontaneous release). Spontaneous and maximal releases were determined in the presence of either medium or 2% Triton X-100, respectively.
Antigen specificity of tumor cell lysis was further determined in a cold target inhibition assay16 by analyzing the capacity of peptide-pulsed unlabeled T2 cells to block lysis of tumor cells at a ratio of 20:1 (inhibitor-to-target ratio).
For antibody blocking experiments, cells were incubated for 30 minutes with 10 μg/mL monoclonal antibody BB7.2 (immunoglobulin G2b [IgG2b]; kindly provided by Stefan Stevanovic, Tübingen, Germany) recognizing HLA-A2 or isotype antibody (ChromPure Mouse IgG; Dianova, Hamburg, Germany; 0.2 μg/mL) before seeding in 96-well plates.
Polyacrylamide gel electrophoresis (PAGE) and Western blotting for detection of survivin protein
Cells were lysed in buffer containing 1% Igepal, 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.5, 150 mM NaCl, 2 mM EDTA (ethylenediaminetetraacetic acid), 10% glycerin, 1 mM PMSF (phenylmethyl sulfonyl fluoride), and 2 μg/mL aprotinin. Protein concentration was determined using a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Total protein (30 μg) was separated on 15% polyacrylamide gel, blotted on nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany), and probed with a survivin-specific antibody (rabbit polyclonal, AF886; R&D). Bands were visualized by electrogenerated chemiluminescence (ECL) staining (Amersham Pharmacia, Freiburg, Germany).
Electroporation of DCs with EGFP in vitro transcript (IVT) or whole tumor–derived RNA
Enhanced green fluorescent protein (EGFP)–IVT was synthesized from the plasmid pSP64 Poly(A) EGFPII (generously provided by VFI Van Tendeloo, Antwerp, Belgium) as described.21 Total RNA was isolated from cell lysates using QIAGEN RNeasy Mini anion-exchange spin columns (QIAGEN) according to the protocol for isolation of total RNA from animal cells provided by the manufacturer. Quantity and purity of RNA was determined by UV spectrophotometry. RNA samples were routinely checked by formaldehyde/agarose gel electrophoresis for size and integrity and stored at -80°C in small aliquots.
Prior to electroporation on day 6, immature DCs were washed twice with serum-free X-VIVO 20 medium (BioWhittaker, Walkersville, MD) and resuspended to a final concentration of 2 × 107 cells/mL. Subsequently, 200 μL cell suspension was mixed with 10 μg total RNA and electroporated in a 4-mm cuvette using an Easyject Plus unit (Peqlab, Erlangen, Germany). The physical parameters were voltage of 300 V, capacitance of 150 μF, resistance of 1540 Ω, and pulse time of 231 ms. After electroporation the cells were immediately transferred into RP10 medium and returned to the incubator. The viability of the cells was more than 80% after electroporation.
Results
Induction of survivin-specific cytotoxic T lymphocytes (CTLs) using peptide-pulsed DCs
Survivin is overexpressed in almost all human malignancies and, therefore, represents a possible universal target antigen for the development of vaccination therapies. As demonstrated by Western blot analysis and RT-PCR, expression of survivin could be detected in all tested human tumor cell lines (Figure 1). Purified B and T lymphocytes were included in the analysis (Figure 1C) as positive (activated lymphocytes) and negative controls (resting lymphocytes).
Survivin expression in human tumor cell lines. (A) RT-PCR using survivin-specific primers was performed to analyze the RNA expression of survivin in human tumor cell lines. β2-Microglobulin–specific primers were used as controls. (B) Western blot analysis using a monoclonal antibody against survivin protein. Peripheral blood mononuclear cells (PBMNCs) and purified B and T lymphocytes were included in the analysis of protein expression as controls (C).
Survivin expression in human tumor cell lines. (A) RT-PCR using survivin-specific primers was performed to analyze the RNA expression of survivin in human tumor cell lines. β2-Microglobulin–specific primers were used as controls. (B) Western blot analysis using a monoclonal antibody against survivin protein. Peripheral blood mononuclear cells (PBMNCs) and purified B and T lymphocytes were included in the analysis of protein expression as controls (C).
To analyze the presentation of survivin-derived T-cell epitopes by these tumor cells we induced survivin-specific CTLs in vitro with the use of DCs derived from adherent PBMNCs of HLA-A2–positive healthy donors. These DCs were pulsed with the described11 HLA-A2–binding antigenic peptide and used as antigen-presenting cells. The cytotoxicity of the in vitro–induced CTLs was assessed in a standard 51Cr-release assay. As shown in Figure 2, the CTL line obtained after several weekly restimulations demonstrated peptide-specific killing. T cells only recognized T2 cells coated with the cognate survivin peptide, whereas they did not lyse target cells pulsed with an irrelevant peptide (adipophilin), confirming the specificity of the cytolytic activity.
Induction of survivin-specific CTL responses in vitro using peptide-pulsed mature DCs as antigen-presenting cells. DCs generated from adherent PBMNCs in the presence of GM-CSF, IL-4, and TNF-α were pulsed with the synthetic peptide derived from the survivin protein and used to induce a CTL response in vitro. Cytotoxic activity of induced CTLs was analyzed in a standard 51Cr-release assay using T2 cells pulsed with the cognate survivin peptide (T2 + Sv, ⋄) or an irrelevant (adipophilin) peptide (T2 + Ad, □) as targets. E:T indicates effector-to-target ratio.
Induction of survivin-specific CTL responses in vitro using peptide-pulsed mature DCs as antigen-presenting cells. DCs generated from adherent PBMNCs in the presence of GM-CSF, IL-4, and TNF-α were pulsed with the synthetic peptide derived from the survivin protein and used to induce a CTL response in vitro. Cytotoxic activity of induced CTLs was analyzed in a standard 51Cr-release assay using T2 cells pulsed with the cognate survivin peptide (T2 + Sv, ⋄) or an irrelevant (adipophilin) peptide (T2 + Ad, □) as targets. E:T indicates effector-to-target ratio.
Survivin-specific CTLs can lyse tumor cells of epithelial and hematologic origin
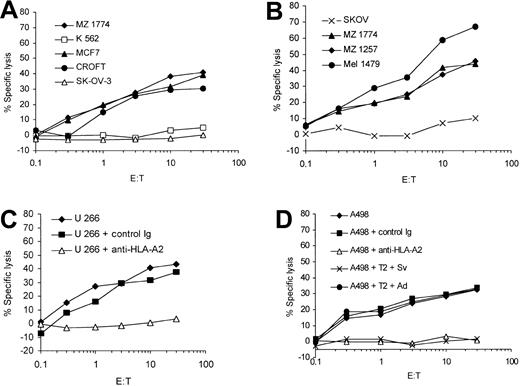
In the next set of experiments we evaluated the ability of the in vitro–induced survivin-specific CTLs to lyse tumor cells endogenously expressing survivin. The survivin- and HLA-A2–expressing cell lines A 498, MZ 1257, and MZ 1774 (renal cell carcinoma, RCC); MCF-7 (breast cancer); Mel 1479 (malignant melanoma); U 266 (multiple myeloma); and EBV-transformed B-cell line Croft were used as target cells in a standard 51Cr-release assay. Survivin-specific CTLs were able to efficiently lyse malignant cells expressing both HLA-A2 and survivin (Figure 3A-D). There was no lysis of the ovarian cancer cells SK-OV-3 (HLA-A3+), demonstrating that the presentation of survivin peptides in context of HLA-A2 molecules on the tumor cells is necessary for the efficient lysis of target cells. Furthermore, these data show for the first time that the survivin-specific CTLs recognize RCC and multiple myeloma cells in an HLA-restricted and antigen-specific manner. The in vitro–induced T cells did not lyse the K 562 cells, indicating that the cytotoxic activity was not NK cell mediated.
Antigen-specific lysis of human tumor cell lines endogenously expressing survivin. Human renal cell carcinoma (MZ 1774, MZ 1257, A 498), melanoma (Mel 1479), breast cancer (MCF-7), multiple myeloma (U 266), and the EBV-immortalized Croft cells expressing survivin and HLA-A2 as well as the ovarian cancer cell line SK-OV-3 (HLA-A2-/survivin+) were used as targets in a standard 51Cr-release assay. K 562 cells were included to determine the NK cell activity (A-C). The HLA-A2 restriction of the CTL lines was analyzed using a HLA-A2–specific monoclonal antibody (U 266 + anti–HLA-A2, 3C; A498 + anti–HLA-A2, 3D). An isotype antibody was used as control (control immunoglobulin). A cold target inhibition assay was performed to analyze the antigen specificity of the CTL lines (D). Unlabeled T2 cells pulsed with the cognate survivin peptide were used to block lysis of A 498 cells (A 498 + T2 + Sv). No inhibition of lysis was detected when using T2 cells pulsed with the irrelevant adipophilin peptide (A 498 + T2 + Ad). The ratio of inhibitor to target cells was 20:1.
Antigen-specific lysis of human tumor cell lines endogenously expressing survivin. Human renal cell carcinoma (MZ 1774, MZ 1257, A 498), melanoma (Mel 1479), breast cancer (MCF-7), multiple myeloma (U 266), and the EBV-immortalized Croft cells expressing survivin and HLA-A2 as well as the ovarian cancer cell line SK-OV-3 (HLA-A2-/survivin+) were used as targets in a standard 51Cr-release assay. K 562 cells were included to determine the NK cell activity (A-C). The HLA-A2 restriction of the CTL lines was analyzed using a HLA-A2–specific monoclonal antibody (U 266 + anti–HLA-A2, 3C; A498 + anti–HLA-A2, 3D). An isotype antibody was used as control (control immunoglobulin). A cold target inhibition assay was performed to analyze the antigen specificity of the CTL lines (D). Unlabeled T2 cells pulsed with the cognate survivin peptide were used to block lysis of A 498 cells (A 498 + T2 + Sv). No inhibition of lysis was detected when using T2 cells pulsed with the irrelevant adipophilin peptide (A 498 + T2 + Ad). The ratio of inhibitor to target cells was 20:1.
To further prove the antigen specificity and major histocompatibility complex (MHC) restriction of the in vitro–induced CTL lines we used an HLA-A2–specific monoclonal antibody and performed cold target inhibition assays. The lysis of the target cells could be blocked by incubation of target cells with the antibody (U 266, Figure 3C, and A 498, Figure 3D) and in cold target inhibition assays (A 498, Figure 3D). The addition of cold (not labeled with 51Cr) T2 cells pulsed with the cognate peptide reduced the lysis of A 498 tumor cells, whereas T2 cells pulsed with an irrelevant peptide (adipophilin) showed no effect.
Moreover, we tested the ability of survivin-specific CTLs to lyse primary human malignant cells isolated from patients with acute myelogenous and lymphoblastic leukemias (AML, ALL) and CLL. As shown in Figure 4 the in vitro–induced CTLs were able to efficiently recognize the primary leukemia cells.
Lysis of primary leukemia cells by survivin-specific CTLs. Primary malignant cells from HLA-A2–positive (CLL-K, AML-Ah, AML-Bu, AML-Na, ALL-Sc) or HLA-A2–negative (CLL-F, AML-Bd, AML-Ra, AML-Fi) patients with acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), and chronic lymphocytic leukemia (CLL) were used as targets in a standard 51Cr-release assay.
Lysis of primary leukemia cells by survivin-specific CTLs. Primary malignant cells from HLA-A2–positive (CLL-K, AML-Ah, AML-Bu, AML-Na, ALL-Sc) or HLA-A2–negative (CLL-F, AML-Bd, AML-Ra, AML-Fi) patients with acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), and chronic lymphocytic leukemia (CLL) were used as targets in a standard 51Cr-release assay.
Survivin-specific CTLs can lyse autologous DCs transfected with whole tumor RNA
To analyze the cytotoxic activity of the CTLs in an autologous setting and to test the presentation of survivin-specific T-cell epitopes on transfection with whole tumor RNA we used autologous DCs, generated from the same PBMNCs that were used for CTL induction, as target cells. As shown in Figure 5, CTLs efficiently lysed autologous mature DCs pulsed with the cognate survivin peptide but spared DCs loaded with an irrelevant peptide (HIV). In addition, the CTL line recognized autologous mature DCs electroporated with the whole tumor RNA isolated from the survivin-expressing A 498 tumor cell line, indicating that survivin peptides are processed and presented after transfection of DCs with RNA derived from survivin-positive tumor cells.
Survivin-specific CTLs recognize autologous dendritic cells transfected with RNA isolated from A 498 tumor cells. Autologous DCs from a healthy HLA-A2+ donor generated from peripheral blood monocytes were pulsed with the cognate survivin peptide (Sv) or electroporated with tumor RNA isolated from the survivin-expressing A 498 tumor cell line and were used as target cells in a standard 51Cr-release assay (▪, •). DCs pulsed with an irrelevant HIV peptide (HIV) or electroporated with EGFP in vitro transcript (IVT) were used as controls in the assay (⋄, ▵).
Survivin-specific CTLs recognize autologous dendritic cells transfected with RNA isolated from A 498 tumor cells. Autologous DCs from a healthy HLA-A2+ donor generated from peripheral blood monocytes were pulsed with the cognate survivin peptide (Sv) or electroporated with tumor RNA isolated from the survivin-expressing A 498 tumor cell line and were used as target cells in a standard 51Cr-release assay (▪, •). DCs pulsed with an irrelevant HIV peptide (HIV) or electroporated with EGFP in vitro transcript (IVT) were used as controls in the assay (⋄, ▵).
Survivin-specific CTLs do not lyse mature dendritic cells or activated B and T lymphocytes
It was recently demonstrated that survivin expression can be induced in cells during activation or differentiation.21,22 We, therefore, analyzed the expression of survivin in human DCs on maturation with TNF-α. We found that survivin protein expression can be up-regulated in DCs on stimulation with TNF-α (Figure 6A). However, the survivin-specific CTLs neither killed autologous immature DCs (generated in the presence of GM-CSF and IL-4) nor DCs activated with TNF-α, whereas they lysed survivin-expressing HLA-A2+ HCT 116 colon carcinoma cells (Figure 6B) and mature DCs pulsed with survivin peptide (Figure 5).
Survivin expression in dendritic cells. (A) Survivin expression in human monocyte-derived dendritic cells was analyzed by Western blotting. (B) Standard 51Cr-release assay was performed using autologous immature DCs and autologous DCs activated with TNF-α from a healthy HLA-A2–positive as well as the HLA-A2– and survivin–positive colon carcinoma cell line HCT 116 as targets for survivin-specific CTLs.
Survivin expression in dendritic cells. (A) Survivin expression in human monocyte-derived dendritic cells was analyzed by Western blotting. (B) Standard 51Cr-release assay was performed using autologous immature DCs and autologous DCs activated with TNF-α from a healthy HLA-A2–positive as well as the HLA-A2– and survivin–positive colon carcinoma cell line HCT 116 as targets for survivin-specific CTLs.
As shown in Figure 1C, survivin is expressed in B and T lymphocytes undergoing activation. To investigate whether these nontransformed-activated lymphocytes are recognized by survivin-specific CTLs, 51Cr-release experiments were performed with the use of activated autologous B and T lymphocytes as targets. No lysis of activated B- and T-cell targets occurred in the assays (Figure 7). However, after pulsing activated B and T lymphocytes with the cognate survivin peptide, they were susceptible to lysis.
Survivin-specific CTLs do not recognize activated B and T lymphocytes. Survivin-specific CTLs were assayed against autologous-activated B and T lymphocytes either pulsed with the cognate survivin (Sv) peptide (filled symbols) or left unpulsed (open symbols).
Survivin-specific CTLs do not recognize activated B and T lymphocytes. Survivin-specific CTLs were assayed against autologous-activated B and T lymphocytes either pulsed with the cognate survivin (Sv) peptide (filled symbols) or left unpulsed (open symbols).
Lysis of primary malignant B-CLL cells by survivin-specific CTLs
In line with a previous report we found that survivin is expressed in malignant B cells from a HLA-A2–positive patient with CLL, who was in remission after treatment with fludarabine (data not shown). We generated survivin-specific CTLs from PBMNCs of this patient and analyzed the presentation of survivin-derived peptides by primary CLL cells that we used as targets in a standard 51Cr-release assay. The expression of survivin in the purified malignant B-cell population that represented more than 90% of the peripheral blood lymphocytes and were cryopreserved before treatment was confirmed by RT-PCR (data not shown). As demonstrated in Figure 8, the induced CTL efficiently lysed autologous DCs from this patient that were pulsed with the cognate peptide and the autologous CLL cells, whereas they spared the nonmalignant B cells or the K 562 cells.
Survivin-specific CTLs can lyse autologous malignant chronic lymphatic leukemia cells. Malignant autologous CLL cells from an HLA-A2+ patient with CLL and autologous immature DCs were pulsed with the cognate survivin peptide (Sv) or an irrelevant adipophilin peptide (Ad) and used as targets. Autologous nonmalignant B cells were included as controls. The antigen specificity of the CTL lines was analyzed in cold target inhibition assays using unlabeled T2 cells pulsed with the cognate survivin (Sv) peptide or the irrelevant adipophilin (Ad) at an inhibitor-to-target ratio of 20:1.
Survivin-specific CTLs can lyse autologous malignant chronic lymphatic leukemia cells. Malignant autologous CLL cells from an HLA-A2+ patient with CLL and autologous immature DCs were pulsed with the cognate survivin peptide (Sv) or an irrelevant adipophilin peptide (Ad) and used as targets. Autologous nonmalignant B cells were included as controls. The antigen specificity of the CTL lines was analyzed in cold target inhibition assays using unlabeled T2 cells pulsed with the cognate survivin (Sv) peptide or the irrelevant adipophilin (Ad) at an inhibitor-to-target ratio of 20:1.
The antigen-specific lysis of CLL cells was confirmed in a cold target inhibition assay using T2 cells pulsed with the cognate or an irrelevant peptide (adipophilin).
Discussion
Survivin is a novel member of the IAP family that is, in contrast to other proteins of this family, overexpressed in malignant cells, whereas it is not expressed in most adult human tissues.1-5 The elevated expression of survivin in tumors is associated with a poor prognosis.6-8 Survivin has been implicated in preservation of cell viability and regulation of mitosis in cancer cells.13,24-29 It suppresses tumor cell apoptosis triggered by chemotherapeutic agents, caspase 3, 7, and 9, or Fas ligand–induced stimuli.30-32 The observation that inhibition of survivin interactions by antisense or dominant-negative approaches in different transformed cell models can induce apoptosis of malignant cells led to the assumption that targeting of survivin could represent a novel strategy to treat cancer patients.
Recently, it was shown that survivin-specific CTLs can be detected in patients with CLL or malignant melanoma and that these CTLs (when isolated by magnetic beads) are able to lyse HLA-matched allogeneic breast cancer and malignant melanoma cells. This finding indicates that survivin-derived epitopes could be applied in immunotherapy of tumors.10 This assumption was strengthened by the identification of a natural processed HLA-A2 binding peptide that could be used for generation of survivin-specific CTLs when pulsed on dendritic cells.11 The CTLs induced in vitro were able to lyse survivin-transfected EBV-immortalized B cells. However, the survivin-specific recognition of autologous tumor cells was not demonstrated in the previous studies.
By using this HLA-A2 peptide for CTL induction in vitro we were able to show that survivin epitopes are expressed on a broad spectrum of epithelial and hematologic malignancies, including renal cell carcinomas, breast cancer, colon cancer, multiple myeloma, and leukemias, thus demonstrating that survivin is a universal tumor antigen.
To analyze whether survivin-derived antigenic peptides are presented by tumor cells endogenously expressing survivin, we induced survivin peptide–specific CTLs and used these CTLs as effector cells. DCs generated from normal HLA-A2+ peripheral blood monocytes in the presence of GM-CSF, IL-4, and TNF-α were pulsed with the survivin peptide and used as antigen-presenting cells (APCs) for CTL priming. The survivin peptide-specific CTL lines obtained after several weekly restimulations were able to lyse not only target cells pulsed with the antigenic peptide but also recognized tumor cells endogenously expressing the survivin protein in an antigen-specific and HLA-A2–restricted manner that was confirmed with the use of monoclonal antibodies and in cold target inhibition assays.
To further determine the specificity of the elicited CTL response we performed the experiments in an autologous setting and used autologous DCs that were either pulsed with the cognate peptide or electroporated with RNA isolated from a survivin-expressing tumor cell line. Survivin peptide-specific CTLs efficiently lysed both, the peptide pulsed autologous DCs and DCs transfected with whole tumor RNA. This finding confirms the specificity of our CTL line and demonstrates that the peptide used for CTL induction is also processed and presented on transfection of DCs with whole tumor RNA and might, therefore, represent an immunodominant epitope.
In the next set of experiments we evaluated the possible application of survivin peptides in patients with malignant diseases. As mentioned earlier, it was previously shown that survivin-reactive T cells can be found using enzyme-linked immuno spot (ELISPOT) analysis in peripheral blood of patients with CLL and that these T cells can recognize allogeneic tumor cells. But the specific recognition of autologous tumor cells by survivin-specific CTLs remained to be proven.
We used peripheral blood from an HLA-A2–positive patient with chronic lymphocytic leukemia who was in remission after treatment with fludarabine for the induction of survivin peptide-specific CTLs. The expression of survivin in malignant B-cell population that represented more than 90% of the peripheral blood lymphocytes and were cryopreserved before treatment was confirmed by RT-PCR (data not shown). The in vitro–induced CTLs efficiently lysed the malignant leukemia cells in an MHC-restricted and antigen-specific manner but spared purified B cells or autologous DCs from this patient. This finding shows that survivin-specific CTLs can be induced in patients with malignancies and that these CTLs are able to recognize the autologous tumor cells.
The pattern of survivin expression during fetal development indicates that it could be a general regulator of mitosis and thus play a regulatory role in adult tissues, particularly on stimulation with growth factors. Recently, it was demonstrated that survivin can be found in the thymus,22 and its expression is up-regulated in CD34+ cells by hematopoietic cytokines and in endothelial cells during angiogenesis stimulated by vascular endothelial growth factor, angiopoetin-1, or basic fibroblast growth factor.23 These results suggest that survivin is not a cancer-specific antiapoptotic antigen and caution is required when targeting this protein in vaccination therapies.
In our study we found that survivin expression can be induced in activated B and T lymphocytes as well as in DCs on treatment with TNF-α. When these cells were used as targets in a standard 51Cr-release assay, the survivin-specific CTLs did not recognize autologous-activated B and T cells or mature DCs. However, the same cells were susceptible to lysis after pulsing with the antigenic peptide. Furthermore, they recognized the TNF-α–activated DCs electroporated with total RNA isolated from tumor cells expressing survivin. So far it is not clear why activated lymphocytes or DCs are not lysed by CTLs against survivin peptide. It might be related to the affinity of the induced T cells and to higher presentation of survivin-derived peptides by tumor cells. Another explanation of this phenomenon might be that nonmalignant cells present a different repertoire of T-cell epitopes as compared with tumor cells. However, when DCs were transfected with RNA isolated from survivin-expressing tumor cells and used as targets in a standard 51Cr-release assay, they were efficiently lysed by survivin-specific CTLs. This situation might lead to the assumption that there are some differences in the processing and presentation of endogenously expressed or exogenously acquired antigens by DCs. Recently, it was shown that immature DCs are susceptible to CTL-induced killing, but they become resistant to the CTL-induced apoptosis on maturation. This protection was achieved by the expression of serine protease inhibitor 6 (SPI-6), a member of the serpin family that inactivates granzyme B.33 However, in our experiments mature DCs treated with TNF-α could efficiently be lysed by CTLs on pulsing with the cognate peptide or electroporation with survivin message containing tumor RNA, thus suggesting that this pathway is not involved in the observed DC protection from CTL-mediated killing.
In several clinical vaccination trials using DCs presenting tumor-associated antigens or adoptive transfer of tumor reactive CTLs generated ex vivo, it was shown that this approach can induce antitumor immunity in patients with malignant diseases.34-38 However, with the exception of some reports in malignant melanoma trials, in which induction of vitiligo was observed after vaccinations with DCs even when antigens like MUC1, Her-2/neu, or telomerase were applied, so far no evidence for the development of autoimmune reactions in these patients was detected. In a phase 1/2 study using DCs pulsed with HLA-A2 binding peptides derived from Her-2/neu or MUC1 tumor antigens, we were able to induce peptide-specific CTLs in patients with metastatic breast and ovarian cancers in vivo without any side effects, especially no induction of anemia.34
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-08-2554.
Supported by a grant from Deutsche Forschungsgemeinschaft (SFB 510).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sylvia Stephan, Bruni Schuster, and Regina Heselmaier for excellent technical assistance. We thank H. Einsele for critical reading of the manuscript.