Abstract
CD30 is a promising target for antibody-based immunotherapy of Hodgkin lymphoma (HL) and anaplastic large cell lymphoma. To overcome the limitations from currently available murine anti-CD30 monoclonal antibodies (mAbs), a new fully human anti-CD30 antibody was generated. Binding properties were evaluated by recombinant CD30 capture enzyme-linked immunosorbent assay (ELISA) and fluorescence-activated cell-sorter (FACS) flow cytometry. Activity of this new mAb was assessed in vitro using growth inhibition and antibody-dependent cellular cytotoxicity (ADCC) assays on several cell lines. In vivo activity was determined in a solid as well as in a disseminated xenografted model of HL in severe combined immunodeficiency (SCID) mice. The mAb 5F11 showed specific binding to CD30 (cluster A). The ADCC assays indicated dose-dependent lysis of L540 cells when 5F11 was combined with human effector cells. Upon cross-linking in vitro, 5F11 inhibited the growth of CD30-expressing cell lines. In vivo, treatment with 5F11 induced a marked growth delay or even a complete regression of established xenografted HL in SCID mice. In the disseminated HL model, a high proportion of 5F11-treated mice experienced long-term survival. The new human anti-CD30 monoclonal antibody 5F11 shows promise as a means of CD30-targeted immunotherapy of malignant lymphomas. Based on these results, a clinical phase 1 study in patients with refractory CD30+ lymphoma has been initiated. (Blood. 2003;102:3737-3742)
Introduction
Hodgkin lymphoma (HL) has become a curable disease after the introduction of improved radiation techniques and polychemotherapy regimens such as ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone).1-4 Although most patients can be cured by standard approaches even in advanced stages, fewer than 30% of those who relapse attain durable disease-free remissions after second-line treatment.5 The outcome is worse for those with primary refractory disease.6 Data from HL and from non-Hodgkin lymphoma (NHL) studies suggest that small numbers of residual tumor cells remaining after first-line treatment can give rise to late relapses.7,8 Thus, eliminating residual malignant lymphoma cells after first-line treatment might further improve outcome in these diseases.
Among the different target antigens on Hodgkin-Reed/Sternberg (H-RS), CD30 seems to be most promising since it is specifically expressed at very high levels.9-12 CD30 is a 120-kDa transmembrane glycoprotein belonging to the tumor necrosis factor (TNF)-receptor superfamily. Physiologically, expression of CD30 is found on virus-infected lymphocytes and on a small subset of activated T cells. Furthermore this receptor is involved in the negative selection process of autoreactive lymphocytes.13 The CD30 ligand (CD30L) is present on activated T cells, resting B cells, and granulocytes. The biologic function of CD30-CD30L interaction is not yet fully understood, but both stimulation of cell growth as well as induction of apoptosis have been described.14 Similar to other members of the TNF family, CD30 seems to be involved in the cell cycle regulation.15,16 CD30 is expressed on a variety of other neoplasms such as mediastinal B-cell lymphoma, anaplastic large cell lymphoma (ALCL), peripheral T-cell lymphoma, and embryonal carcinoma.17-20 Based on the rationale of potential clinical use, a vast number of murine anti-CD30 monoclonal antibodies (mAbs) have been developed. Some of these antibodies have demonstrated in vitro and in vivo activity on Hodgkin-derived tumors; however, mAbs containing murine components can generate a human antimouse antibody (HAMA) response when administered to patients, thus limiting their utility.11,21,22 Apart from these diverse interactions between CD30 and anti-CD30 mAbs, targeting this receptor with human antibodies might recruit effector mechanisms including complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) resulting in tumor rejection. These immune mechanisms have proven effective in the antibody-based treatment of indolent and aggressive B-cell NHL.23-25 Therefore, we generated several human anti-CD30 antibodies using the human monoclonal antibody (HuMAb) mouse, of which the clone 5F11 (MDX-060) (both from Medarex, Bloomsbury, NJ) was chosen for further development. Here we report on the characteristics of this first fully human anti-CD30 antibody.
Materials and methods
Antibody generation
The human CD30 hybridoma, 5F11, was derived by fusing murine P3X63Ag8-653 myeloma cells (ATCC CRL 1580; American Type Culture Collection, Manassas, VA) with the spleen cells from a HuMAb mouse that had been immunized twice intraperitoneally with 107 L540 cells in phosphate-buffered saline (PBS) and then boosted with 0.1 mg purified recombinant CD30 that was emulsified in incomplete Freund adjuvant. A final immunization was given intravenously with 0.1 mg purified CD30 in PBS. Four days after the final boost, the spleen cells from the immunized mouse were harvested and fused with P3X63Ag8-653 myeloma cells using polyethyleneglycol (PEG) as fusionogen using standard procedures. After fusion, the cells were plated in 96-well plates and hybridomas were selected in hypoxanthine-aminopterin-thymidine (HAT)-containing media. Hybridoma lines that grew out of the selection media were screened for CD30 reactivity by enzyme-linked immunosorbent assay (ELISA) and flow cytometry. Hybridoma 5F11 secretes human immunoglobulin G1 (IgG1) with kappa light chains.
Cells and reagents
Soluble CD30 was generated by transiently (COS cells) or stably (NS0 cells) transfecting cells with a vector that contains cDNA that encodes the extracellular portion of CD30 fused to a histidine tag. The molecule was purified by metal affinity chromatography. Alternatively, a soluble CD30-Fc fusion protein was purchased from R&D Systems (Minneapolis, MN). Cell lines including the HL-derived cell lines L540, L540Cy, L428, and L1236, and the ALCL cell line KARPAS-299 were cultivated in RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS), 50 μg/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine. All cells were cultured at 37°C in a 5% CO2 atmosphere.
Binding studies
ELISA. Microtiter plates were coated with a recombinant CD30-Fc fusion protein (R&D Systems). After blocking the wells with 5% bovine serum albumin (BSA) solution, protein A-purified 5F11 was incubated at varying concentrations at 37°C. After 1 hour the wells were washed with PBS-Tween and the bound antibodies were detected by incubating the cells with an alkaline phosphatase-labeled goat anti-human IgG F(ab)2-specific probe at 37°C. The excess probe was washed from the wells and the plate was developed with pNPP (p-nitrophenyl phosphate). The optical density at 405 to 650 nm was determined using a microtiter plate reader.
Flow cytometry. Protein A-purified 5F11 or an isotype control was incubated at varying concentrations with the Hodgkin tumor cell line L540 at 4°C. After 1 hour the cells were washed with PBS containing 0.1% BSA and 0.05% NaN3, and the bound antibodies were detected by incubating the cells with a fluorescein isothiocyanate (FITC)-labeled goat anti-human IgG Fc-specific probe at 4°C. The excess probe was washed from the cells with PBS and the cell-associated fluorescence was determined by analysis using a FACSCalibur instrument (Becton Dickinson, Franklin Lakes, NJ). Alternatively, direct binding of FITC-labeled 5F11 was assessed by incubation with the CD30+ cell line, L540, or the CD30 line, Daudi, for 1 hour on ice, washed, and then the cell-associated fluorescence was determined by FACSCalibur.
Real-time binding analysis. Real-time binding analysis, using surface plasmon resonance, was performed on a Biacore 2000 system (Biacore, Uppsala, Sweden). The antigen sCD30-Fc (R&D Systems) was immobilized on a CM5 sensor chip (Biacore) by amine coupling using the amine coupling kit (Biacore) according to the manufacturer's instructions. The surface density of sCD30-Fc was 566 response units (RUs) after immobilization. Antibodies 5F11 and Ki4 were injected at flow rates of 30 μL/minute at various concentrations. Flow cells were regenerated between injections using 6 M guanidine-HCl/0.2 M acetic acid. Kinetic analysis of affinity constants was performed using the software BIAevaluation 3.1 (Biacore). The values of the negative control, an empty flow cell, were subtracted before analysis. Curves were fitted applying the predefined model for 1:1 Langmuir binding.
Antibody-dependent cellular cytotoxicity
L540 cells were used as targets for lysis by fresh human mononuclear cells or by interferon γ (IFN-γ)-treated human monocytes. Mononuclear cells were purified from heparinized whole blood by ficoll hypaque density centrifugation. Monocytes were purified from normal adult source leukopacs (Biological Specialty Corp, Colmar, PA) were cultured in macrophage serum-free medium (M-SFM; Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) and IFN-γ (1000 u/mL, R&D Systems) for 2 days. Target cells were labeled with 100 μCi (3.7 MBq) of 51Cr for 1 to 2 hours prior to combining with effector cells and human monoclonal antibody (HuMAb) in a U-bottom microtiter plate. After incubation for 16 hours at 37°C supernatants were collected and analyzed for radioactivity. Cytotoxicity was calculated by the following formula where CPM indicates counts per minute: % lysis = (experimental CPM - target leak CPM)/(detergent lysis CPM - target leak CPM) × 100. Specific lysis = % lysis with HuMAb - % lysis without HuMAb. Assays were performed in triplicates.
Epitope mapping
FITC-labeled 5F11 (0.25 μg/mL) was incubated with L540 cells in the presence of 10- to 20-fold excess of unlabeled antibody for 1 hour on ice. Blocking antibodies included the CD30 cluster A-specific antibodies, Ki-4 and BerH2, the CD30 cluster B-specific antibody Ki-1, or the CD30 cluster C-specific antibody, AC10. The excess probe was washed from the cells with PBS and the cell-associated fluorescence was determined by analysis using a FACScalibur instrument.
In vitro growth inhibition
Metabolism of the yellow tetrazolium salt (XTT) was determined according to the manufacturers instructions (Roche Molecular Biochemicals, Mannheim, Germany). Briefly, various dilutions of the mAb 5F11 with or without 10-fold excess of GAH-IgG (goat anti-human immunoglobulin G) were distributed in 100-μL aliquots in 96-well plates. Target cells (2-4 × 104; L540, L1236, Karpas 299) in 100-μL aliquots of complete medium were added and the plates incubated for 48 hours at 37°C in a 5% CO2 atmosphere. Then, cell cultures were pulsed with 100 μL fresh culture medium supplemented with XTT and N-methyl dibenzopyrazine methyl sulfate (final concentrations of 1.49 mM and 0.025 mM, respectively) for 4 hours. The spectrophotometric absorbance of the samples were measured at 450 nm and 650 nm (reference wave length) with an ELISA reader (MWG Biotech, Ebersberg, Germany). Negative controls were done using the GAH-IgG mAbs only together with the above-mentioned target cells and using the combination of the 5F11 and the cross-linking antibody GAH-IgG on a CD30- cell line (BL38). Cell viability relative to untreated control cultures was calculated using the following formula: (test value/untreated value) × 100. All measurements were done in triplicates and repeated twice.
Xenograft models
Pathogen-free female C.B-17/Icr severe combined immunodeficiency (SCID) mice (FOX CHASE SCID; Taconic M&B A/S, Ry, Denmark) were maintained under pathogen-free conditions and fed autoclaved standard chow and water. To evaluate the effects of the mAb 5F11 treatment on the survival of SCID mice challenged with human HL L540Cy cells, a subcutaneous and a disseminated model was used. For the disseminated model, 1 × 107 exponentially growing L540Cy cells were injected via the tail vein of the 3- to 4-week-old SCID mice. One day after injection of L540Cy cells, mice received 100 μg 5F11 intraperitoneally diluted in 200 μL PBS every 4 days for a total of 4 injections. Control groups included PBS only, 5F11 + 10-fold excess GAH-IgG mAbs, and GAH-IgG mAbs alone using the same treatment schedule. Mice with signs of progressive disease (ruffled fur, inactivity, skull deformation) were killed. After gross examination, organs that were macroscopically infiltrated with L540Cy cells were fixed in formalin. Animals surviving up to 200 days were killed at that time.
Subcutaneous solid L540Cy tumors were established by injection of L540Cy cells (1 × 107) resuspended in 200 μL PBS into the right flank. Tumor development was measured every 3 days and tumor volume determined using the formula (length × width × height)/2. Animals with established tumors of 4 to 6 mm in the largest diameter were divided randomly into the different groups and received 100 μg 5F11 (in 200 μL PBS intraperitoneally) every 4 days for a total of 4 injections. Control mice received PBS only. The experiment was stopped and the mice killed when the median tumor diameter in the control group exceeded 20 mm (day 107). The treatment and control group consisted of 5 animals each and results were confirmed by a second set of experiments.
Statistical methods
Survival analyses were performed by the Kaplan-Meier method, and statistical significance was compared by the log-rank test.
Results
Binding to recombinant CD30 by ELISA and flow cytometry
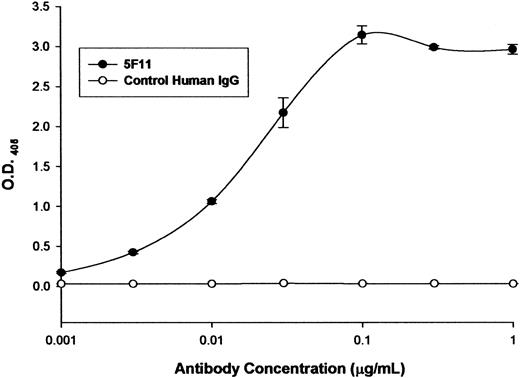
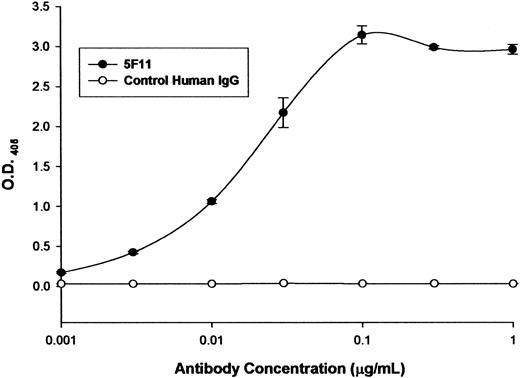
The ability of the selected HuMAb to bind to recombinant CD30 was investigated by ELISA (Figure 1). The 5F11 showed dose-dependent binding in the ELISA, indicating that it recognized the recombinant CD30. A control human antibody showed no reactivity with CD30. The ability of 5F11 to bind to CD30 on Hodgkin tumor cells was investigated by flow cytometry (Figure 2). L540 is a Hodgkin lymphoma cell line that overexpresses CD30. The 5F11 demonstrated binding to L540 cells with saturation at concentrations below 1 μg/mL (Figure 2A). The histogram shown in Figure 2B showed that 5F11 bound to all of the L540 cells and could bind in the presence of 33% human plasma (black line). No binding of 5F11 was seen on the CD30 line Daudi (data not shown). These data demonstrate that this antibody binds efficiently to native CD30 expressed on live tumor cells. In addition, 5F11 can bind in the presence of human plasma, an important observation when considering treating patients bearing CD30+ lymphomas.
Binding to recombinant CD30 by ELISA. Microtiter plates were coated with a recombinant CD30-Fc fusion protein (R&D Systems). After blocking the wells with 5% BSA solution, protein A-purified 5F11 was incubated at varying concentrations at 37°C. After 1 hour the wells were washed with PBS-tween and the bound antibodies were detected by incubating the cells with an alkaline phosphatase-labeled goat anti-human IgG F(ab)2-specific probe at 37°C. The excess probe was washed from the wells and the plate was developed with pNPP. The optical density (O.D.) at 405 to 650 nm was determined using a microtiter plate reader. All measurements were done in triplicate and error bars represent standard deviations.
Binding to recombinant CD30 by ELISA. Microtiter plates were coated with a recombinant CD30-Fc fusion protein (R&D Systems). After blocking the wells with 5% BSA solution, protein A-purified 5F11 was incubated at varying concentrations at 37°C. After 1 hour the wells were washed with PBS-tween and the bound antibodies were detected by incubating the cells with an alkaline phosphatase-labeled goat anti-human IgG F(ab)2-specific probe at 37°C. The excess probe was washed from the wells and the plate was developed with pNPP. The optical density (O.D.) at 405 to 650 nm was determined using a microtiter plate reader. All measurements were done in triplicate and error bars represent standard deviations.
Binding of 5F11 TO CD30-expressing L540 cells. (A) Protein A-purified 5F11 or an isotype control were incubated at varying concentrations with the Hodgkin tumor cell line L540 at 4°C and detected by incubating the cells with an FITC-labeled goat anti-human IgG Fc-specific probe. The excess probe was washed from the cells with PBS and the cell-associated fluorescence was determined by analysis using a FACScalibur instrument. All measurements were done in duplicate and error bars represent standard deviations. (B) FITC-labeled 5F11 or an FITC-labeled control human IgG (shaded) were incubated with L540 cells for 1 hour on ice in the presence (black line) or absence (gray line) of 33% human plasma. Cells were washed and analyzed by FACSCalibur.
Binding of 5F11 TO CD30-expressing L540 cells. (A) Protein A-purified 5F11 or an isotype control were incubated at varying concentrations with the Hodgkin tumor cell line L540 at 4°C and detected by incubating the cells with an FITC-labeled goat anti-human IgG Fc-specific probe. The excess probe was washed from the cells with PBS and the cell-associated fluorescence was determined by analysis using a FACScalibur instrument. All measurements were done in duplicate and error bars represent standard deviations. (B) FITC-labeled 5F11 or an FITC-labeled control human IgG (shaded) were incubated with L540 cells for 1 hour on ice in the presence (black line) or absence (gray line) of 33% human plasma. Cells were washed and analyzed by FACSCalibur.
Kinetic affinity measurements
The 5F11 and Ki4 bound specifically to immobilized sCD30. Specificity was confirmed since the antibodies did not bind to a flow cell that did not contain immobilized antigen. At flow rates of 30 μL/minute there was no indication for mass transfer limitation. A global fit of 8 binding curves from injections between 0.5 μg/mL and 100 μg/mL resulted in a calculated dissociation constant for 5F11 of kd = 6.1 × 10-10 M. The on-rate ka was 5.1 × 105 M and the off-rate kd was 3.1 × 10-4 M. Binding curves of 5F11 and Ki-4 were almost identical (data not shown).
Epitope mapping
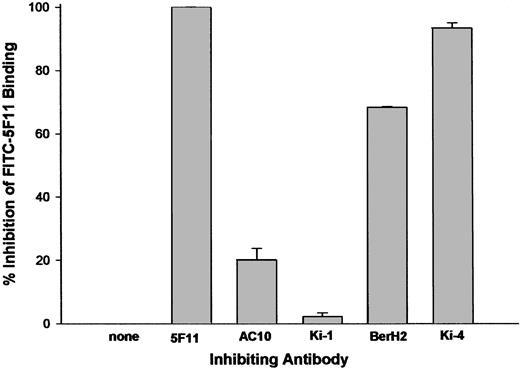
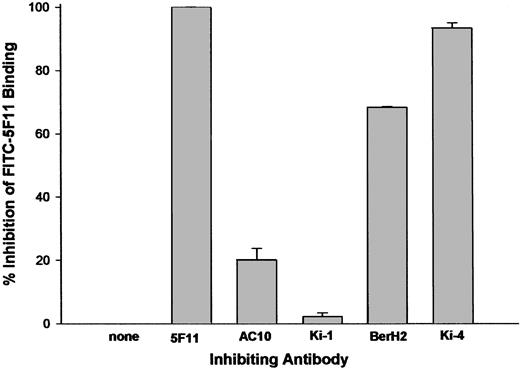
CD30 has been shown to contain at least 3 serologically defined clusters: A, B, and C. We examined the abilities of unlabeled antibodies to either cluster A (Ki-4 and BerH2), cluster B (Ki-1), or cluster C (AC10) to inhibit the binding of FITC-labeled 5F11. The data in Figure 3 reveal that antibodies to cluster A were able to inhibit FITC-5F11 binding to L540 cells, whereas antibodies to clusters B or C could not, implicating that 5F11 binds to or near the cluster A epitope. No inhibitory interaction could be observed between the mAb 5F11 and the cluster A mAbs Ki-2 and HRS-3, the cluster B mAb Ki-1, and the cluster C mAb Ki-3 (data not shown).
Competition FACS flow cytometry. FITC-labeled 5F11 (0.25 μg/mL) was incubated with L540 cells in the presence of 10- to 20-fold excess of unlabeled antibody for 1 hour on ice. The excess probe was washed from the cells with PBS and the cell-associated fluorescence was determined by analysis using a FACScalibur instrument. All measurements were done in duplicate and error bars represent standard deviations.
Competition FACS flow cytometry. FITC-labeled 5F11 (0.25 μg/mL) was incubated with L540 cells in the presence of 10- to 20-fold excess of unlabeled antibody for 1 hour on ice. The excess probe was washed from the cells with PBS and the cell-associated fluorescence was determined by analysis using a FACScalibur instrument. All measurements were done in duplicate and error bars represent standard deviations.
In vitro activity
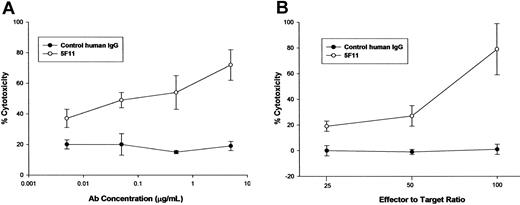
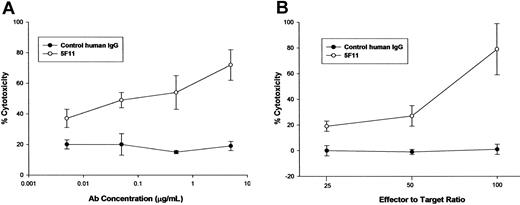
ADCC. The capacity of the 5F11 to mediate lysis of CD30-expressing tumor cells was investigated using a 51Cr-release assay. Healthy human monocytes were activated in culture with IFN-γ to up-regulate Fc receptors and cytolytic activity. The 5F11 mediated dose-dependent lysis of the Hodgkin tumor-derived L540 cells, whereas a control human IgG had minimal effect (Figure 4A). In addition, freshly isolated mononuclear cells were isolated and used as effector cells for ADCC. The data depicted in Figure 4B indicate the potent ability of 5F11 to mediate ADCC at 3 different effector-to-target ratios. These results demonstrate that this HuMAb is capable of targeting CD30-expressing tumor cells to nonactivated effector cells, most likely natural killer cells (NK), for Fc receptor-mediated lysis.
The 5F11 mediates potent ADCC OF CD30+ tumor cells. (A) L540 cells were used as targets for lysis by IFN-γ-treated human monocytes. Monocytes were purified from normal adult source leukopacs (Biological Specialty Corp) and cultured in macrophage serum-free medium (M-SFM; Gibco) supplemented with 10% FBS and IFN-γ (1000 u/mL; R&D Systems) for 2 days. Target cells were labeled with 100 μCi (3.7 MBq) of 51Cr for 1 to 2 hours prior to combining with effector cells and HuMAb and then incubated for 16 hours at 37°C. Cytotoxicity was calculated by the formula: % lysis = (experimental CPM - target leak CPM)/(detergent lysis CPM - target leak CPM) × 100. Specific lysis = % lysis with HuMAb - % lysis without HuMAb. All measurements were done in triplicate and error bars represent standard deviations. (B) L540 cells were used as targets for lysis by fresh human mononuclear cells purified from heparinized whole blood by ficoll hypaque density centrifugation. Target cells were labeled with 100 μCi (3.7 MBq) of 51Cr for 1 to 2 hours prior to combining with effector cells and HuMAb. After incubation for 16 hours at 37°C supernatants were collected and analyzed for radioactivity. Cytotoxicity was calculated as in panel A. All measurements were done in triplicate and error bars represent standard deviations.
The 5F11 mediates potent ADCC OF CD30+ tumor cells. (A) L540 cells were used as targets for lysis by IFN-γ-treated human monocytes. Monocytes were purified from normal adult source leukopacs (Biological Specialty Corp) and cultured in macrophage serum-free medium (M-SFM; Gibco) supplemented with 10% FBS and IFN-γ (1000 u/mL; R&D Systems) for 2 days. Target cells were labeled with 100 μCi (3.7 MBq) of 51Cr for 1 to 2 hours prior to combining with effector cells and HuMAb and then incubated for 16 hours at 37°C. Cytotoxicity was calculated by the formula: % lysis = (experimental CPM - target leak CPM)/(detergent lysis CPM - target leak CPM) × 100. Specific lysis = % lysis with HuMAb - % lysis without HuMAb. All measurements were done in triplicate and error bars represent standard deviations. (B) L540 cells were used as targets for lysis by fresh human mononuclear cells purified from heparinized whole blood by ficoll hypaque density centrifugation. Target cells were labeled with 100 μCi (3.7 MBq) of 51Cr for 1 to 2 hours prior to combining with effector cells and HuMAb. After incubation for 16 hours at 37°C supernatants were collected and analyzed for radioactivity. Cytotoxicity was calculated as in panel A. All measurements were done in triplicate and error bars represent standard deviations.
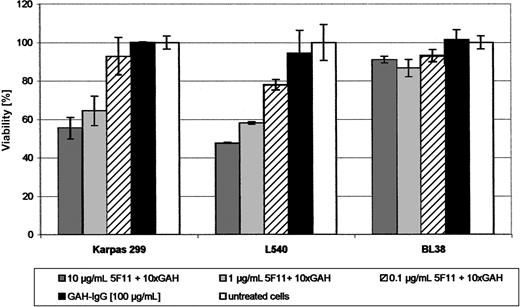
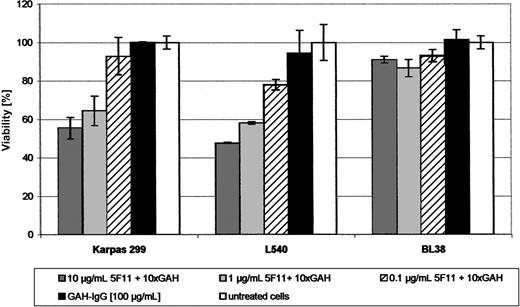
Growth inhibition. The ability of 5F11 to inhibit growth of tumor cells in vitro was demonstrated by a cell viability assay using XTT. A clear and dose-dependent effect on cell metabolism could be shown after cross-linking the antibody for the HL cell line L540 and the ALCL cell line KARPAS-299 (Figure 5). Neither the GAH-IgG mAb nor the 5F11 antibody alone showed any effect. No activity could be observed for the L428 and L1236 cells as well as for the control cell line BL38.
Activity of the mAb 5F11 in vitro (XTT-ASSAY). Indicated dilutions of the mAb 5F11 with 10-fold excess of GAH-IgG were distributed in 100-μL aliquots in 96-well plates. Target cells (2-4 × 104; L540, L1236, Karpas 299) in 100-μL aliquots of complete medium were added and the plates incubated for 48 hours at 37°C in a 5% CO2 atmosphere. Negative controls were done using the GAH-IgG mAb only together with the above-mentioned target cells and using the combination of the 5F11 and GAH-IgG on a CD30- cell line (BL38). Cell viability relative to untreated control cultures was calculated using the following formula: (test value/untreated value) × 100. All measurements were done in triplicate and error bars represent standard deviations.
Activity of the mAb 5F11 in vitro (XTT-ASSAY). Indicated dilutions of the mAb 5F11 with 10-fold excess of GAH-IgG were distributed in 100-μL aliquots in 96-well plates. Target cells (2-4 × 104; L540, L1236, Karpas 299) in 100-μL aliquots of complete medium were added and the plates incubated for 48 hours at 37°C in a 5% CO2 atmosphere. Negative controls were done using the GAH-IgG mAb only together with the above-mentioned target cells and using the combination of the 5F11 and GAH-IgG on a CD30- cell line (BL38). Cell viability relative to untreated control cultures was calculated using the following formula: (test value/untreated value) × 100. All measurements were done in triplicate and error bars represent standard deviations.
In vivo activity
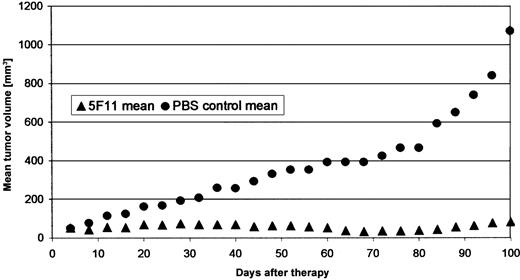
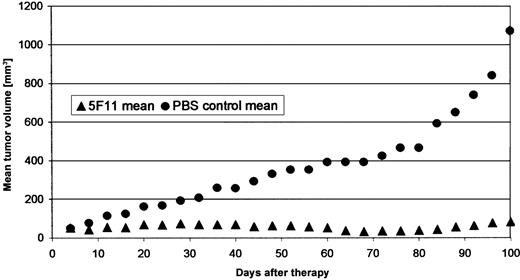
Localized tumor model. Subcutaneous CD30-expressing human tumors were established in the flanks of animals by injecting L540Cy cells in PBS. All animals in the control groups developed tumors larger than 20 mm in diameter with central necrosis within 100 days. Treatment with the 5F11 mAb resulted in substantial tumor regression with complete disappearance in 2 of 5 animals at day 70 and growth inhibition in the remaining mice (Figure 6). The mice were killed on day 100 and no macroscopic signs of disease were found in 2 animals, suggesting a curative potential of the 5F11 antibody in this model.
Effect of 5F11 treatment on subcutaneous L540CY tumors in SCID mice. Subcutaneous solid L540Cy tumors were established by injection of L540Cy cells (1 × 107) resuspended in 200 μL PBS into the right flank. Tumor development was measured every 3 days and tumor volume determined using the formula (length × width × height)/2. Animals with established tumors of 4 to 6 mm in the largest diameter were divided randomly into the different groups and received 100 μg 5F11 (in 200 μL PBS intraperitoneally) every 4 days for a total of 4 injections. Control mice received PBS only. The experiment was stopped and the mice killed when the median tumor diameter in the control group exceeded 20 mm (day 107). The treatment and control group consisted of 5 animals. Data are calculated as the mean tumor volume of all animals per group.
Effect of 5F11 treatment on subcutaneous L540CY tumors in SCID mice. Subcutaneous solid L540Cy tumors were established by injection of L540Cy cells (1 × 107) resuspended in 200 μL PBS into the right flank. Tumor development was measured every 3 days and tumor volume determined using the formula (length × width × height)/2. Animals with established tumors of 4 to 6 mm in the largest diameter were divided randomly into the different groups and received 100 μg 5F11 (in 200 μL PBS intraperitoneally) every 4 days for a total of 4 injections. Control mice received PBS only. The experiment was stopped and the mice killed when the median tumor diameter in the control group exceeded 20 mm (day 107). The treatment and control group consisted of 5 animals. Data are calculated as the mean tumor volume of all animals per group.
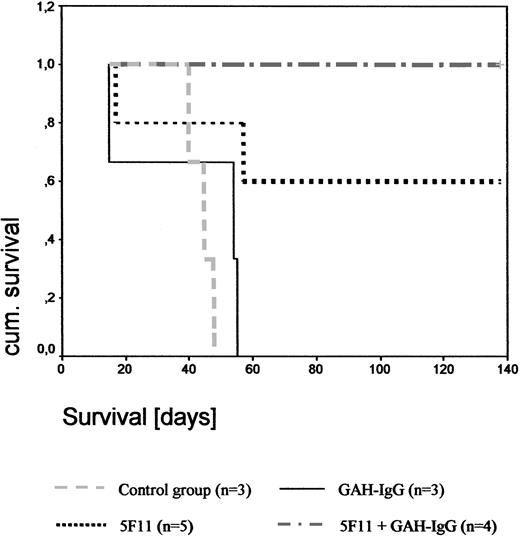
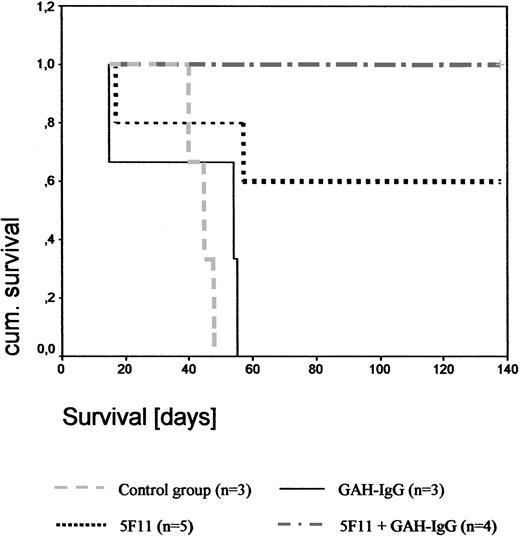
Disseminated tumor model. As documented by Kaplan-Meier analysis (Figure 7), the mean survival time of the PBS-treated control group was 43 days (range, 40-46 days) and in the GAH-IgG-treated group 39 days (range, 15-51 days. In the 5F11 treatment group, 3 of 5 mice were long-term survivors and showed no signs of disease upon autopsy. One animal died on day 17 without developing any macroscopic signs of disease, suggesting that the cause of death was unrelated to the tumor. A second mouse died on day 57 from progressive disease. Upon cross-linking with GAH-IgG mAbs, 4 of 4 mice did not develop any signs of tumor and were killed on day 200. Thus, 5F11 treatment was curative for a high proportion of animals in this xenograft model of HL (P = .01 and P = .046 for the 5F11 + GAH-IgG and 5F11 treatment groups, respectively). Results were confirmed by a second experiment as indicated in Table 1.
Effect of 5F11 treatment on disseminated L540CY tumors in SCID mice. To evaluate the effects of the mAb 5F11 treatment on the survival of SCID mice challenged with human HL cells L540Cy, 1 × 107 exponentially growing L540Cy cells were injected via the tail vein into the 3- to 4-week-old SCID mice. One day after injection of L540Cy cells, mice received 100 μg 5F11 intraperitoneally diluted in 200 μL PBS every 4 days for a total of 4 injections. Control groups included PBS only, 5F11 + 10-fold excess GAH-IgG mAbs, and GAH-IgG mAbs alone using the same treatment schedule. Survival was plotted using the Kaplan-Meier method.
Effect of 5F11 treatment on disseminated L540CY tumors in SCID mice. To evaluate the effects of the mAb 5F11 treatment on the survival of SCID mice challenged with human HL cells L540Cy, 1 × 107 exponentially growing L540Cy cells were injected via the tail vein into the 3- to 4-week-old SCID mice. One day after injection of L540Cy cells, mice received 100 μg 5F11 intraperitoneally diluted in 200 μL PBS every 4 days for a total of 4 injections. Control groups included PBS only, 5F11 + 10-fold excess GAH-IgG mAbs, and GAH-IgG mAbs alone using the same treatment schedule. Survival was plotted using the Kaplan-Meier method.
Discussion
We report here the first fully human anti-CD30 mAb (5F11) with substantial antitumor activity against CD30-expressing human tumor cells. The major characteristics of this first human anti-CD30 antibody are as follows: (1) The mAb 5F11 binds specifically to CD30. Partial cross-reactivity with the murine Ki-4 mAb suggests binding to cluster A. (2) The antibody effectively mediates ADCC in vitro. (3) Upon cross-linking, the antibody shows growth-inhibitory activity against the CD30+ lymphoma cell lines L540 and KARPAS-299 in vitro with an inhibitory concentration at which 50% of cells are inhibited (IC50) of 10 μg/mL. (4) The 5F11 treatment of human Hodgkin tumors in SCID mice results in regression of established subcutaneous tumors and has a curative potential in the disseminated tumor model.
CD30 is a membrane-anchored receptor with a very restricted distribution on normal cells and has therefore been recognized as a potential target for a selective immunotherapy. Several reports indicate an important role of the CD30 receptor in malignant lymphoma. Recently it has been shown that ligand-independent activation of CD30 signaling drives nuclear factor-kappa B (NF-κB) activation and results in constitutive cytokine expression of H-RS cells.26 Deletion of its cytoplasmic domain results in apoptosis of H-RS cells. CD30 cross-linking induces decreased proliferative activity in H-RS cells.27 Activation of CD30 leads to the induction of apoptotic death of ALCL cells.28 Overall, targeting CD30 with mAbs may be a promising approach to directly affect the cell cycle regulation of CD30+ malignant cells. After the first description of this lymphocyte activation marker, numerous murine mAbs have been developed.29 However, the only unmodified anti-CD30 antibody clinically tested so far (the murine mAb Ber-H2) did not show any effect in advanced HL patients.30 It has been suggested that H-RS cells are resistant to induction of apoptosis upon CD30 ligation since they constitutively overexpress NF-κB.28 In addition, anti-CD30 antibodies have very different effects depending on their binding epitope. For example, CD30/CD30L interaction might be completely blocked upon anti-CD30 antibody binding or is not affected at all.31 Accordingly, CD30 shedding can be induced as well as inhibited.10 Thus, we developed new human anti-CD30 antibodies, of which the clone 5F11 was chosen for further development based on its biologic activity.
In general, human antibodies are more effective than their murine counterparts in recruiting immune effector mechanisms such as ADCC. Here we show activity of the human 5F11 mAb against H-RS cells with human peripheral blood leukocytes (PBLs) and monocytes as effector cells in vitro, suggesting that this mechanism of action might be active in vivo as well. However, clinical studies are required to determine the impact of this finding.
Although binding of the 5F11 mAb alone had no impact on cell viability in vitro, marked activity against the HL line L540 and the ALCL line KARPAS-299 was seen upon cross-linking. The B-cell-type HL lines L428 and L1236 were resistant to 5F11 treatment, although this may be explained by their constitutive NF-κB expression at high levels.32,33 Receptor cross-linking of members of the TNF superfamily has been shown to be essential for signal transduction and might play a role in HL as well.26,34,35 Our findings are in accordance with those from Wahl et al22 reported recently for the anti-CD30 mAb SGN-30. In addition, cross-linking by Fc-receptor-bearing cells might play a role in vivo, as it could be shown for a humanized anti-Fas mAb in a SCID mouse model recently.36 Importantly, antitumor activity of the 5F11 antibody was documented in different xenograft models of HL in SCID mice. In the disseminated model, the antibody was curative for all animals when administered together with a GAH-IgG and still active when given as a single agent. Furthermore, treatment with 5F11 induced a marked growth delay or even a complete regression of established xenografted HL in SCID mice. These results are promising, although both xenografted HL models are artificial and do neither reflect the biology of HL in humans nor the potential antibody-mediated human effector mechanisms, emphasizing the importance of clinical studies.
In summary, the first fully human anti-CD30 mAb 5F11 shows specific cytotoxic activity in different preclinical models. On the basis of these results, a clinical phase 1 study in patients with refractory CD30+ lymphomas has been initiated.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-02-0515.
Supported in part by the Deutsche Forschungsgemeinschaft, Grant TFB30.
Several of the authors (J.F.T., H.-f. Z., T.D., T.K., and R.F.G.) are employees of a company (Medarex Inc) whose potential product (the monoclonal antibody 5F11) was studied in the present work.
P.B. and J.F.T. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Mrs Gisela Schön and Mr Samir Tawadros for their excellent technical assistance.