Abstract
Thymocyte development is a non–cell-autonomous process that requires signals provided by the thymic stroma. Bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs) derived from thymic stroma have been implicated as possible regulators of T-cell development. Using thymic organ culture, this study demonstrates that both BMP4 and FGF7/FGF10 arrest early T-cell development at the CD4-CD8-CD44+CD25- (double-negative 1 [DN1]) population and at the CD4-CD8- double-negative (DN) to CD4+CD8+ double-positive (DP) transition in a stromal compartment–dependent manner. Furthermore, BMP4 functions upstream of FGF7/FGF10, as the effects of BMP can be suppressed by cotreatment with an FGF receptor antagonist. BMP4 also acts directly on the thymic stroma to up-regulate the stroma-specific transcription factor Foxn1 and stroma-expressed chemokines. Taken together, the data in this report demonstrate that BMP acts upstream of FGF in the regulation of early T-cell development and that BMP4 acts primarily through the thymic stroma, thereby altering the thymic microenvironment and affecting thymopoiesis.
Introduction
The thymus is a heterogeneous organ composed of thymocytes, epithelia, and neural crest–derived mesenchymal cells.1,2 Thymic epithelial cells (TECs) are derived from interaction between the third pharyngeal pouch and cleft during midgestation3-6 and play a critical role in supporting the development of blood-borne precursors to differentiated thymocytes. Upon entering the thymus, T-cell precursors proceed through a complex differentiation program. They progress through a series of CD4-CD8- (double-negative [DN]) stages, from CD44+CD25-CD4-/CD8- (DN1), CD44+CD25+CD4-CD8- (DN2), and CD44-CD25+CD4-CD8- (DN3) to CD44-CD25-CD4-CD8- (DN4) progenitor cells,7 with ordered changes in cell-surface phenotypes, proliferation status, and function. DN4 cells are immediate precursors of CD4+CD8+ (double-positive [DP]) thymocytes from which CD4+ (CD4 single-positive [CD4SP]) and CD8+ (CD8SP) thymocytes are generated.
Although considerable progress has been made in the understanding of the regulation of thymopoiesis, much remains unknown about the molecules that have direct and indirect effects on this process. Indeed, this understanding is complicated further by the cross-talk between stromal cell and thymocyte compartments during thymopoiesis. Two families of molecules, bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs), have been implicated in participating in this cross-talk and in regulating T-cell development.8
BMPs are members of the transforming growth factor–β (TGF-β) superfamily and are known to be involved in cell-fate determination and patterning of the embryo.9 BMP4 and its receptors, BMPRI and BMPRII, are expressed in the developing thymus.10 BMP signaling is regulated by a number of extracellular inhibitors — including Noggin, Chordin,11 and Twisted Gastrulation (Tsg)12 — all of which can prevent BMP binding to its cell surface receptors.13 Chordin and Tsg are both expressed in the developing thymus.14,15
FGFs are ligands for a family of receptor tyrosine kinases that have essential roles in embryonic patterning and development. FGF7 and FGF10 are produced by mesenchymal cells and act on a wide range of epithelial-derived cells that express FGF receptor-2 IIIb (FGFR2IIIb).16 FGF7, FGF10, and FGFR2IIIb are all expressed in the fetal thymus,17 with FGF7 expressed in thymocytes and stromal mesenchyme, FGF10 in the stromal mesenchyme, and FGFR2IIIb in TECs.18
The roles of BMPs and FGFs in regulating thymic patterning have been studied genetically. Expression of soluble dominant-negative FGFR2IIIb in transgenic mice leads to thymic dysgenesis.19 Furthermore, in mice lacking either FGF10 or FGFR2IIIb, the thymus is greatly reduced in size, indicating a role for these molecules in epithelial-mesenchymal interactions during thymic development,20,21 especially for proper generation of the thymic stroma. Mice null for BMP4 or BMP receptors I and II (BMPRI and II), on the other hand, die between embryonic day 6.5 and 9.5, precluding study of their functions in thymic development.22-24 However, mice lacking the BMP4 inhibitor Tsg display an atrophic thymus with lymphopenia, indicating an essential role for BMP4 in thymopoiesis.25
In this report, we demonstrate that both BMP4 and FGFs have effects on thymocyte development in fetal thymic organ culture (FTOC), thereby expounding on recent studies that have demonstrated roles for BMPs and FGFs in thymocyte differentiation.9,15,26
Our study also illustrates a previously unknown interaction between BMP4 and FGF signaling and indicates that BMP4 acts upstream of FGF signaling. Furthermore, we provide evidence that BMP4 regulates thymocyte development, in significant part, via a stromal and FGF signaling–dependent mechanism.
Materials and methods
Mice
Swiss/Webster mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in the vivarium of the University of California at Los Angeles (UCLA) Division of Laboratory Animal Medicine (Los Angeles, CA). The presence of a vaginal plug was designated as day 0.5 of gestation.
Fetal thymic organ culture
Fetal thymic organ cultures (FTOCs) were established according to the protocol described by Jenkinson et al.27 Briefly, fetal thymic lobes from 15-day-old Thy-1.1 Swiss/Webster embryos (E15) were harvested aseptically, dissected free from extraneous tissue, and cultured for 7 days in the absence (untreated) or presence (treated) of the following soluble factors either individually or in combination: BMP4 (30 ng/mL), FGF7 (166 ng/mL), FGF10 (1.6 μg/mL), Noggin (500 ng/mL), FGFR2β (IIIb)/Fc (1.6 μg/mL), neutralizing BMP4 antibody (3 μg/mL). Lobes were cultured on filter (Millipore, Billerica, MA) (0.8-μm pore size)/gelfoam rafts in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 5 × 10-5 M 2-β-mercaptoethanol, 100 U/mL streptomycin, and 100 U/mL penicillin, and placed in a humidified, 5% CO2/air incubator at 37°C. For reconstitution assays of treated stroma, lobes were cultured for 5 days with or without growth factors and 1.35 mM deoxyguanosine to deplete endogenous thymocytes. The lobes were then rinsed and incubated with donor cells in hanging drop cultures in Terasaki plates (Fisher, Tustin, CA) for 48 hours. Subsequently, lobes were transferred to FTOC and incubated for 3 weeks. For reconstitution with treated donor cells, lobes were treated with 1.35 mM deoxyguanosine for 5 days. The lobes were then rinsed and incubated with donor cells that had been previously treated for 8 hours prior to hanging drop culture in Terasaki plates for 48 hours. The lobes were then transferred to FTOC as described and incubated for 3 weeks (bone marrow [BM] and DN1), 2 weeks (DN3), and 1 week (total DN). Cell proliferation and differentiation in FTOC was assessed at regular intervals following culture initiation, as we will describe. Statistical analysis was performed by means of Microsoft Excel analysis software; P values were derived from single-tailed t test.
Flow cytometry and antibodies
Cells were analyzed for the expression of the following cell surface determinants: CD4, CD8, CD25, and CD44 (Pharmingen, San Diego, CA), with the use of antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), or Cy-chrome. After staining, cells were analyzed on a Becton Dickinson FACScan (Becton Dickinson, Franklin Lakes, NJ).
Cell culture
All cultured cells used in this study were cultured in Dulbecco Minimum Essential Medium (DMEM) supplemented with 10% FCS, 2 mM l-glutamine, 100 U/mL streptomycin, and 100 U/mL penicillin; 427 thymic stromal cells were provided by Dr Barbara B. Knowles.28
Northern analysis, RT-PCR, and Taqman PCR
First, 427 cells or thymic stroma were treated with BMP4 with or without FGFR2IIIb/Fc and harvested 36 or 48 hours after treatment, respectively. RNA was generated by means of RNeasy mini-columns (Qiagen, Valencia, CA). Foxn1 probe was generated by digesting Foxn1 cDNA with BstX1; FGFR2IIIb probe encompasses exon IIIb. RNA for reverse-transcription polymerase chain reaction (RT-PCR) was generated as described or through RNAqueous-4PCR (Ambion, Austin, TX). Thymic stroma were generated by treatment of embryonic 15-day thymic lobes with 1.35 mM deoxyguanosine for 2 days in the presence or absence of soluble factors. First strand was generated by means of the Superscript first-strand synthesis method (Invitrogen, Carlsbad, CA). PCR used the following primers: Foxn1: forward, ccccagccaggaacacaacc; reverse, ggtaggtctgcccatagcag. BMPRI: forward, gcttgcggccaatcgtgtctaa; reverse, gcagcctgtgaagatgtagagg. FGFR2IIIb: forward, cttgctgtttgggcaggaca; reverse, cactcggggataaatagctc. β-actin: forward, gtgggccgctctaggcacca; reverse, cggttggccttagggttcagggggg. Eotaxin: forward, atgccacaaagcacctggac; reverse, tccctcagagcacgtcttagga. EB-11 ligand (ELC): forward, gaaagccttccgctaccttctt; reverse, tgttgcctttgttcttggca. Taqman PCR used first strand as template and was performed on ABI Prism 7900 (Applied Biosystems, Foster City, CA). Probes were labeled with 6-FAM (carboxyfluorescein) and nonfluorescent quencher. The following primers were used: FGFR2IIIb: forward, cccgtcagacaaaggcaact; reverse, caggtggtaggtgtggttgatg. Actin: forward, cgtgaaaagatgacccagatca; reverse, cacagcctggatggctacgt. Probes used were as follows: FGFR2IIIb, cacctgcctggtggagaatgaatacgg; actin, ttgagaccttcaacaccccagccatg. Quantifi-cation was performed with Quantity One (Bio-Rad, Hercules, CA) and ImageQuant (Amersham Biosciences, Freiburg, Germany) analysis software. Statistical analysis was performed with Microsoft Excel analysis software (Microsoft, Seattle, WA); P values are derived from single-tailed t tests.
Results
BMP4 treatment arrests early T-cell development
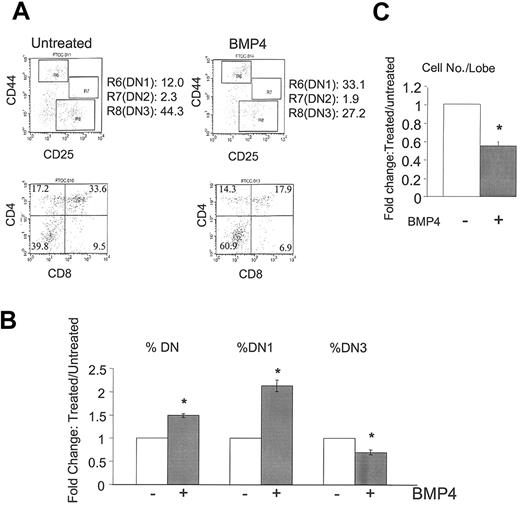
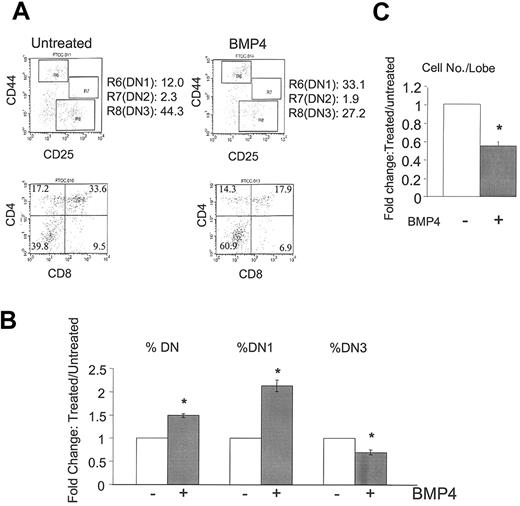
To investigate the role of BMP4 in thymocyte development, we treated intact thymic lobes with BMP4 and observed a significant change in the developmental progression of thymocytes. First, we observed an increased percentage of DN thymocytes with a corresponding decrease in the DP thymocyte population (Figure 1A-B). We further subdivided the DN group by CD25 and CD44 staining and observed a significant increase in the DN1 subset (Figure 1A-B). Consequently, a marked decrease in a later developmental stage, the DN3 population, was observed (Figure 1A). In addition, BMP4-treated intact lobes consistently yielded reduced numbers of thymocytes as compared with untreated lobes (Figure 1C). These BMP4-induced changes in thymocyte development could be abrogated with the addition of the BMP4 inhibitor Noggin (data not shown). To examine if the increased DN1 population was a result of increased apoptosis in more differentiated populations, cell death in all thymocyte populations were examined, but no differences between BMP4-treated and untreated populations were observed (data not shown). Taken together, these data suggest that BMP4 controls thymopoiesis by inhibiting DN1 differentiation and by inhibiting the DN-to-DP transition.
BMP4 arrest of T-cell development. E15 FTOC was cultured for 7 days in the presence or absence of BMP4 (30 μg/mL). (A) Representative fluorescence-activated cell sorter (FACS) dot plots of CD44/CD25 (upper panels) and CD4/CD8 (lower panels) expression by thymocytes in untreated (left panels) and BMP4-treated (right panels) FTOCs. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. (B) Bars represent the relative change in total DN, DN1, and DN3 subpopulations in a total of 22 experiments. Changes are significant (*) with greater than 99.9% confidence by P values. (C) Bars represent the relative change in total cell number per lobe in these cultures in a total of 14 experiments. The decrease with BMP4 treatment is again significant (*) with greater than 99.9% confidence. Error bars indicate mean ± SD.
BMP4 arrest of T-cell development. E15 FTOC was cultured for 7 days in the presence or absence of BMP4 (30 μg/mL). (A) Representative fluorescence-activated cell sorter (FACS) dot plots of CD44/CD25 (upper panels) and CD4/CD8 (lower panels) expression by thymocytes in untreated (left panels) and BMP4-treated (right panels) FTOCs. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. (B) Bars represent the relative change in total DN, DN1, and DN3 subpopulations in a total of 22 experiments. Changes are significant (*) with greater than 99.9% confidence by P values. (C) Bars represent the relative change in total cell number per lobe in these cultures in a total of 14 experiments. The decrease with BMP4 treatment is again significant (*) with greater than 99.9% confidence. Error bars indicate mean ± SD.
BMP4's inhibitory activities can be mediated through thymic stroma
To understand how BMP4 affects thymocyte development, we examined the compartment-specific expression of BMP4 and its receptors in adult thymocyte subsets and E15 thymic stroma. RT-PCR analysis demonstrates that BMP4 is expressed in the stromal compartment while BMPRI and BMPRII are expressed in all thymocyte subsets in addition to the stromal compartment (Figure 2A). The widespread expression of BMP4 receptors indicates that BMP4 may act through both thymocyte and stromal compartments to block thymocyte differentiation.
Dependence of BMP4 action on stroma and thymocytes. (A) RT-PCR analysis of BMP4 and BMP4 receptor expression in stroma and thymocyte compartments. The cDNAs were prepared from DN1, total DN, and DP thymocytes and dexoyguanosine-treated E15 thymic lobes. (B-C) Representative FACS plots of CD44/CD25 and CD4/CD8 expression by thymocytes from reconstitution experiments. Flow cytometric analysis was performed 2.5 to 3 weeks after FTOC. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. Panel B shows untreated (left panels) and BMP4 (30 μg/mL)–treated (right panels) deoxyguanosine-treated lobes (for 5 days) that were later reconstituted with untreated whole bone marrow. Panel C shows untreated thymocyte-depleted thymic lobes reconstituted with untreated (left panels) and BMP4 (30 μg/mL)–treated (right panels) whole bone marrow (for 8 hours prior to reconstitution) (D) Untreated thymocyte-depleted thymic lobes reconstituted with untreated (upper panels) and BMP4 (30 μg/mL)–treated (lower panels) DN1 thymocytes (treated for 8 hours prior to reconstitution).
Dependence of BMP4 action on stroma and thymocytes. (A) RT-PCR analysis of BMP4 and BMP4 receptor expression in stroma and thymocyte compartments. The cDNAs were prepared from DN1, total DN, and DP thymocytes and dexoyguanosine-treated E15 thymic lobes. (B-C) Representative FACS plots of CD44/CD25 and CD4/CD8 expression by thymocytes from reconstitution experiments. Flow cytometric analysis was performed 2.5 to 3 weeks after FTOC. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. Panel B shows untreated (left panels) and BMP4 (30 μg/mL)–treated (right panels) deoxyguanosine-treated lobes (for 5 days) that were later reconstituted with untreated whole bone marrow. Panel C shows untreated thymocyte-depleted thymic lobes reconstituted with untreated (left panels) and BMP4 (30 μg/mL)–treated (right panels) whole bone marrow (for 8 hours prior to reconstitution) (D) Untreated thymocyte-depleted thymic lobes reconstituted with untreated (upper panels) and BMP4 (30 μg/mL)–treated (lower panels) DN1 thymocytes (treated for 8 hours prior to reconstitution).
To test whether the effect of BMP4 might be mediated through the stromal compartment, we repopulated BMP4-treated, thymocyte-depleted lobes with untreated adult bone marrow. Similarly to intact lobes, DN1 and DN thymocyte populations are increased when compared with untreated controls (Figure 2B; Table 1), with a corresponding decrease in the DN3 subpopulation (Figure 2B). Repopulation of BMP4-treated stroma with DN thymocytes also show elevated DN1 and DN populations (data not shown). Taken together, these data indicate that BMP4's inhibitory activity can be mediated by the stromal compartment.
BMP4's direct effects on thymocytes affect only DN1 development
To examine whether BMP4 might also act directly on thymocyte progenitors, we reconstituted untreated, thymocyte-depleted lobes with BMP4-treated adult bone marrow. These reconstitutions yield an increased DN1 subpopulation (Figure 2C; Table 1). However, reconstitution with BMP4-treated bone marrow does not result in the increased DN population seen in reconstitution with both BMP4-treated stroma and BMP4-treated intact lobes. These data suggest that BMP4's effect on the DN population is mainly a stroma-dependent event.
We further examined BMP4's effects on thymocytes by reconstituting untreated, thymocyte-depleted stroma with BMP4-treated DN1 and DN3 subpopulations. These experiments further confirm that BMP4's thymocyte effects are limited to the DN1 population (Figure 2D; Table 1). In reconstitution with the DN1 subpopulation, BMP4 treatment inhibits differentiation only at this noncommitted stage, while in reconstitution with DN3 cells, no significant changes are seen between the untreated and treated groups (Table 1).
The fact that BMP4 treatment results in an increase in the DN1 population but not in the total DN population suggests that the increases in the total DN population—seen in BMP4-treated intact FTOC and in reconstitution of BMP4-treated stroma—do not result secondarily from an increase in the DN1 subpopulation. Confirming this result, in untreated stroma reconstituted with BMP4-treated total DN population, an increase in the DN1 population is seen without an increase in the total DN population (Table 1). Thus, BMP4 has 2 distinct effects: (1) on the DN1 population, mediated by both the stromal and thymocyte compartments, and (2) at the DN to DP transition, mediated exclusively by the thymic stroma.
FGF7 and FGF10 arrest early T-cell development
FGFs have been demonstrated to have a role in thymus development in vivo. To examine the possible effects of FGFs on thymocyte development, we treated intact E15 FTOC with either FGF7 or FGF10. Surprisingly, the effects of either FGF greatly resemble the effects seen with BMP4 treatment, with significant increases in both DN and DN1 cell populations (Figure 3A-B). Also similarly to BMP4 treatment, FGFs decrease the percentage of the DN3 subpopulation (Figure 3A-B) as well as the total number of cells recovered per lobe (Figure 3C).
FGF7 and FGF10 arrest of T-cell development. E15 FTOC was cultured for 7 days in the presence or absence of FGF7 or FGF10. (A) Representative FACS dot plots of CD44/CD25 (upper panels) and CD4/CD8 (lower panels) expression by thymocytes from untreated (left panels) and FGF7 (166 ng/mL)–treated (right panels) FTOC. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. (B) Bars represent the relative change of total DN, DN1, and DN3 subpopulations in these cultures in a total of 6 experiments. Increases are significant (*) with P values denoting greater than 99.9% confidence. (C) Bars represent the relative change in total cell number per lobe in these cultures in a total of 4 experiments with each factor. Decreases with FGF7 and FGF10 treatment are significant (*) with greater than 99.9% confidence. Error bars indicate mean ± SD.
FGF7 and FGF10 arrest of T-cell development. E15 FTOC was cultured for 7 days in the presence or absence of FGF7 or FGF10. (A) Representative FACS dot plots of CD44/CD25 (upper panels) and CD4/CD8 (lower panels) expression by thymocytes from untreated (left panels) and FGF7 (166 ng/mL)–treated (right panels) FTOC. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. (B) Bars represent the relative change of total DN, DN1, and DN3 subpopulations in these cultures in a total of 6 experiments. Increases are significant (*) with P values denoting greater than 99.9% confidence. (C) Bars represent the relative change in total cell number per lobe in these cultures in a total of 4 experiments with each factor. Decreases with FGF7 and FGF10 treatment are significant (*) with greater than 99.9% confidence. Error bars indicate mean ± SD.
To confirm that these observations are FGF signaling dependent, we blocked FGFR2IIIb function with FGFR2IIIb/Fc, which contains the extracellular domain of FGFR2IIIb fused to the carboxy-terminal Fc region of human immunoglobulin G (IgG).29,30 Addition of FGFR2IIIb/Fc almost completely reverses the effects seen with FGF treatment (data not shown). Taken together, these data demonstrate that FGF7/FGF10 and BMP4 have similar effects on thymocyte development.
Blocking FGF suppresses BMP4's inhibitory activity
The similar effects of FGF7/FGF10 and BMP4 treatment on thymocyte differentiation indicate that these molecules could act either in parallel or upstream/downstream of one another. To distinguish between these 2 possibilities, intact E15 lobes were treated in FTOC with BMP4 alone or in combination with the FGFR2IIIb-Fc inhibitor. BMP4 treatment increases the DN population as well as the DN1 subpopulation, with relative decreases in total cell numbers (Figure 4). Addition of FGFR2IIIb/Fc reverses BMP4's effects, restoring the percentage of the DN population to control levels (Figure 4A-B). Furthermore, treatment with FGFR2IIIb/Fc significantly rescues the total cell numbers generated per lobe, though not completely to control levels (Figure 4C). Interestingly, blocking FGF signaling has less of an effect on the DN1 subset (Figure 4A-B). These data suggest that BMP4's effects on the DN1 subset are partially FGF independent while its effects on the DN to DP transition are FGF dependent. Taken together, the data demonstrate that BMP4 is probably acting upstream of FGF signaling in the regulation of thymocyte development.
Suppression of BMP4 activity by FGF inhibitor. E15 FTOC was cultured for 7 days in the presence of BMP4 alone or BMP4 plus FGFR2IIIb/Fc inhibitor. (A) Representative FACS dot plots of CD44/CD25 (upper panels) and CD4/CD8 (lower panels) expression by thymocytes from untreated (left panels), BMP4-treated (middle panels), and BMP4 plus FGFR2IIIb/Fc inhibitor–treated (right panels) FTOC. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. (B) Bars represent the relative change in total DN, DN1, and DN3 subpopulations in these cultures. Restoration of DN population with FGFR2IIIb/Fc addition is significant in a total of 3 experiments; *P < .0115. (C) Bars represent the relative change in total cell number per lobe in these cultures. Restoration of cell numbers with FGFR2IIIb/Fc addition is significant (*), with greater than 99.9% confidence, from a total of 3 experiments. Error bars indicate mean ± SD.
Suppression of BMP4 activity by FGF inhibitor. E15 FTOC was cultured for 7 days in the presence of BMP4 alone or BMP4 plus FGFR2IIIb/Fc inhibitor. (A) Representative FACS dot plots of CD44/CD25 (upper panels) and CD4/CD8 (lower panels) expression by thymocytes from untreated (left panels), BMP4-treated (middle panels), and BMP4 plus FGFR2IIIb/Fc inhibitor–treated (right panels) FTOC. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. (B) Bars represent the relative change in total DN, DN1, and DN3 subpopulations in these cultures. Restoration of DN population with FGFR2IIIb/Fc addition is significant in a total of 3 experiments; *P < .0115. (C) Bars represent the relative change in total cell number per lobe in these cultures. Restoration of cell numbers with FGFR2IIIb/Fc addition is significant (*), with greater than 99.9% confidence, from a total of 3 experiments. Error bars indicate mean ± SD.
BMP4 inhibitors fail to suppress FGF7 activity
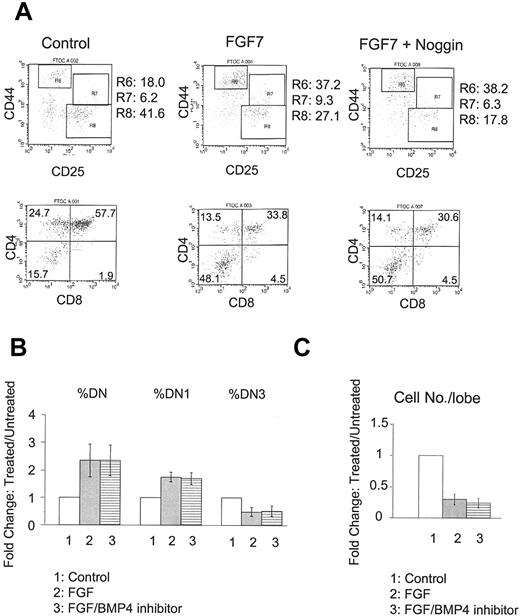
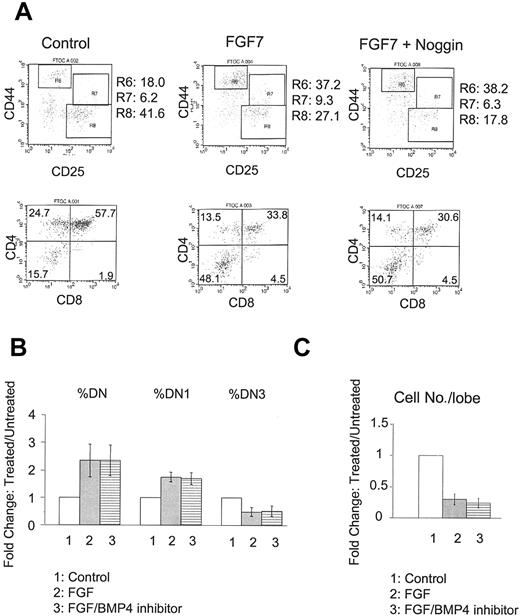
To further confirm that BMP4 is acting upstream of FGF, we performed the reciprocal experiments using the BMP4 inhibitors Noggin or anti-BMP4 neutralizing antibody on FGF7-treated FTOC. As shown before, FGF7 treatment increases both DN and DN1 subpopulations, accompanied by decreased total cell numbers (Figure 5). Addition of BMP4 inhibitors does not change FGF's inhibitory effects appreciably (Figure 5), suggesting that BMP4 indeed acts upstream of FGF and that its inhibition of thymocyte differentiation is mediated largely by the FGF-signaling pathway.
Failure of BMP4 antagonists to suppress activity of FGF7. E15 FTOC was cultured for 7 days with FGF7 in the presence or absence of BMP4 inhibitor. (A) Representative FACS dot plots of CD44/CD25 (upper panels) and CD4/CD8 (lower panels) expression by thymocytes from untreated (left), FGF7 only–treated (middle), and FGF7 plus Noggin–treated (right) FTOC. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. (B) Bars represent relative changes in total DN, DN1, and DN3 subpopulations in control, FGF7-treated, and FGF7 plus BMP4 inhibitor–treated versus untreated cultures in a total of 4 experiments. Differences with BMP4 inhibitor addition were not significant. (C) Bars represent relative changes in total cell number per lobe in these cultures in a total of 4 experiments. Differences with BMP4 inhibitor addition were not significant. Error bars indicate mean ± SD.
Failure of BMP4 antagonists to suppress activity of FGF7. E15 FTOC was cultured for 7 days with FGF7 in the presence or absence of BMP4 inhibitor. (A) Representative FACS dot plots of CD44/CD25 (upper panels) and CD4/CD8 (lower panels) expression by thymocytes from untreated (left), FGF7 only–treated (middle), and FGF7 plus Noggin–treated (right) FTOC. R6, R7, and R8 denote DN1, DN2, and DN3 subpopulations, respectively. (B) Bars represent relative changes in total DN, DN1, and DN3 subpopulations in control, FGF7-treated, and FGF7 plus BMP4 inhibitor–treated versus untreated cultures in a total of 4 experiments. Differences with BMP4 inhibitor addition were not significant. (C) Bars represent relative changes in total cell number per lobe in these cultures in a total of 4 experiments. Differences with BMP4 inhibitor addition were not significant. Error bars indicate mean ± SD.
BMP4 up-regulates FGFR2IIIb in the thymic stroma
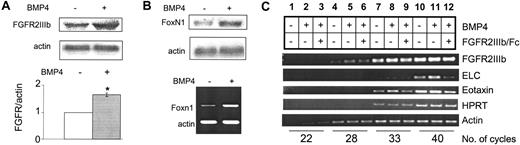
To better understand the mechanism by which the BMP4- and FGF7/10–signaling pathways interact, we examined the effects of BMP4 on the FGF7/10 receptor. Since the effects of BMP4 are significantly mediated by the thymic stroma and because FGFR2IIIb is expressed exclusively in the epithelial compartment of the thymic stroma,20 we hypothesized that BMP4 may modulate FGFR2IIIb function in a thymocyte-independent and stroma-dependent manner. We first examined the levels of FGFR2IIIb in response to BMP4 treatment using a 427 thymic stromal cell line.28 BMP4 treatment increases levels of FGFR2IIIb by 1.63-fold (Figure 6A, upper panel). We then examined FGFR2IIIb levels in thymocyte-depleted lobes. BMP4 treatment also significantly up-regulates the expression of FGFR2IIIb (Figure 6A, lower panel, and 6C), on the basis of Taqman real-time PCR analysis. These data demonstrate that BMP4 up-regulates levels of FGFR2IIIb through direct action on the thymic stroma.
BMP4 up-regulation of FGFR2IIIb and other TEC-specific factors. (A) Northern blot analysis in 427 cell lines (upper panel) and Taqman real-time RT-PCR analysis of dexoyguanosine-treated FTOC (lower panel) for FGFR2IIIb expression with BMP4 treatment. Increase in FGFR2IIIb levels in 427 cell line is approximately 1.63-fold with BMP4 treatment. Increase with Taqman analysis is significant (*) with greater than 99.9% confidence. (B) Northern blot analysis of 427 cells (upper panel) and RT-PCR analysis of deoxyguanosine-treated FTOC (lower panel) for Foxn1 expression with BMP4 treatment. Increase in Foxn1 levels in 427 cell lines is approximately 1.7-fold with BMP4 treatment; quantitation was performed with Quantity One analysis software. (C) Semiquantitative RT-PCR analysis of deoxyguanosine-treated FTOC with BMP4 alone or BMP4 plus FGFR2IIIb/Fc inhibitor for FGFR2IIIb, ELC, and eotaxin with hypoxanthine phosphoribosyltransferase (HPRT) and actin as controls. Note: FGFR2IIIb/Fc inhibits BMP4-stimulated ELC and eotaxin expression (lanes 8 versus 9, 11 versus 12). Changes in ELC (lanes 10-12) and eotaxin (lanes 7-9) are quantified as shown. Quantification was performed by means of ImageQuant software analysis. Error bars indicate mean ± SD.
BMP4 up-regulation of FGFR2IIIb and other TEC-specific factors. (A) Northern blot analysis in 427 cell lines (upper panel) and Taqman real-time RT-PCR analysis of dexoyguanosine-treated FTOC (lower panel) for FGFR2IIIb expression with BMP4 treatment. Increase in FGFR2IIIb levels in 427 cell line is approximately 1.63-fold with BMP4 treatment. Increase with Taqman analysis is significant (*) with greater than 99.9% confidence. (B) Northern blot analysis of 427 cells (upper panel) and RT-PCR analysis of deoxyguanosine-treated FTOC (lower panel) for Foxn1 expression with BMP4 treatment. Increase in Foxn1 levels in 427 cell lines is approximately 1.7-fold with BMP4 treatment; quantitation was performed with Quantity One analysis software. (C) Semiquantitative RT-PCR analysis of deoxyguanosine-treated FTOC with BMP4 alone or BMP4 plus FGFR2IIIb/Fc inhibitor for FGFR2IIIb, ELC, and eotaxin with hypoxanthine phosphoribosyltransferase (HPRT) and actin as controls. Note: FGFR2IIIb/Fc inhibits BMP4-stimulated ELC and eotaxin expression (lanes 8 versus 9, 11 versus 12). Changes in ELC (lanes 10-12) and eotaxin (lanes 7-9) are quantified as shown. Quantification was performed by means of ImageQuant software analysis. Error bars indicate mean ± SD.
BMP4 affects Foxn1 and chemokine expression in the thymic stroma
The effects of BMP4 appear to be mediated largely through a stroma-dependent mechanism, indicating that BMP4 is acting directly on the thymic stroma. To further assess this effect, we examined both intracellular and extracellular factors produced by the stroma. Foxn1 (formerly known as winged helix nude)31-33 is a Forkhead family transcription factor expressed specifically in the thymic epithelium and is essential for proper TEC development.31-33 Lack of Foxn1 in mouse, rat, or human leads to an athymic phenotype owing to failure of TEC proliferation and differentiation and a deficiency in T-progenitor cell homing and proliferation/differentiation.34 In the 427 thymic stromal cell line (Figure 6B, upper panel) and purified stroma (Figure 6B, lower panel), BMP4 treatment leads to up-regulation of Foxn1. These data further indicate that BMP4 is acting directly on the thymic stroma.
Stroma-expressed chemokines are essential mediators of stromathymocyte interactions. Expressed by the stroma, these small polypeptides play roles in regulating thymocyte migration during development.35,36 To further assess BMP4's actions on the thymic stroma, we examined the levels of the chemokines eotaxin and ELC in BMP4-treated thymocyte-depleted lobes. BMP4 affects both chemokines in an FGF signaling–dependent manner (Figure 6C; Table 2). These data further indicate that BMP4 can act directly on the thymic stroma, thereby affecting thymocyte development.
Discussion
In this study, we demonstrate that BMP4 controls thymocyte development at 2 distinct stages. BMP4 blocks the DN1 to DN2 progression and the DN to DP transition. We also demonstrate that BMP4 treatment leads to a decrease in the total cell numbers generated per thymic lobe, an effect that results primarily from BMP4's effects at the DN to DP transition. Two recent publications have also implicated BMP4 in regulating thymocyte development at the DN1 stage and at the DN to DP transition, but neither shows a decrease in total cell number.9,15 This difference may result from the differences in the duration of FTOC. Whereas the published reports examined their FTOC after only 5 days, the FTOC in this study was analyzed after 7 days.
Our study provides the first evidence that BMP4 influences T-cell development in primarily a thymic stroma-dependent manner. Since BMP receptors are expressed in both thymocyte and stromal compartments, we separated these compartments and treated each independently with BMP4. Using FTOC reconstitution assays, we demonstrate that while both compartments can mediate the inhibitory effects of BMP4 on the DN1 subset, only the thymic stroma mediates BMP4's effects on the DN to DP transition.
We also demonstrate that FGF7 and FGF10 have very similar effects on thymocyte development when compared with BMP4. FGF7 and FGF10 regulate the development of the DN1 subpopulation and the DN to DP transition and decrease the total cell numbers generated per lobe. These data further extend the previous conclusion that FGF7 regulates the transition from DN to DP stages, although FGF10 was not observed to exert such an effect.26 The subtle differences seen with FGF7 and FGF10 treatment may be attributable to protein activities and concentrations, in addition to possible affinity differences in ligand-receptor binding in the thymocyte populations.
Most importantly, however, this study provides the first evidence that BMP4 acts upstream of FGF7/FGF10 to control T-lymphoid development. Blocking FGF signaling results in a rescue of BMP4 effects on the total DN population and a partial rescue of the DN1 population, while inhibition of BMP4 could not rescue the effects of FGF7 on any of these parameters. Interestingly, blocking FGF signaling appears to rescue those BMP4 effects that are mediated exclusively by the thymic stroma, while having less of an effect on those that are both thymocyte and stroma dependent, consistent with a role for FGF signaling in mediating BMP4's stroma-dependent effects on thymopoiesis.
Of note, the interactions between BMP and FGF family members in controlling development are not limited to the control of thymopoiesis described in this study. BMP signaling has been implicated in the regulation of FGF expression during limb development.37,38 Also, BMP4 has been demonstrated to up-regulate FGFR2 expression in avian feather bud formation.39,40 Similarly, we demonstrate that BMP4 up-regulates FGFR2IIIb, which may increase the sensitivity of TECs to FGF7 and FGF10 stimulation.
BMP4 also controls the expression of the stroma-specific transcription factor Foxn1, further supporting that BMP4 acts directly on the thymic stroma to control thymopoiesis. BMP4 also appears to up-regulate Foxn1 expression in the murine hair follicle.41 In Msx2-Noggin transgenic mice, Foxn1 expression is severely reduced in the transgenic hair follicles.42 Furthermore, comparison of thymic phenotypes in Foxn1 and FGF mutants suggests that Foxn1 may fall upstream of the FGF-signaling pathway, as the thymus phenotype in nude mice is more severe than that seen in FGF10 and FGFR2IIIb null mice.20 In addition, FGF7 treatment of nude mice normalizes the follicular keratinization defect normally seen in these mice.43 Since Foxn1 and FGFR2IIIb are expressed exclusively in TECs and since Foxn1 is transcriptionally regulated by BMP4 independently of FGF signaling, Foxn1 may be a mediator of BMP4's regulation of FGFR2IIIb. Indeed, our unpublished results suggest that overexpression of Foxn1 leads to up-regulated FGFR2 expression (P.T.T., unpublished results, May 2003).
BMP4 also up-regulates the stromal chemokines eotaxin and ELC in an FGF signaling–dependent manner. Since these chemokines are also regulated by FGF7,26 these data further support that BMP4 acts upstream of FGF signaling. Furthermore, modulation of these chemokines may contribute to BMP4's effects on thymocyte development, since alterations in chemokine expression can disrupt thymocyte trafficking, thereby preventing thymocytes from receiving the correct signals for survival, proliferation, or differentiation. In vivo examination of the function of these chemokines in BMP4-regulated thymopoiesis would be an interesting avenue for future study.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-05-1657.
Supported in part by the Medical Scientist Training Program training grant (P.T.T. and R.A.L.). H.W. is a V Foundation scholar and an Assistant Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ken Dorshkind for technical assistance and for helpful comments on this manuscript; Drs Yoshiko Hashimoto and Encarnacion Montecino-Rodriguez for technical assistance; and Dr Barbara Knowles for her gift of the 427 thymic stromal cell line.