Abstract
Phosphatidylserine (PS) is normally confined to the cytoplasmic leaflet of the red blood cell (RBC) membrane, but some sickle RBCs expose PS in the outer leaflet (PS+ cells). This study examined the relationships among PS externalization, fetal hemoglobin content, hydration state, and cell age. Sickle RBCs exhibit a wide range of PS externalization. Those with low-level exposure (type 1 PS+) include many young transferrin-receptor-positive (TfR+) cells. This is not specific for sickle cell disease because many nonsickle TfR+ cells are also PS+. RBCs with higher PS exposure (type 2 PS+) appear to be more specific for sickle cell disease. Their formation is most likely sickling dependent because type 2 PS+ dense sickle cells have a lower percentage of fetal hemoglobin (HbF) than PS- cells in the same density fraction (1.7 vs 2.9; n = 8; P < .01). In vivo experiments using biotin-labeled sickle cells showed a sharp decrease in the percentage of circulating, labeled PS+ cells in the first 24 hours after reinfusion. This decrease was confined to type 1 PS+ cells and was thus consistent with the reversal of PS exposure in very young cells. As the labeled cells aged in the circulation, the percentages of type 1 and type 2 PS+ cells increased. These studies indicate that PS externalization in sickle cells may be low level, as observed in many immature cells, or high level, which is associated with dehydration and appears to be more specific for sickle RBCs. (Blood. 2003;102: 365-370)
Introduction
Phosphatidylserine (PS), normally confined to the cytoplasmic red blood cell (RBC) membrane leaflet, escapes to the outer leaflet in some sickle RBCs.1-3 This abnormal loss of phospholipid asymmetry was first demonstrated using phospholipase-based assays and more recently demonstrated with fluorochrome-conjugated annexin V and flow cytometry. The latter analysis has shown that loss of asymmetry is confined to a subpopulation of cells (PS+). The number of PS+ cells varies widely among patients, changes considerably with time in individual patients, and decreases after transfusion.2 When sickle cells are separated by density, the lightest and densest fractions tend to have the highest percentages of PS+ cells.4 Loss of phospholipid asymmetry in dense cells may be a consequence of increased sickling or of the deactivation of an adenosine triphosphate (ATP)-dependent aminophospholipid translocase that is inhibited by calcium and plays an important role in maintaining normal asymmetry by returning PS from the outer to the inner leaflet.4,5 In vitro, red cell phospholipid asymmetry is lost and PS is externalized after ionophore-mediated calcium entry.
The loss of normal membrane phospholipid asymmetry may play a role in the pathophysiology of sickle cell disease. Studies demonstrating the recognition of PS by macrophages6-8 have led to the proposal that PS externalization contributes to the decreased lifespan of sickle cells. However, data obtained using biotin-labeled RBCs in patients with sickle cell disease9 indicate that the exposure of PS does not lead to the immediate removal of high-density sickle RBCs from the circulation. A murine sickle model was interpreted as showing an important contribution of externalized PS to decreased red cell survival.10 However, most of the decreased red cell survival in this model appeared to be independent of PS externalization. External PS may also be involved in thrombogenesis. Chiu et al11 showed that the dense fraction of sickle cells, which typically contains a high number of PS+ cells, has procoagulant activity in vitro. Finally, PS+ RBCs may contribute to vaso-occlusion by adhering to endothelial cells (ECs) as part of a complex series of events that includes increased RBC lysophosphatidic acid, EC retraction, and actin redistribution.12 Higher PS exposure in children with sickle cell anemia may be associated with an increased risk for stroke.13
Reticuloctyes can be divided into younger cells that are transferrin-receptor positive (TfR+) and slightly older cells that are transferrin-receptor negative (TfR-). Wood et al2 showed, and it has recently been confirmed,4 that many TfR+ sickle reticulocytes are PS+. This raises several important issues, including the possibility of TfR+PS+ cells in nonsickle states, the potential for reversal of PS externalization, and the prospect of PS- mediated removal of reticulocytes from the circulation.
In addition to sickle cell hemoglobin (HbS), a discrete population of sickle RBCs contains fetal hemoglobin (HbF), a potent inhibitor of HbS polymerization.14,15 These cells are less prone to sickle, and, because increased calcium flux into sickle cells is thought to depend on sickling,16 it is likely that they experience lower calcium traffic across the membrane and accumulate less intracellular calcium.17 If PS externalization is dependent on sickling, calcium accumulation, or both, it would be reasonable to expect a lower percentage of F cells to be PS+ and, conversely, that PS+ cells would contain less HbF.
Based on this background, we have studied the associations between PS exposure and other cellular properties thought to be important in sickle cell disease, including HbF content, maturity, age, and hydration state. Sickle cells exhibit a wide range of PS externalization. In this investigation we introduce the importance of distinguishing between cells with relatively low exposure and those with high exposure.
Materials and methods
Buffers
All concentrations are in millimolar: buffer A, 10 HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 140 NaCl, 2.5 CaCl2, pH 7.4; buffer D, buffer A with 0.5% bovine serum albumin (BSA) and vacuum degassed; phosphate-buffered saline (PBS), 140 NaCl, 1.4 NaH2PO4, 8.6 Na2HPO4, pH 7.4.
Materials
Materials included annexin V-fluorescein isothiocyanate (FITC; R&D Systems, Minneapolis, MN), streptavidin-phycoerythrin (SA-PE; Molecular Probes, Eugene, OR), streptavidin-coated MicroBeads, LS+ magnetic separation columns, magnet (Midimac; Miltenyi Biotec, Auburn, CA), and ArabiNIM arabinogalactan cell separation media and diluent (Cardinal Associates, Santa Fe, NM). Antibodies included biotin-conjugated mouse monoclonal anti-FITC (Sigma, St Louis, MO) and mouse antihuman TfR (CD71)-phycoerythrin (anti-TfR-PE; Lienco Technologies, Ballwin, MO).
Blood samples
Blood (in EDTA [ethylenediaminetetraacetic acid] or heparin) was obtained with informed consent as approved by the institutional review board of the University of Cincinnati College of Medicine according to the Declaration of Helsinki from adult patients with homozygous sickle cell disease in clinical steady state; patients had not undergone transfusion for at least 3 months. Additional blood samples were obtained from anemic nonsickle patients with reticulocytosis.
Determination of PS externalization and percentage HbF
Sickle RBCs from 20 adult patients were assayed to determine the percentage of PS+ cells and the percentage of HbF. This group included 4 patients who were taking hydroxyurea. Sickle RBCs were washed twice in PBS and once in buffer A and were resuspended at 1% in buffer A. Ten microliters annexin V-FITC (10 mg/mL) were added to 100 μL of the 1% cell suspension, and the mixture was incubated for 15 minutes in the dark. The percentage of PS+ cells was determined by flow cytometry.9 Sickle RBC lysate HbF content was measured by ion-exchange high-performance liquid chromatography (HPLC).18
Density fractionation of sickle RBCs
Sickle RBCs were separated into density fractions using discontinuous arabino galactan gradients. Patients in these studies were not taking hydroxyurea. To prepare gradients, 2 mL each arabinogalactan density mixture was placed into a polyallomer ultracentrifuge tube measuring 14 × 89 mm using an underlayer technique. Cells were resuspended to 1 mL with PBS (45%-50% hematocrit [Hct]) and were gently layered atop the gradient. Tubes were spun at 20 000 rpm for 45 minutes at room temperature. Separated fractions of red cells (less than 1.073; 1.073-1.083; 1.083-1.090; 1.090-1.094; 1.094-1.100; greater than 1.100 g/mL) were collected, washed twice in PBS, and washed once in the appropriate buffer for further studies. Less than 10% of normal RBCs are denser than 1.094 in this system.19
Isolation of dense PS+ sickle RBCs
Dense PS+ cells from 8 patients were isolated using a modification of the method of Kuypers et al.3 Six microliters RBCs (greater than 1.094 g/mL) were washed once in buffer A, resuspended in 600 μL of the same buffer, and incubated with 60 μL annexin V-FITC (10 μg/mL) for 15 minutes in the dark. Samples were washed twice in buffer A, resuspended in 200 μL buffer D, mixed with 5 μL (1:10 in buffer A) biotin-conjugated anti-FITC, and slowly rotated for 30 minutes. Cells were washed twice and resuspended to 180 μL in buffer D. Twenty microliters streptavidin-coated magnetic beads were added, and the samples were incubated on ice for 15 minutes. After 2 washes in buffer D, the cells were resuspended to 1 mL in the same buffer and were loaded onto a magnetized separation column that had been prewashed with buffer D. The column was washed with 9 mL buffer, and the cells eluted at this step were labeled as nonadherent. The column was removed from the magnet and was flushed with 5 mL buffer D to recover the adherent cells. Adherent cells were subjected to a second identical magnetic column isolation to further purify PS+ cells. Small aliquots of adherent and nonadherent cells were washed twice in calcium-free PBS to remove the annexin complex, then once in buffer A, and were resuspended in buffer A. Each of these cell suspensions was incubated with annexin V-FITC (50 μL of a 1% cell suspension incubated with 5 μL annexin V-FITC [10 μg/mL]) for 15 minutes in the dark and was analyzed by flow cytometry to determine the purity of the isolated PS+ cells. The percentage of HbF was determined by HPLC for samples of unfractionated cells, all dense cells, and PS+ dense cells.
Double labeling with anti-TfR-PE and annexin V-FITC
To evaluate PS externalization in sickle and nonsickle TfR+ reticulocytes, blood samples were taken from 7 patients with sickle cell disease and 6 patients with reticulocytosis without sickle cell disease. RBCs were washed twice in PBS and were resuspended in PBS to 1% suspension. Then 10 μL PBS/1% BSA and 10 μL anti-TfR-PE (0.1 mg/mL) were added to 80 μL cell suspension, followed by 30-minute incubation in the dark at room temperature. Cells were washed once in PBS and once in buffer A, then resuspended in buffer A to a 1% suspension. One hundred microliters of this suspension was incubated with 10 μL annexin V-FITC for 15 minutes, brought up to a volume of 1 mL in buffer A without washing, and analyzed by 2-color flow cytometry.
Biotin label studies
The biotin label has been use by us9,19 and others20 to follow the in vivo fate of human red blood cells. For 6 patients, sickle cells were labeled with biotin, reinfused, and identified in subsequent blood samples as previously described.19 The PS+ cells in the unlabeled and labeled populations were evaluated using a 2-color flow cytometric method.9
Results
Wide range of PS exposure in sickle cells
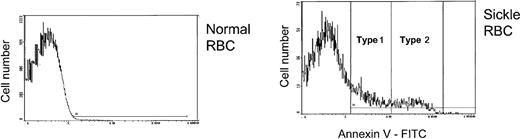
Two broad levels of PS externalization were observed in patients with sickle cell disease, and, based on this pattern, PS+ cells were designated as either type 1 (those with relatively low PS exposure) or type 2 (those with higher PS exposure) as shown in Figure 1. The region for type 1 cells ranged from negative (based on the fluorescence of normal cells with annexin V-FITC) to approximately 1 log higher than normal. For many patients, the high end of the type 1 range corresponded to a minimum on the cell frequency histogram. For type 2, the region extended for approximately 1 log higher. Therefore, the median annexin V-FITC fluorescence of type 2 cells was approximately 10 times higher than the corresponding value for type 1 cells.
Normal or sickle RBCs were reacted with annexin V-FITC to detect external PS. Positive cells were divided into those with low (type 1) or high (type 2) external PS. The horizontal line in each panel shows the gate for PS+ cells.
Normal or sickle RBCs were reacted with annexin V-FITC to detect external PS. Positive cells were divided into those with low (type 1) or high (type 2) external PS. The horizontal line in each panel shows the gate for PS+ cells.
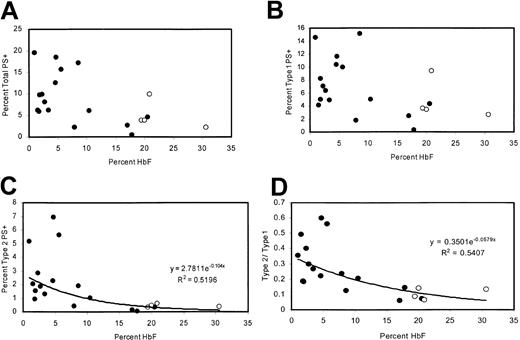
Patients with high levels of HbF have fewer type 2 PS+ cells
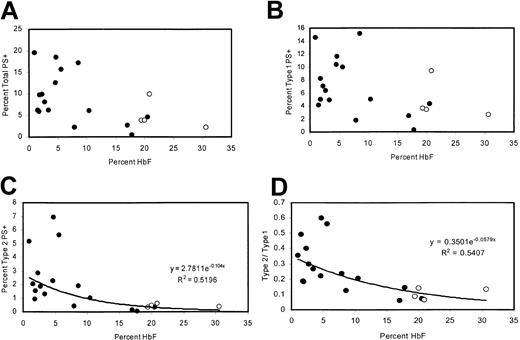
The percentage of PS+ cells was measured in 20 adult patients with homozygous sickle cell disease with a wide range of HbF levels, including 4 patients taking hydroxyurea. There was a negative correlation between percentage of PS+ cells (type 1 + type 2) and the percentage of HbF (Figure 2A). This correlation was similar to that previously reported21 and was consistent with sickling-dependent PS exposure. However, because reticulocytes tend to be type 1 PS+ (“Many TfR+ reticulocytes are type 1 PS+”), this correlation could also be attributed to the lower rate of erythropoiesis associated with high levels of HbF. Therefore, we also examined the percentages of type 1 and type 2 PS+ cells individually. Type 1 cell percentages tended to be lower for high values of HbF, consistent with the lower number of reticulocytes (Figure 2B). Type 2 cells exhibited a tighter correlation with HbF (Figure 2C) and were uniformly less than 1% for HbF values greater than 11%. At low HbF, the ratio of type 2 to type 1 cells was higher (Figure 2D), even though there would be more type 1 PS+ reticulocytes under these conditions.
Correlations between HbF content and levels of PS positivity. (A) Percentages of total PS+ cells, (B) type 1 PS+ cells, (C) type 2 PS+ cells, and (D) ratio of type 2 to type 1 as a function of HbF content. Open circles represent patients taking hydroxyurea.
Correlations between HbF content and levels of PS positivity. (A) Percentages of total PS+ cells, (B) type 1 PS+ cells, (C) type 2 PS+ cells, and (D) ratio of type 2 to type 1 as a function of HbF content. Open circles represent patients taking hydroxyurea.
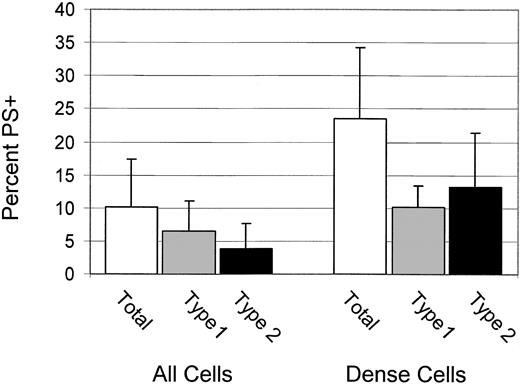
High percentages of dense cells are type 2 PS+
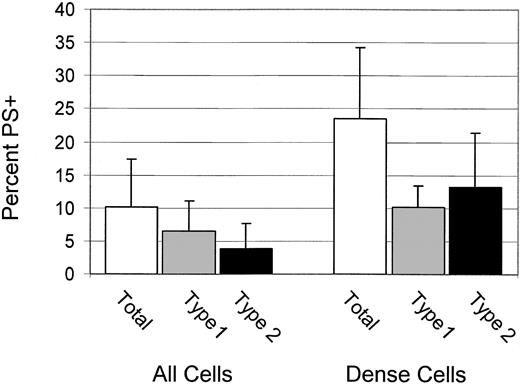
For 6 patients, the percentages of total, type 1, and type 2 PS+ cells were determined for dense (greater than 1.100 g/mL) cells. These data, shown in Figure 3, confirm the previous finding4 that dense cells have a higher percentage of PS+ cells, and they demonstrate that most PS+ dense cells are type 2.
Dense cells have high PS exposure and are type 2 PS+ Percentages of total, type 1, and type 2 PS+ for all cells and dense cells. Error bars represent 1 SD.
Dense cells have high PS exposure and are type 2 PS+ Percentages of total, type 1, and type 2 PS+ for all cells and dense cells. Error bars represent 1 SD.
Type2PS+ dense cells have a low level of HbF
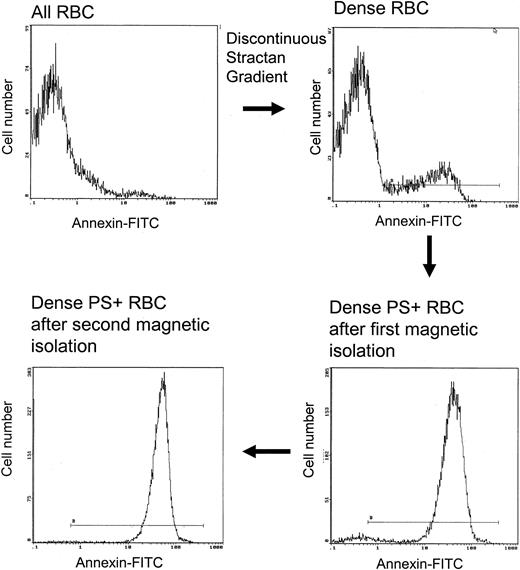
Given that PS+ cells are more prevalent in the lightest and densest cells, we performed studies to help define the properties associated with PS exposure in these fractions. Dense sickle cells have been shown to contain less HbF than unfractionated cells,22 and this is confirmed by the data in Table 1. As demonstrated in Figure 4, a highly enriched population of Type 2 PS+ cells was isolated from the dense cells by means of the magnetic procedure described in “Materials and methods.” The lack of type 1 cells in the isolate indicates that cells with low external PS do not bind efficiently to the magnetic column. Dense type 2 PS+ isolates contained a significantly lower percentage of HbF than the entire population of dense cells (Table 1; P < .01). This is consistent with a sickling-dependent component of PS exposure. However, PS+ isolates were not devoid of HbF, indicating that the presence of HbF does not completely prevent high-level PS exposure in dense sickle cells.
Magnetic isolation of type 2 PS+ cells from the dense sickle cell fraction. Two identical isolation procedures were performed in series. The horizontal line in each panel shows the gate for PS+ cells.
Magnetic isolation of type 2 PS+ cells from the dense sickle cell fraction. Two identical isolation procedures were performed in series. The horizontal line in each panel shows the gate for PS+ cells.
Many TfR+ reticulocytes are type 1 PS+
There is a higher percentage of PS+ cells in the light fraction of sickle cells, and, based on previous data,2 we suspected that these were very young cells. Therefore, experiments were performed to quantitate PS exposure in TfR+ cells for patients with and without sickle cell disease and with elevated reticulocyte counts. TfR+ cells had a higher percentage of PS+ than TfR- cells (Figure 5). Furthermore, TfR+PS+ reticulocytes were not specific for sickle cell disease, and nonsickle TfR+ reticulocytes followed a similar pattern. In sickle cells and nonsickle cells, TfR+PS+ reticulocytes had a lower level of PS exposure (type 1 PS+). There were few, if any, TfR+ type 2 cells.
PS exposure in sickle and nonsickle TfR+ reticulocytes. Percentages of PS+ cells in the TfR- (black bars) and TfR+ (gray bars) populations for nonsickle high reticulocyte (NSHR) states and sickle cell disease. Error bars represent 1 SD.
PS exposure in sickle and nonsickle TfR+ reticulocytes. Percentages of PS+ cells in the TfR- (black bars) and TfR+ (gray bars) populations for nonsickle high reticulocyte (NSHR) states and sickle cell disease. Error bars represent 1 SD.
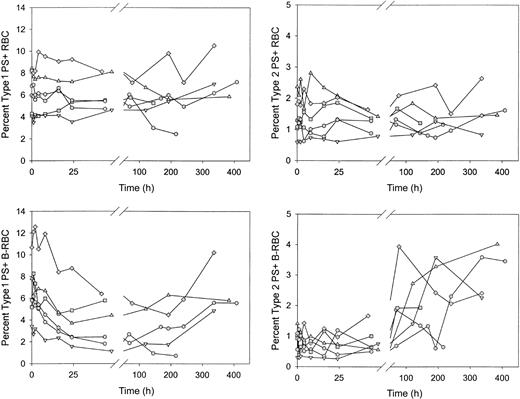
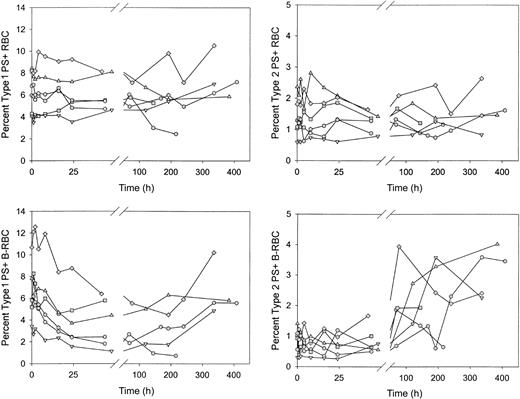
Type 1, but not type 2, PS+ cells decreased rapidly after reinfusion of biotin-labeled sickle cells
After reinfusion of biotin-labeled sickle RBCs, the percentages of unlabeled and labeled PS+ cells were determined as the labeled cells aged in the circulation. Unlabeled type 1 and type 2 PS+ cells demonstrated no consistent increase or decrease (Figure 6A-B). The percentage of labeled type 1 PS+ cells decreased by more than 40% in the first 48 hours after reinfusion (6.3% ± 2.5% vs 3.7% ± 2.2%; P < .02 by paired t test; Figure 6C), then leveled off and tended to increase at later times. The percentage of labeled type 2 PS+ cells was consistently lower immediately after reinfusion compared with unlabeled cells. This may be attributed to losses during labeling because these cells are probably less stable during in vitro manipulations. The percentage of labeled type 2 PS+ cells did not change in the first 48 hours after reinfusion but did increase 2- to 4-fold at later times (Figure 6D). The decrease in the labeled type 1 PS+ cells after infusion could have been caused by removal from the circulation or by reversal of PS externalization. Because there was no change in labeled type 2 PS+ during this time period (48 hours), it is unlikely that the decrease in type 1 cells, which exposed fewer PS molecules, was a result of erythrophagocytosis mediated by PS receptors. On the other hand, if the decrease during the first 24 hours were a result of reticulocyte maturation and reversal of PS externalization, the change would have occurred in the type 1 cells and thus would have been consistent with the observed changes.
Sickle red blood cells from 6 subjects were biotin labeled and reinfused. Percentages of type 1 and type 2 PS+ cells were determined at each time point for unlabeled (RBC) and labeled (B-RBC) cells.
Sickle red blood cells from 6 subjects were biotin labeled and reinfused. Percentages of type 1 and type 2 PS+ cells were determined at each time point for unlabeled (RBC) and labeled (B-RBC) cells.
Discussion
Sickle cells exhibit a wide range of PS externalization, with the number of PS molecules in the outer lipid leaflet of PS+ cells varying by 2 orders of magnitude. In previous studies, cells with relatively low levels of PS externalization (type 1) were not distinguished from those with high levels (type 2). Type 2 PS+ cells are of particular interest because they appear to be more specific for sickle cell disease. Of paramount importance is the possibility that correlations with clinical states, such as that reported for increased transcranial Doppler flow velocity,13 will be stronger and more predictive if made with the apparently pathognomonic type 2 PS+ cells.
The data presented here, in agreement with a recent study,21 show that patients with high HbF levels have fewer PS+ cells. In addition, we show that the PS+ cells in patients with high HbF levels tend to be in the type 1 subpopulation. The low level of type 2 PS+ cells in patients with high HbF most likely reflects the smaller number of non-F cells available to become type 2 PS+ cells. Other factors present in patients with high HbF levels, such as lower erythropoietic stress or more normal circulatory conditions, may also contribute.
Correlations between HbF and total (type 1 + type 2) PS exposure must be interpreted with care. The presence of a higher level of HbF, and therefore many cells that contain HbF, reduces the percentage of cells that sickle under physiological conditions. This would decrease the amount of sickling-dependent PS exposure. However, patients with high levels of HbF tend to have a lower percentage of reticulocytes, which would also decrease the number of PS+ cells.
Compared with unfractionated cells, dense cells contain a higher percentage of PS+ cells,4 now shown to include many type 2 cells. We investigated the relationship between propensity to sickle and PS externalization in this fraction by isolating the type 2 PS+ cells magnetically and measuring their HbF content. As expected, dense cells had lower HbF concentration than unfractionated cells, and an essentially pure population of type 2 PS+ cells, isolated from the dense cell fraction, had significantly less HbF than dense cells. Therefore, F cells are less likely to appear in the dense fraction, and those that do are less likely to be PS+. This is consistent with a sickling-dependent mechanism for type 2 PS exposure. However, the presence of HbF does not preclude PS exposure because some of the dense PS+ cells contain HbF.
Previous studies2,4 indicated that a relatively high percentage of TfR+ sickle cells are PS+. We now show that type 1 PS externalization is a common feature of young erythroid cells and that it is not specific for sickle cells. This makes it less likely that a low level of PS exposure functions as a recognition signal for removal from the circulation by macrophages.23 However, it is possible that macrophage interaction could selectively remove PS+ portions of the membrane. In a previous study,9 we examined changes in the percentage of PS+ cells after the reinfusion of biotin-labeled, high-density-enriched sickle cells. In these experiments, almost all the light cells were removed before labeling so that only the relatively small number of reticulocytes with high density were included. In contrast to the early decrease in PS+ cells for the unfractionated cells reported in the current study, there was no consistent decrease in the studies with high-density-enriched cells. The fact that PS+ cells could have been removed while new ones were formed does not rule out a decreased lifespan for PS+ cells. However, it does place a limit on the degree of shortened survival because if PS+ cells had a markedly shortened survival and were continuously replenished by the conversion of PS- to PS+ cells, the entire pool of biotin-labeled cells would be quickly consumed. For example, we described9 a patient for whom the isolated high-density-enriched cells were approximately 25% PS+ when reinfused and had a survival time longer than 100 hours. After reinfusion, the percentage of PS+ biotin-labeled cells ranged between 20% and 34%. For this patient, with a dense cell survival of more than 4 days, the survival of PS+ dense cells could not have been less than approximately 1 day.
In 2 recent studies,10,24 investigators used the biotin-label method to examine the survival of PS+ red cells in the mouse model of sickle cell disease developed by Paszty et al,25 and they concluded that PS exposure is an important factor contributing to decreased red cell lifespan. However, the potential conversion of PS+ reticulocytes to PS- mature cells makes it difficult to interpret these data. de Jong et al10 labeled all the circulating red cells with biotin in vivo on day 0, and the subsequent rate of replacement of labeled with unlabeled RBCs in the circulation was determined. Wild-type mouse RBCs had a 50% survival time of 20 days. Mouse sickle RBCs, including PS+ and PS- cells, had a 50% survival of 2 days. Given that only 2% to 5% of the cells were PS+, this 90% decrease in survival cannot be attributed to PS status. The 50% survival of PS+ cells, when examined separately, was decreased further to approximately 1.25 days. It was concluded that PS+ cells have shorter survival times than PS- cells. However, these survival values assume there is no change in PS status subsequent to labeling. Conversion of labeled PS- cells to PS+ would cause the survival of PS+ cells to be overestimated because labeled PS+ cells that were removed from the circulation would have been replaced with newly formed labeled PS+ cells. The authors discussed this possibility. However, it is also possible that PS+ cells could convert to PS- cells, and this would have the opposite effect, making the survival of PS+ cells look worse. An average of 38% reticulocytes was present in the sickle mice, and approximately the same percentage of reticulocytes and mature RBCs were PS+. Therefore, at the time of labeling, approximately 38% of the PS+ cells were reticulocytes. These PS+ reticulocytes (which were also present in wild-type animals in smaller numbers) may become PS- as they mature. This progression from PS+ to PS- with erythroid differentiation would be consistent with the data in the same article showing that bone marrow and spleen erythroid precursors, in wild-type and sickle mice, have levels of PS externalization that are higher than the corresponding reticulocytes. Kean et al24 also demonstrate an extremely short red cell survival in sickle mice (t ½ = 0.8 days). As would be expected with such rapid removal from the circulation, many cells did not survive the reticulocyte stage. The percentage of surviving labeled cells that had external PS decreased with time, and this was taken as evidence for PS- mediated removal from the circulation. However, the alternative possibility of a reversal of PS externalization as part of the reticulocyte remodeling process was not eliminated.
It would seem premature to conclude from these studies that external PS is a major determinant of sickle hemolysis because (1) most of the decreased survival of mouse sickle cells appears to be unrelated to PS status, (2) the disappearance of some PS+ biotin-labeled cells may reflect reticulocyte maturation—especially in mouse models in which cell survival and reticulocyte maturation occur in the same time frame—and (3) in dense sickle cells, the percentage of PS+ biotin-labeled cells remains constant as the number of biotin-labeled cells declines.
In the experiments described here, the relative behavior of type 1 and type 2 cells during the first 48 hours after reinfusion suggests that PS- mediated hemolysis, adhesion, or both are not responsible for the early drop in type 1 cells. If this were the case, we would have expected an equal or a greater decrease for type 2 cells during this time period because of their greater exposure to PS. Therefore, it appears that a time-dependent change in the properties of the labeled cells, rather than physical removal from the circulation, is responsible for the observed early type 1 decrease. Given that many TfR+ reticulocytes are type 1 PS+ and reticulocytes differentiate to mature RBCs within 48 to 72 hours, the early decrease in labeled type 1 PS+ cells is most likely caused by the reversal of PS exposure during reticulocyte maturation.
In the current studies we show that PS+ sickle cells can be divided into those with low PS externalization (type 1) and those with high externalization (type 2). Some, but not all, type 1 cells are reticulocytes and are seen in high reticulocyte states unrelated to sickle cell disease. Type 2 cells, however, appear to be specific for sickle disease. Type 2 cells deserve further study because of this and because many type 2 cells are in the dense fraction of sickle cells known to have short in vivo survival and the higher level of PS exposure in these cells is more likely to lead to pathologic sequelae. The distinction between type 1 and type 2 PS+ cells may be important in clinical studies because the inclusion of type 1 PS+ reticulocytes in a correlation between PS+ cells and clinical variables (eg, red cell survival, stroke, thrombophilia) may obscure any relationship. Given that it is the type 2 cells that are more specific for sickle cell disease and are more severely affected, this subpopulation may be the best measure of sickling-dependent loss of phospholipid asymmetry.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-11-3416.
Supported by National Institutes of Health grants RO1 HL51174, RO1 HL57614, and P60 HL58421.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.