Abstract
Human neutrophils were found to express members of the inhibitor of apoptosis (IAP) family, namely cellular IAP1 (cIAP1), cIAP2, and X-linked IAP. Among these members, cIAP2 expression was selectively up-regulated by stimulation with granulocyte colony-stimulating factor (G-CSF), but not with granulocyte-macrophage CSF. The increased expression of cIAP2 mRNA was detected as early as 30 minutes after in vitro stimulation with G-CSF, and the elevated level of cIAP2 protein was detected at 1 hour. The elevated level of cIAP2 protein was also detected in peripheral blood neutrophils obtained from healthy donors receiving G-CSF administration. G-CSF–induced up-regulation of cIAP2 mRNA and protein, phosphorylation of signal transducer and activator of transcription 3 (STAT3), and the antiapoptotic effects were inhibited by pretreatment of cells with AG490, a specific inhibitor of Janus kinase 2 (JAK2). Mature neutrophils from a patient with chronic neutrophilic leukemia exhibited remarkable overexpression of cIAP2 mRNA and prolongation of survival, whereas cIAP2 mRNA expression and survival in mature neutrophils from patients with chronic myelogenous leukemia were essentially similar to those in normal neutrophils. These findings suggest that cIAP2 expression is up-regulated by G-CSF through activation of the JAK2-STAT3 pathway, and increased expression of cIAP2 protein may contribute to G-CSF–mediated antiapoptosis. In addition, overexpression of cIAP2 may be partly responsible for sustained neutrophilia at least in some cases of chronic neutrophilic leukemia.
Introduction
Human neutrophils are short-lived cells and undergo spontaneous apoptosis during culture. Spontaneous neutrophil apoptosis is delayed or accelerated in the presence of various cytokines, such as granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage CSF (GM-CSF), and tumor necrosis factor (TNF).1-3Modulation of neutrophil apoptosis by inflammatory cytokines may be closely associated with the outcome of inflammation. However, neither the mechanisms regulating spontaneous neutrophil apoptosis nor the mechanisms by which cytokines exert an apoptotic or antiapoptotic effect on neutrophils are fully understood. Possibilities may include up- or down-regulation of molecules associated with apoptosis, which may include the Bcl-2 family proteins such as Bcl-XL, Mcl-1, A1, and Bax.4-9 For example, it has been reported that G-CSF up-regulates the expression of A15 and down-regulates the expression of Bax.6 GM-CSF up-regulates the expression of Mcl-14,8,9 and down-regulates the expression of Bax-α,10 whereas TNF down-regulates the expression of Bcl-XL.10 However, the results reported are contradictory.8,11 In regard to the signaling pathways, tyrosine kinase Lyn, extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K), and Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) have been proposed to be involved in the GM-CSF–mediated antiapoptotic effect on human neutrophils.9,10,12,13 Our recent study showed that G-CSF appears to prolong neutrophil survival by inhibiting caspase activation, and G-CSF–mediated antiapoptosis is completely abolished by cycloheximide, suggesting that protein synthesis is required for the antiapoptotic effect of G-CSF.1
The inhibitor of apoptosis (IAP) proteins were first discovered in baculoviruses and were shown to be involved in suppressing the host cell-death response to viral infection. The human IAP family includes cellular IAP1 (cIAP1), cIAP2, X-linked IAP (XIAP), neuronal apoptosis inhibitor protein, and survivin.14,15 These proteins are characterized by the presence of the baculoviral IAP repeat, zinc ring finger, and caspase recruitment domains and have been shown to inhibit active caspase-3 and caspase-7 directly and to inhibit activation of pro–caspase-9.14,15 The IAP family proteins are expressed in various types of cells, including hematopoietic cells. It has been reported that TNF up-regulates human IAP1, also known as cIAP2, through activation of nuclear factor (NF)–κB in Jurkat cells.16XIAP and cIAP1 are reported to be expressed in most leukemia cell lines, whereas the expression of cIAP2 is restricted to some cell lines.17 XIAP protein is expressed in leukemia cells from most patients with acute myelogenous leukemia (AML), and the low level of XIAP protein is associated with longer survival and a tendency toward longer remission duration.17 More recently, it has been shown that survivin is expressed in normal human CD34+ cells, myeloid leukemia cell lines, and most primary AML samples, and the expression of survivin protein is increased by stimulation with G-CSF, GM-CSF, and stem cell factor alone or in combination.18,19 Furthermore, it has been shown that XIAP is one of the antiapoptotic regulators that confers resistance on murine macrophages.20
In this paper, we show that XIAP, cIAP1, and cIAP2 are expressed in human neutrophils, and among these molecules cIAP2 expression is selectively up-regulated by stimulation with G-CSF, but not with GM-CSF, at the protein as well as mRNA levels. In addition, the results suggest that cIAP2 expression is up-regulated by G-CSF through, at least in part, activation of the JAK2-STAT3 pathway, and increased expression of cIAP2 may contribute to G-CSF–mediated antiapoptosis. Furthermore, we found that mature neutrophils from a patient with chronic neutrophilic leukemia (CNL) exhibited remarkable overexpression of cIAP2 mRNA and prolongation of survival, raising the possibility that the overexpression of cIAP2 may be partly responsible for sustained neutrophilia, at least in some cases of CNL.
Patients, materials, and methods
Reagents
Highly purified recombinant human G-CSF, GM-CSF, interleukin-1β (IL-1β), and TNF produced by Escherichia coli were provided by Kirin Brewery (Tokyo, Japan), Schering-Plough (Osaka, Japan), Otsuka Pharmaceutical (Tokushima, Japan), and Dainippon Pharmaceutical (Osaka, Japan), respectively. Endotoxin contamination of each preparation was less than 100 pg per milligram protein. SB203580, an inhibitor of p38 mitogen-activated protein kinase (MAPK), and AG490, an inhibitor of JAK2, were purchased from Calbiochem (San Diego, CA). Cytochrome c type III, N-formyl-methionyl-leucyl-phenylalanine (FMLP), superoxide dismutase, and wortmannin were purchased from Sigma (St Louis, MO). Conray was purchased from Mallinckrodt (St Louis, MO). Ficoll and the enhanced chemiluminescence (ECL) Western blotting system were purchased from Amersham Pharmacia Biotech (Buckinghamshire, England). Rabbit polyclonal antibodies against cIAP1 and cIAP2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies against p38 MAPK, STAT3, Tyr705-phosphorylated STAT3, and Thr202/Tyr204-phosphorylated ERK1/ERK2, and PD98059, an inhibitor of MAPK/ERK kinase (MEK), were purchased from Cell Signaling Technology (Beverley, MA). Rabbit polyclonal antibody against Mcl-1 was purchased from BD PharMingen (San Diego, CA).
Preparation of cells
Human neutrophils and mononuclear cells were prepared from healthy adult donors as described previously,21 using dextran sedimentation, centrifugation with Conray-Ficoll, and hypotonic lysis of contaminated erythrocytes. Mature neutrophils were also prepared from a patient with CNL, 6 patients with chronic myelogenous leukemia (CML), and 4 healthy donors receiving G-CSF administration for allogeneic peripheral blood stem cell transplantation using the same procedure described in the previous sentence.21Informed consent was obtained from all subjects. Monocytes and lymphocytes were purified from mononuclear cells by centrifugal elutriation in a Hitachi SRR6Y elutriation rotor (Hitachi, Tokyo, Japan), as described previously.22 Cells were suspended in Hanks balanced salt solution (HBSS) containing 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4). For the experiments with cell cultivation, cells were suspended in RPMI 1640 supplemented with 10% fetal calf serum (FCS), penicillin (100 U/mL), and streptomycin (100 μg/mL). Morphologic assessment of the cells was performed using the cytospin preparations stained with May-Grünwald and Giemsa. Neutrophil fractions contained more than 98% neutrophils and less than 2% eosinophils, monocyte fractions contained 90% to 95% monocytes and 5% to 10% lymphocytes, and lymphocyte fractions contained 100% lymphocytes.
A case of CNL
CNL was diagnosed in an afebrile 58-year-old Japanese man with the following hematologic findings: white blood cell count 36.5 × 109/L (92.0% mature neutrophils), hemoglobin 11.1 g/dL, and platelets 342 × 109/L. Bone marrow examination revealed myeloid hyperplasia without dysplastic features. Cytogenetic analysis of bone marrow cells showed normal karyotype, and bcr gene rearrangement was not detected by reverse transcription–polymerase chain reaction (RT-PCR) analysis. The serum concentrations of G-CSF and GM-CSF were below the detectable levels (less than 10 pg/mL and less than 8 pg/mL, respectively). The physical and laboratory findings were compatible with the diagnosis of CNL.23
Cell culture and determination of apoptosis
Neutrophils (5 × 106/mL) suspended in RPMI 1640 were cultivated in 5% CO2/95% humidified air at 37°C. When required, G-CSF (50 ng/mL), GM-CSF (5 ng/mL), PD98059 (50 μM), SB203580 (10 μM), wortmannin (100 nM), or AG490 (50 μM) was added to the culture medium. HL-60 cells were grown in RPMI 1640 supplemented with 10% FCS, penicillin (100 U/mL), and streptomycin (100 μg/mL). DNA content in the cells was determined by propidium iodide staining and flow cytometry with FACS Calibur (Becton Dickinson, Mountain View, CA), as described previously.1 For determination of DNA content, cells (1 × 106) were placed in 70% ethanol in phosphate-buffered saline and stored at −20°C until use. Cells were treated with DNase-free RNase (50 μg/mL) and propidium iodide (50 μg/mL) for 15 minutes at room temperature. Samples were kept at 4°C in the dark until analysis. Cells with decreased DNA content (hypodiploid cells) were considered as cells undergoing apoptosis-associated DNA degradation.1
Western blotting
Human neutrophils suspended in HBSS were prewarmed for 10 minutes at 37°C and were then stimulated with cytokines for 10 minutes at 37°C. When required, cells were preincubated with AG490 (50 μM) for 20 minutes at 37°C before stimulation with cytokines. The reactions were terminated by rapid centrifugation, and the pellets were frozen in liquid nitrogen after aspiration of the supernatant. The cell pellets were resuspended in ice-cold solution containing 50 mM HEPES (pH 7.4), 1% Triton X-100, 2 mM sodium orthovanadate, 100 mM sodium fluoride, 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM EGTA (ethyleneglycoltetraacetic acid), 1 mM phenylmethylsulfonyl fluoride, 100 μg/mL aprotinin, and 10 μg/mL leupeptin, and were lysed for 10 minutes at 4°C. After rapid centrifugation, the supernatant was mixed 1:1 with 2 × sample buffer (4% sodium dodecyl sulfate [SDS], 20% glycerol, 10% mercaptoethanol, and a trace amount of bromophenol blue dye in 125 mM Tris-HCl, pH 6.8), heated at 100°C for 5 minutes, and then frozen at −80°C until use. Samples were subjected to 10% SDS gel electrophoresis. After electrophoresis, proteins were electrophoretically transferred from the gel onto a nitrocellulose membrane in a buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol at 2 mA/cm2 for 1.5 hours at 25°C. Residual binding sites on the membrane were blocked by incubating the membrane in Tris-buffered saline (pH 7.6) containing 0.1% Tween 20 and 5% nonfat dry milk for 2 hours at 25°C. The blots were washed in Tris-buffered saline containing 0.1% Tween 20 and then incubated with appropriate antibody overnight at 4°C. After washing, the membrane was incubated with antirabbit IgG antibody conjugated with horseradish peroxidase, and the antibody complexes were visualized by the ECL detection system as directed by the manufacturer. Immunoreactive bands were quantified by a National Institutes of Health (NIH) Image program on a Macintosh computer.
RT-PCR analysis
Total RNA was isolated from neutrophils with the RNeasy Mini Kit (Qiagen, Hilden, Germany). To generate cDNA, we used 100 ng RNA for each reaction. The reaction mixtures (10 μL) contained RNA, random primer pd(N)6 (1 μM), RNase inhibitor (0.5 U/μL) (Roche Molecular Biochemicals, Mannheim, Germany), dNTP mixture (500 μM of each dNTP), and Omniscript reverse transcriptase (0.2 U/μL) (Qiagen); the reaction mixtures were incubated for 60 minutes at 37°C. Semiquantitative RT-PCR analysis was performed using a GeneAmp PCR system model 9700 (Perkin Elmer, Norwalk, CT). PCR reaction mixtures (25 μL) contained cDNA, dNTP mixture (200 μM of each dNTP), MgCl2 (1.5 mM), Taq DNA polymerase (0.02 U/μL) (Fermentas AB, Vilnius, Lithuania), and the forward and reverse primers.
The following primer pairs were used. For each set, the forward and reverse primers and the accession number are listed: cIAP1: 5′-AGC TGT TGT CAA CTT CAG ATA CCA CT-3′, 5′-TGT TTC ACC AGG TCT CTA TTA AAG CC-3′, U45879; cIAP2: 5′-ACT TGA ACA GCT GCT ATC CAC ATC-3′, 5′-GTT GCT AGG ATT TTT CTC TGA ACT GTC-3′, U45878; XIAP: 5′-GGT TCA GTT TCA AGG ACA TT-3′, 5′-CAA GGA ACA AAA ACG ATA GC-3′, U45880; Mcl-1: 5′-CTC TCA TTT CTT TTG GTG CCT-3′, 5′-ATT CCT GAT GCC ACC TTC TA-3′, AF198614; A1: 5′-GTT TGA AGA CGG CAT CAT T-3′, 5′-ACA AAG CCA TTT TCC CAG-3′, U27467; Bcl-2: 5′-GCC CTG TGG ATG ACT GAG TA-3′, 5′-ACT TGT GGC TCA GAT AGG CA-3′, M13994; Bcl-XL: 5′-GCA GGT ATT GGT GAG TCG G-3′, 5′-CTG AAG AGT GAG CCC AGC A-3′, Z23115; β2-microglobulin: 5′-GCT ATG TGT CTG GGT TTC-3′, 5′-TAC ATG TCT CGA TCC CAC-3′, V00567; β-actin: 5′-CCA ACC GCG AGA AGA TGA C-3′, 5′-GGA AGG AAG GCT GGA AGA GT-3′,X00351; and 18S rRNA: 5′-TCC GAT AAC GAA CGA GAC TC-3′, 5′-CAG GGA CTT AAT CAA CGC AA-3′, M10098.
The conditions for PCR amplification were as follows: denaturation for 30 seconds at 94°C, annealing for 30 seconds at 56°C, and elongation for 30 seconds at 72°C, with 18 cycles for 18S rRNA; 28 cycles for β2-microglobulin and β-actin; 33 cycles for cIAP1, cIAP2, XIAP, Mcl-1, and A1; and 35 cycles for Bcl-XL and Bcl-2. The PCR products were analyzed by electrophoresis on a 2% agarose gel containing ethidium bromide. The PCR products were purified and the nucleotide sequence was analyzed for confirmation.
Real-time PCR analysis
For quantitative determination of the transcripts, real-time PCR analysis was performed with LightCycler (Roche) as directed by the manufacturer. The reaction mixtures (20 μL) contained MgCl2 (3 mM), the forward and reverse primers (0.5 μM), the fluorescein isothiocyanate (FITC)–conjugated probe (0.2 μM), LightCycler Red 640 (LC Red 640)–conjugated probe (0.4 μM), LightCycler-DNA master hybridization probe (0.4 μM), and the cDNA (1 μL).24 As an internal control, 18S rRNA was used. The primer sequences are described 2 paragraphs above, and the hybridization probe sets were as follows: cIAP1: 5′-CAG GTG TAT TCA TCA TGA CAG CAT CTT C-[FITC]-3′ and 5′-[LC Red 640]-GAA GAA CTT TCT CCA GGT CCA AAA TGA-[phosphate]-3′; cIAP2: 5′-GAT TGC ATC TTC TGA ATG GTC TTC TCC-[FITC]-3′ and 5′-[LC Red 640]-GGT TCC AAA TGG ATA ATT GAT GAC TCT G-[phosphate]-3′; and 18S rRNA: 5′-GGA CAT CTA AGG GCA TCA CAG ACC T-[FITC]-3′ and 5′-[LC Red 640]-TTA TTG CTC AAT CTC GGG TGG CT-[phosphate]-3′.
Determination of superoxide (O) release
O was assayed by superoxide dismutase–inhibitable reduction of ferricytochrome c, and the continuous assay was performed in a Hitachi 557 spectrophotometer (a double wavelength spectrophotometer) equipped with a thermostated cuvette holder (37°C), as described previously.21 22 The final concentration of cytochrome c was 100 μM, and the final cell concentration was 1 × 106 cells/mL. The amount of O release was calculated from cytochromec reduced for 5 minutes after the addition of FMLP (10−7 M). When required, neutrophils were pretreated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), TNF (100 U/mL), or IL-1β (300 U/mL) for 10 minutes at 37°C before stimulation with FMLP.
Statistical analysis
Analysis of variance followed by a multiple comparison test or the Student t test was used to determine statistical significance.
Results
Expression of the IAP family in neutrophils and selective up-regulation of cIAP2 by G-CSF
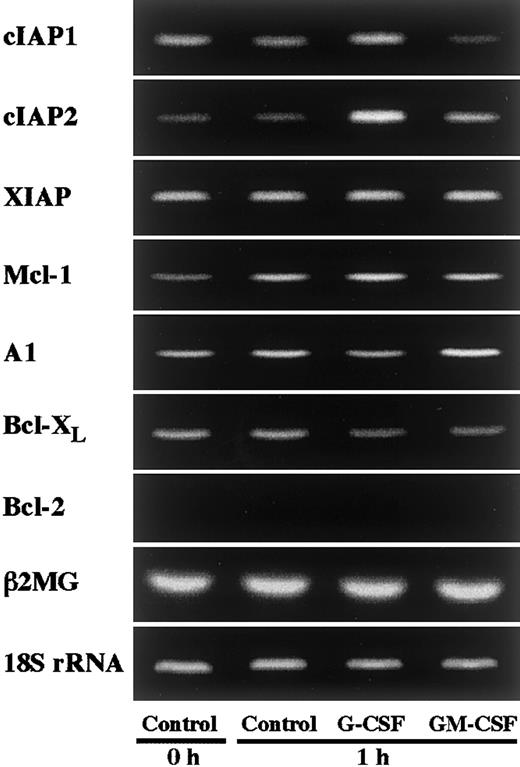
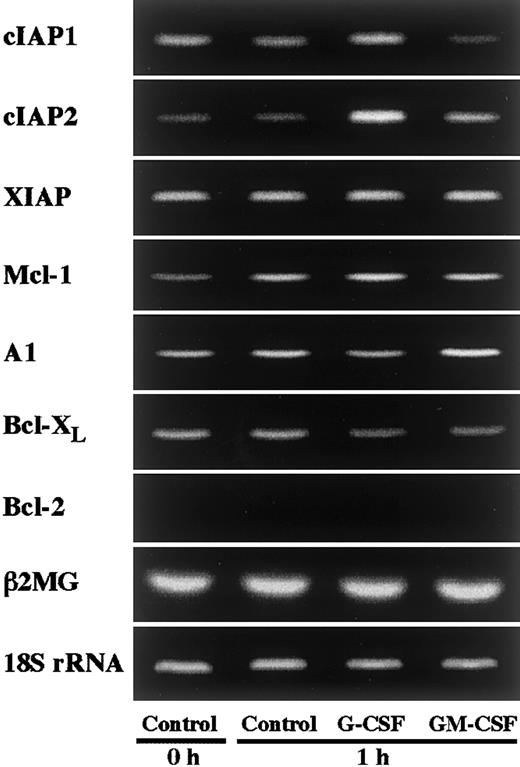
In an initial experiment, we studied the expression of apoptosis-related gene transcripts in human neutrophils by RT-PCR. As shown in Figure 1, human neutrophils expressed Mcl-1, A1, and Bcl-XL, but not Bcl-2, in agreement with previous reports.4-10 In addition to these transcripts, human neutrophils were found to express members of the IAP family, namely cIAP1, cIAP2, and XIAP (Figure 1). To determine the gene transcripts up-regulated by G-CSF or GM-CSF stimulation, we stimulated neutrophils with G-CSF (50 ng/mL) or GM-CSF (5 ng/mL) for 1 hour and thereafter analyzed the expression of the transcripts of these molecules by semiquantitative RT-PCR. Among these transcripts, cIAP2 expression was markedly and selectively up-regulated by stimulation with G-CSF, but not with GM-CSF (Figure 1). Under the same conditions, no dramatic increase in the expression of other molecules, including Mcl-1 and A1, was detected. Then, in the following study, we addressed the role of cIAP2 up-regulation in the G-CSF–mediated effect on human neutrophils.
Effects of G-CSF and GM-CSF on expression of apoptosis-related gene transcripts in neutrophils.
Neutrophils were cultivated in the presence or absence of G-CSF (50 ng/mL) or GM-CSF (5 ng/mL) for 1 hour, and thereafter the expression of cIAP1, cIAP2, XIAP, Mcl-1, A1, Bcl-XL, and Bcl-2 mRNA was analyzed by semiquantitative RT-PCR. As internal controls, β2-microglobulin (β2MG) and 18S rRNA were used. The results shown are representative of 3 independent experiments.
Effects of G-CSF and GM-CSF on expression of apoptosis-related gene transcripts in neutrophils.
Neutrophils were cultivated in the presence or absence of G-CSF (50 ng/mL) or GM-CSF (5 ng/mL) for 1 hour, and thereafter the expression of cIAP1, cIAP2, XIAP, Mcl-1, A1, Bcl-XL, and Bcl-2 mRNA was analyzed by semiquantitative RT-PCR. As internal controls, β2-microglobulin (β2MG) and 18S rRNA were used. The results shown are representative of 3 independent experiments.
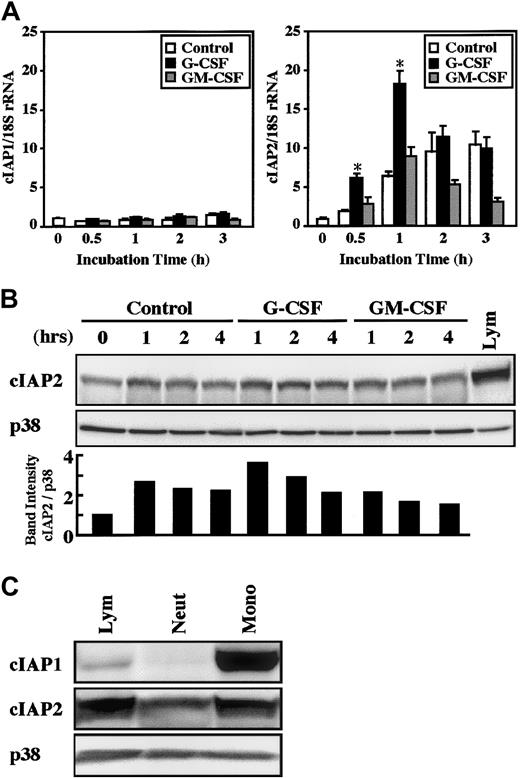
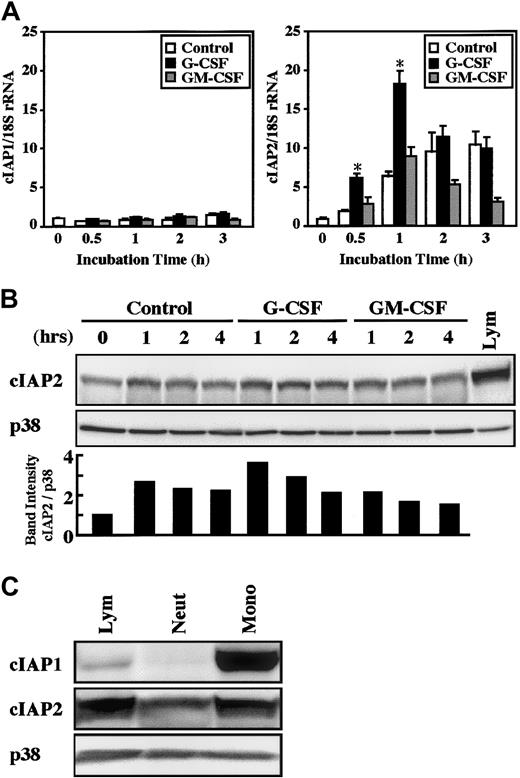
The real-time PCR analysis revealed that a significant increase in cIAP2 mRNA expression was detected as early as 30 minutes after stimulation with G-CSF, and the maximal increase was obtained at 1 hour, followed by a gradual decrease in the level (Figure2A). In contrast, no significant increase in cIAP2 mRNA expression was induced by stimulation with GM-CSF. The expression of cIAP2 mRNA was also slightly, but significantly (already significant at 30 minutes, P < .05), increased by cultivation alone, presumably because of stresses such as cell preparation procedures and in vitro culture of cells.25 In contrast to cIAP2, the expression of cIAP1 mRNA was not altered by stimulation with G-CSF or GM-CSF, even when the cultivation time was prolonged up to 3 hours (Figure 2A).
Effects of G-CSF and GM-CSF on expression of cIAP1 and cIAP2 mRNA and proteins in neutrophils.
(A) Neutrophils were cultivated in the presence or absence of G-CSF (50 ng/mL) or GM-CSF (5 ng/mL). After cultivation for 0.5 to 3 hours, the expression of cIAP1 and cIAP2 mRNA was analyzed by the real-time PCR. As an internal control, 18S rRNA was used. The ratios of cIAP1 to 18S rRNA and cIAP2 to 18S rRNA were calculated, and the ratio at hour 0 is expressed as 1. The data are expressed as means ± standard errors of the mean of 5 experiments. *Significantly increased as compared with control cells (P < .01). (B) Neutrophils were cultivated in the presence or absence of G-CSF (50 ng/mL) or GM-CSF (5 ng/mL). After cultivation for 1 to 4 hours, the expression of cIAP2 and p38 proteins was analyzed by immunoblotting using antibodies against each protein. The cell lysates equivalent to 2.5 × 106 cells were loaded onto each lane. As a control, p38 was used. The ratio of cIAP2 to p38 band intensity was calculated, and the ratio at hour 0 is expressed as 1 (lower panel). The results shown are representative of 3 independent experiments. Lym indicates lymphocytes (2.5 × 106 cells). (C) The expression of cIAP1, cIAP2, and p38 proteins in freshly isolated neutrophils (Neut), monocytes (Mono), and lymphocytes (Lym) was analyzed by immunoblotting using antibodies against each protein. The cell lysates from an equal number of cells (2.7 × 106) were loaded onto each lane. The results shown are representative of 3 independent experiments.
Effects of G-CSF and GM-CSF on expression of cIAP1 and cIAP2 mRNA and proteins in neutrophils.
(A) Neutrophils were cultivated in the presence or absence of G-CSF (50 ng/mL) or GM-CSF (5 ng/mL). After cultivation for 0.5 to 3 hours, the expression of cIAP1 and cIAP2 mRNA was analyzed by the real-time PCR. As an internal control, 18S rRNA was used. The ratios of cIAP1 to 18S rRNA and cIAP2 to 18S rRNA were calculated, and the ratio at hour 0 is expressed as 1. The data are expressed as means ± standard errors of the mean of 5 experiments. *Significantly increased as compared with control cells (P < .01). (B) Neutrophils were cultivated in the presence or absence of G-CSF (50 ng/mL) or GM-CSF (5 ng/mL). After cultivation for 1 to 4 hours, the expression of cIAP2 and p38 proteins was analyzed by immunoblotting using antibodies against each protein. The cell lysates equivalent to 2.5 × 106 cells were loaded onto each lane. As a control, p38 was used. The ratio of cIAP2 to p38 band intensity was calculated, and the ratio at hour 0 is expressed as 1 (lower panel). The results shown are representative of 3 independent experiments. Lym indicates lymphocytes (2.5 × 106 cells). (C) The expression of cIAP1, cIAP2, and p38 proteins in freshly isolated neutrophils (Neut), monocytes (Mono), and lymphocytes (Lym) was analyzed by immunoblotting using antibodies against each protein. The cell lysates from an equal number of cells (2.7 × 106) were loaded onto each lane. The results shown are representative of 3 independent experiments.
Consistent with the changes in cIAP1 and cIAP2 mRNA expression, stimulation of neutrophils with G-CSF resulted in an elevated level of cIAP2, but not cIAP1, protein (Figures 2B and 5). The elevated level of cIAP2 protein was already detected at 1 hour and was still detected at 2 hours after G-CSF stimulation, a finding consistent with the fact that the increased level of cIAP2 mRNA was detected at 30 minutes after G-CSF stimulation (Figure 2A). In contrast, no significant increase in the level of cIAP2 protein was detected in neutrophils stimulated by GM-CSF. A significant increase in the level of cIAP2 protein during culture was also sometimes detected in control cells, in accordance with the increased level of cIAP2 mRNA (Figures 2B and 5). Monocytes and lymphocytes were also found to express cIAP1 and cIAP2 mRNA and proteins. The expression of these proteins in monocytes and lymphocytes was greater than that in neutrophils on a cell basis, and cIAP1 protein was strongly expressed in monocytes (Figures 2C and9).
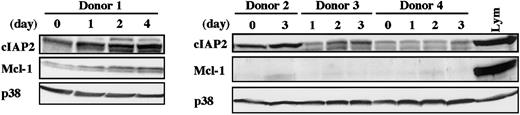
Elevated level of cIAP2 protein in neutrophils from donors receiving G-CSF administration
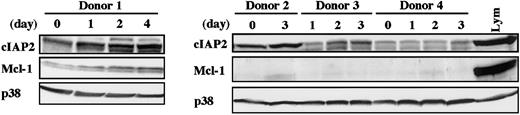
To determine whether cIAP2 expression in human neutrophils is also up-regulated by in vivo stimulation with G-CSF, we isolated peripheral blood neutrophils from healthy donors receiving G-CSF administration and analyzed the level of cIAP2 protein by Western blotting. As shown in Figure 3, the level of cIAP2 protein was consistently elevated in all 4 samples tested, with some variations according to the donors. The level of cIAP2 protein appeared to be gradually increased over the span of 4 days. Under the same conditions, no consistent increase in Mcl-1 protein was detected; only in donor 1 was Mcl-1 protein gradually increased in parallel with cIAP2 protein.
Elevated level of cIAP2 protein in neutrophils from donors receiving G-CSF administration.
Peripheral blood neutrophils were isolated from 4 healthy donors receiving subcutaneous administration of G-CSF (200 μg/m2) twice daily for allogeneic peripheral blood stem cell transplantation. At the indicated days after the start of G-CSF administration, venous blood was obtained just before the injection of G-CSF. The expression of cIAP2, Mcl-1, and p38 proteins was analyzed by immunoblotting using antibodies against each protein. The cell lysates equivalent to 3.7 × 106 cells were loaded onto each lane. Lym indicates lymphocytes (3.7 × 106 cells).
Elevated level of cIAP2 protein in neutrophils from donors receiving G-CSF administration.
Peripheral blood neutrophils were isolated from 4 healthy donors receiving subcutaneous administration of G-CSF (200 μg/m2) twice daily for allogeneic peripheral blood stem cell transplantation. At the indicated days after the start of G-CSF administration, venous blood was obtained just before the injection of G-CSF. The expression of cIAP2, Mcl-1, and p38 proteins was analyzed by immunoblotting using antibodies against each protein. The cell lysates equivalent to 3.7 × 106 cells were loaded onto each lane. Lym indicates lymphocytes (3.7 × 106 cells).
G-CSF–induced up-regulation of cIAP2 and antiapoptosis are inhibited by AG490
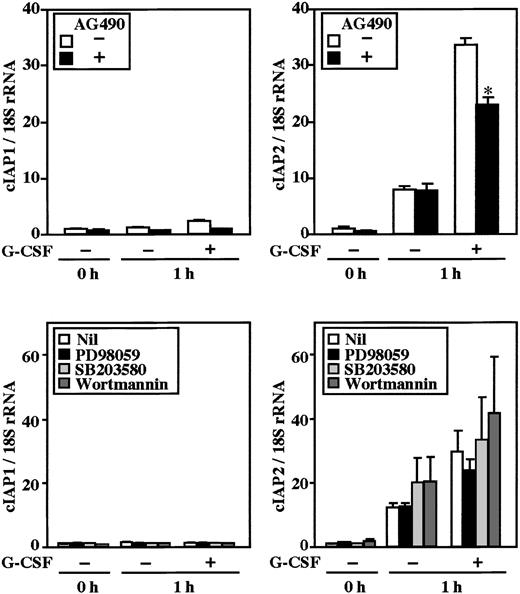
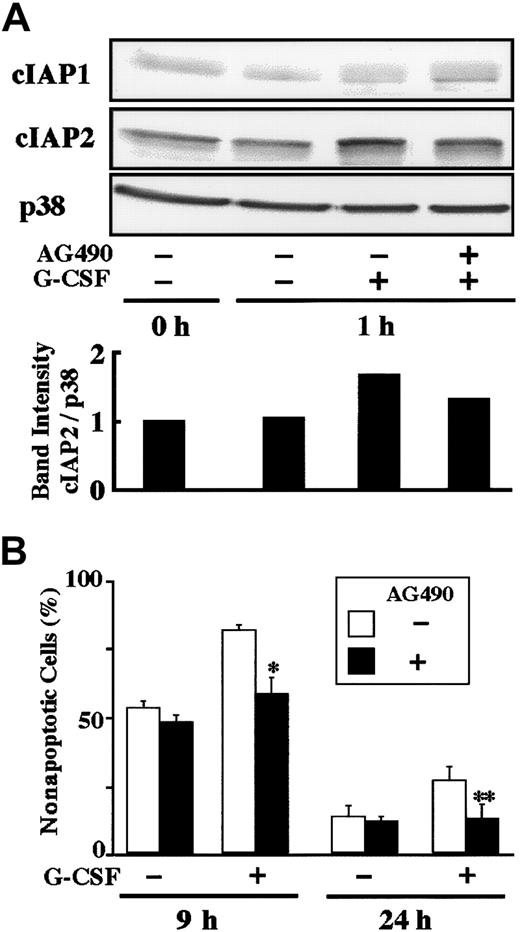
Stimulation of human neutrophils with G-CSF results in activation of the MEK-ERK and JAK-STAT3 pathways.25-27Possible involvement of these pathways in G-CSF–induced up-regulation of cIAP2 was explored using specific inhibitors for these pathways. As shown in Figure 4, G-CSF–induced up-regulation of cIAP2 mRNA was significantly inhibited by pretreatment of cells with AG490 (JAK2 inhibitor), but not with PD98059 (MEK inhibitor), SB203580 (p38 MAPK inhibitor), or wortmannin (PI3K inhibitor). None of the inhibitors affected the expression of cIAP1 mRNA. Consistent with this, G-CSF–induced up-regulation of cIAP2 protein, but not cIAP1 protein, was significantly inhibited by AG490 (Figure 5A). Furthermore, the G-CSF–induced antiapoptotic effect on human neutrophils was also significantly inhibited by AG490 (Figure 5B), but was not affected by PD98059, SB203580, or wortmannin (data not shown).
Effects of AG490, PD98059, SB203580, and wortmannin on expression of cIAP1 and cIAP2 mRNA in neutrophils stimulated by G-CSF.
Neutrophils were preincubated with AG490 (50 μM), PD98059 (50 μM), SB203580 (10 μM), or wortmannin (100 nM) for 20 minutes, and thereafter cultivated in the presence or absence of G-CSF (50 ng/mL) for 1 hour. The expression of cIAP1 and cIAP2 mRNA was analyzed by real-time PCR. As an internal control, 18S rRNA was used. The ratios of cIAP1 to 18S rRNA and cIAP2 to 18S rRNA were calculated, and the ratio at hour 0 is expressed as 1. The data are expressed as means ± standard errors of the mean of 3 to 5 experiments. *Significantly inhibited by AG490 (P < .05).
Effects of AG490, PD98059, SB203580, and wortmannin on expression of cIAP1 and cIAP2 mRNA in neutrophils stimulated by G-CSF.
Neutrophils were preincubated with AG490 (50 μM), PD98059 (50 μM), SB203580 (10 μM), or wortmannin (100 nM) for 20 minutes, and thereafter cultivated in the presence or absence of G-CSF (50 ng/mL) for 1 hour. The expression of cIAP1 and cIAP2 mRNA was analyzed by real-time PCR. As an internal control, 18S rRNA was used. The ratios of cIAP1 to 18S rRNA and cIAP2 to 18S rRNA were calculated, and the ratio at hour 0 is expressed as 1. The data are expressed as means ± standard errors of the mean of 3 to 5 experiments. *Significantly inhibited by AG490 (P < .05).
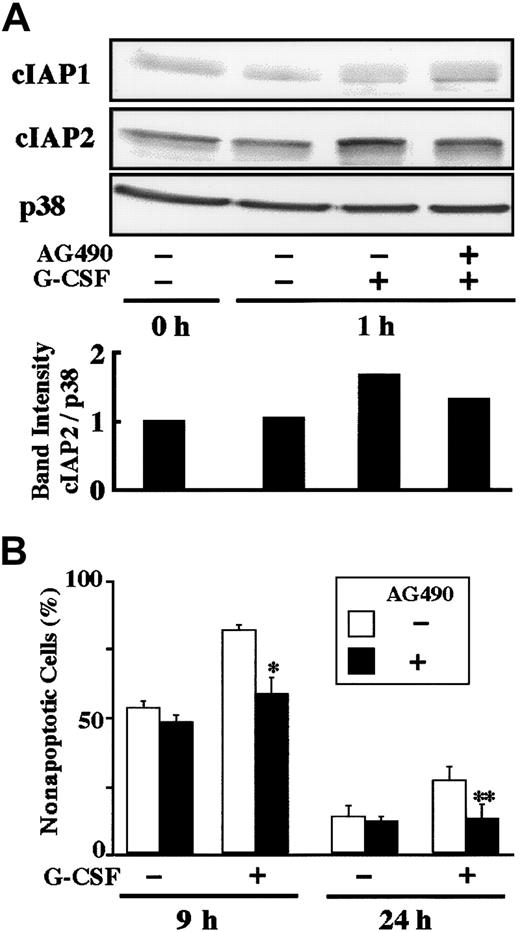
Effect of AG490 on G-CSF–induced up-regulation of cIAP2 protein and antiapoptosis.
(A) Neutrophils were preincubated with AG490 (50 μM) for 20 minutes, and thereafter cultivated in the presence or absence of G-CSF (50 ng/mL) for 1 hour. The expression of cIAP1 and cIAP2 proteins was analyzed by immunoblotting using antibodies against each protein. As a control, p38 was used. The ratio of cIAP2 to p38 band intensity was calculated, and the ratio at hour 0 is expressed as 1 (lower panel). The cell lysates equivalent to 2.5 × 106 cells were loaded onto each lane. The results shown are representative of 3 independent experiments. (B) Neutrophils were preincubated with AG490 (50 μM) for 20 minutes, and thereafter cultivated in the presence or absence of G-CSF (50 ng/mL). After cultivation for 9 or 24 hours, apoptotic cells were determined by flow cytometry. The data are expressed as means ± standard deviations of 3 experiments. * and ** indicate significantly inhibited by AG490 (*P < .05; **P < .01).
Effect of AG490 on G-CSF–induced up-regulation of cIAP2 protein and antiapoptosis.
(A) Neutrophils were preincubated with AG490 (50 μM) for 20 minutes, and thereafter cultivated in the presence or absence of G-CSF (50 ng/mL) for 1 hour. The expression of cIAP1 and cIAP2 proteins was analyzed by immunoblotting using antibodies against each protein. As a control, p38 was used. The ratio of cIAP2 to p38 band intensity was calculated, and the ratio at hour 0 is expressed as 1 (lower panel). The cell lysates equivalent to 2.5 × 106 cells were loaded onto each lane. The results shown are representative of 3 independent experiments. (B) Neutrophils were preincubated with AG490 (50 μM) for 20 minutes, and thereafter cultivated in the presence or absence of G-CSF (50 ng/mL). After cultivation for 9 or 24 hours, apoptotic cells were determined by flow cytometry. The data are expressed as means ± standard deviations of 3 experiments. * and ** indicate significantly inhibited by AG490 (*P < .05; **P < .01).
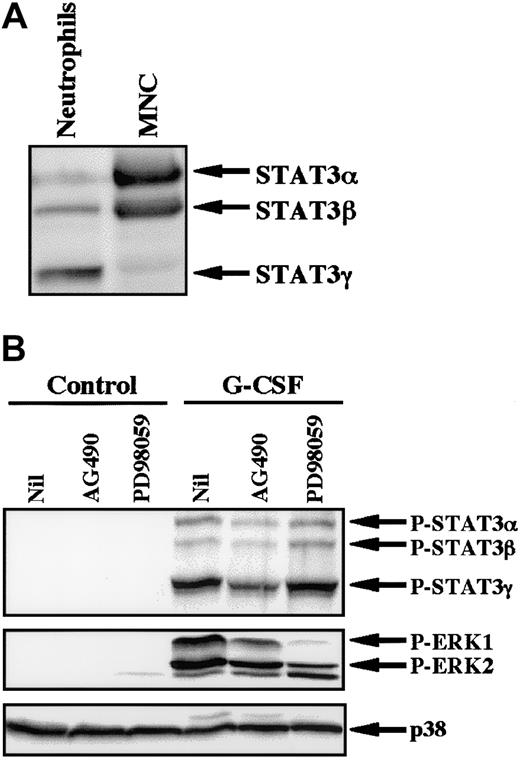
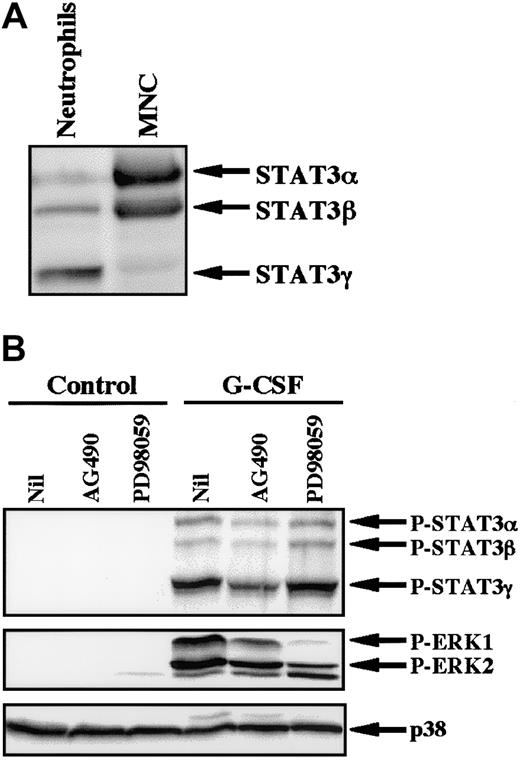
Because AG490 is a potent inhibitor of JAK2,28 we studied the effect of AG490 on phosphorylation of STAT3 in neutrophils stimulated by G-CSF. In human neutrophils, 3 isoforms of STAT3 (STAT3α, β, and γ) were detected, with STAT3γ being predominantly expressed at the protein level (Figure6A).27 This profile contrasts with that of mononuclear cells, in which STAT3α was predominantly expressed. STAT3γ may be derived from STAT3α by limited proteolysis and lacks the C-terminal portion of STAT3α.27 G-CSF induced tyrosine phosphorylation of all isoforms of STAT3, with STAT3γ being predominantly phosphorylated. G-CSF–induced phosphorylation of STAT3 was significantly inhibited by pretreatment of cells with AG490, but not with PD98059, indicating that the JAK2-STAT3 pathway is activated in neutrophils stimulated by G-CSF (Figure 6B). In addition to STAT3, stimulation of neutrophils with G-CSF resulted in phosphorylation of ERK, which was also slightly inhibited by AG490 and was markedly inhibited by PD98059 (Figure6B).26 In considering that PD98059 had no effect on G-CSF–induced up-regulation of cIAP2 mRNA (Figure 4), these findings indicate that the JAK2-STAT3 pathway, but not the MEK-ERK pathway, may be involved in G-CSF–induced up-regulation of cIAP2 in neutrophils.
Effects of AG490 and PD98059 on phosphorylation of STAT3 and ERK in neutrophils stimulated by G-CSF.
(A) Differential expression of STAT3 isoforms in neutrophils and mononuclear cells (MNC). The cell lysates from an equal number of cells (2.5 × 106) were analyzed by immunoblotting using antibody against STAT3. The results shown are representative of 3 independent experiments. (B) Neutrophils were preincubated with AG490 (50 μM) for 20 minutes, and thereafter stimulated with G-CSF (50 ng/mL) for 10 minutes at 37°C. The cell lysates from an equal number of cells (2.8 × 106) were analyzed by immunoblotting using antibodies against phosphorylated forms of STAT3 and ERK. The equal loading of proteins onto each lane was confirmed by immunoblotting using antibody against p38. The results shown are representative of 3 independent experiments.
Effects of AG490 and PD98059 on phosphorylation of STAT3 and ERK in neutrophils stimulated by G-CSF.
(A) Differential expression of STAT3 isoforms in neutrophils and mononuclear cells (MNC). The cell lysates from an equal number of cells (2.5 × 106) were analyzed by immunoblotting using antibody against STAT3. The results shown are representative of 3 independent experiments. (B) Neutrophils were preincubated with AG490 (50 μM) for 20 minutes, and thereafter stimulated with G-CSF (50 ng/mL) for 10 minutes at 37°C. The cell lysates from an equal number of cells (2.8 × 106) were analyzed by immunoblotting using antibodies against phosphorylated forms of STAT3 and ERK. The equal loading of proteins onto each lane was confirmed by immunoblotting using antibody against p38. The results shown are representative of 3 independent experiments.
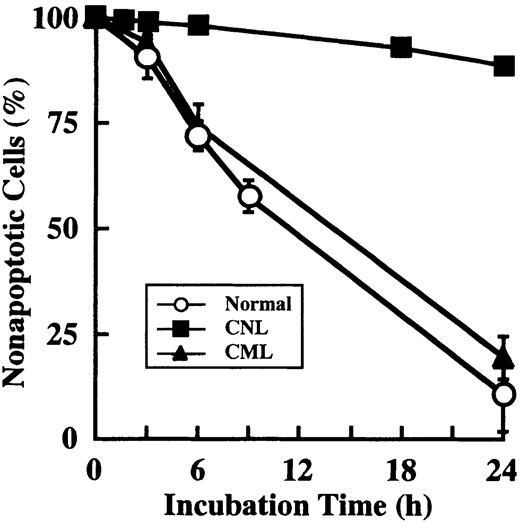
Prolonged survival and overexpression of cIAP2 mRNA in CNL neutrophils
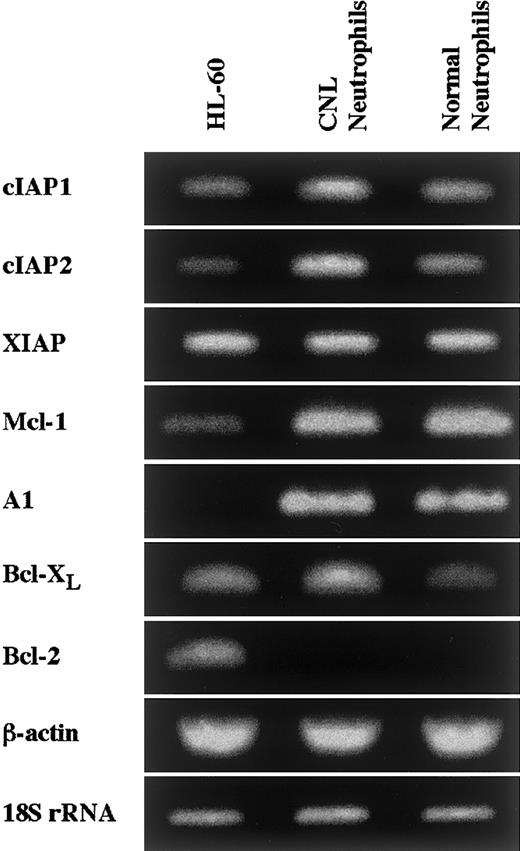
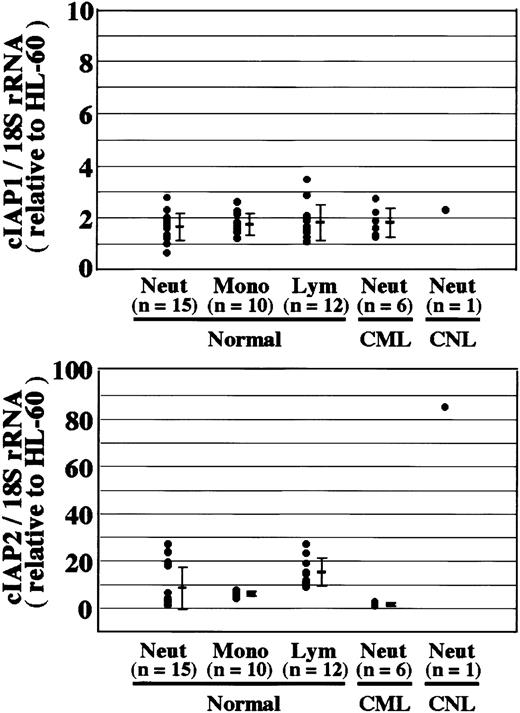
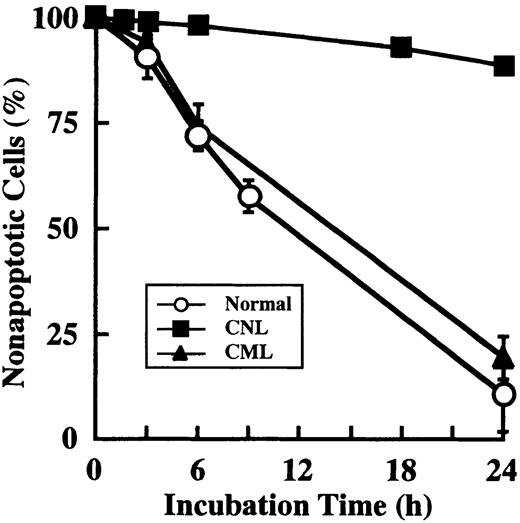
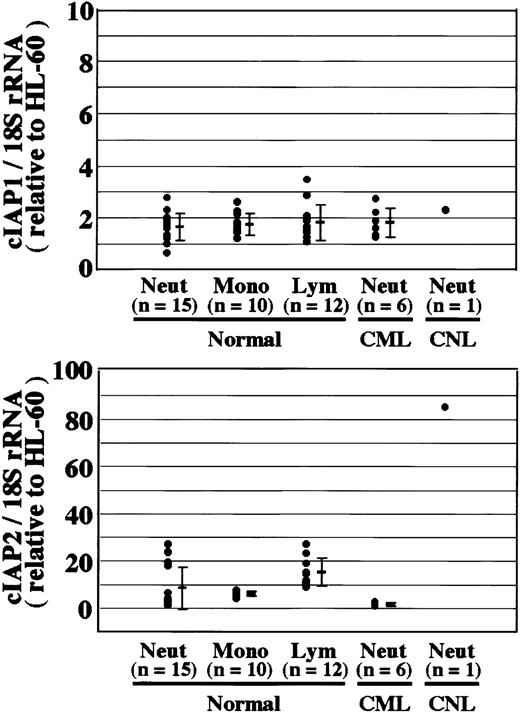
Neutrophils from a patient with CNL showed prolonged survival as compared with normal and CML neutrophils (Figure7), in agreement with a recent report.29 The survival rates in normal and CML neutrophils were essentially similar to each other. Prolonged neutrophil survival might be caused by altered expression of genes associated with apoptosis. Therefore, the expression of apoptosis-related gene transcripts was analyzed by semiquantitative RT-PCR. As shown in Figure8, among the transcripts examined, cIAP2 mRNA was apparently overexpressed in neutrophils from this patient as compared with normal neutrophils. The expression of XIAP, Mcl-1, and A1 in CNL neutrophils was essentially the same as that in normal neutrophils, whereas the expression of cIAP1 and Bcl-XL in CNL neutrophils appeared to be slightly greater than that in normal neutrophils. Bcl-2 was not expressed in CNL neutrophils or normal neutrophils. To assess more quantitatively the increased expression of cIAP2 mRNA in CNL neutrophils, we performed real-time PCR analysis using 18S rRNA as an internal control. As shown in Figure9, a marked increase in cIAP2 mRNA expression was found in neutrophils from this CNL patient as compared with normal and CML neutrophils. The expression of cIAP1 mRNA in neutrophils from this patient was essentially the same as that in normal and CML neutrophils. On the other hand, the expression of cIAP1 and cIAP2 mRNA in CML neutrophils was the same as that in normal neutrophils. In addition, the expression levels of cIAP1 and cIAP2 mRNA in normal neutrophils, monocytes, and lymphocytes were similar to one another when the ratios of cIAP1 to 18S rRNA and cIAP2 to 18S rRNA were used for comparison (Figure 9).
Prolonged survival of CNL neutrophils.
Neutrophils were prepared from 1 patient with CNL, 3 patients with CML, and 3 healthy volunteers. After cultivation for the indicated periods, apoptotic cells were determined by flow cytometry. The data for CML and normal neutrophils are expressed as means ± standard deviations of 3 experiments, and the data for CNL neutrophils are expressed as means of a single experiment.
Prolonged survival of CNL neutrophils.
Neutrophils were prepared from 1 patient with CNL, 3 patients with CML, and 3 healthy volunteers. After cultivation for the indicated periods, apoptotic cells were determined by flow cytometry. The data for CML and normal neutrophils are expressed as means ± standard deviations of 3 experiments, and the data for CNL neutrophils are expressed as means of a single experiment.
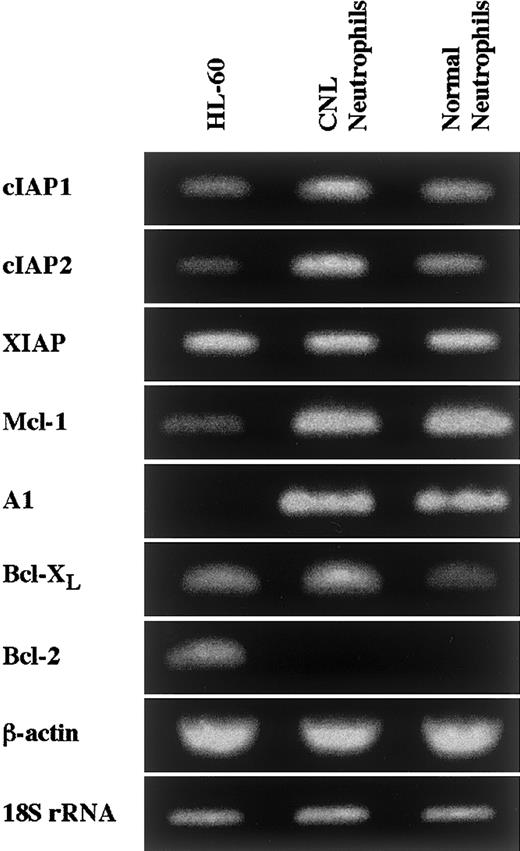
Expression of apoptosis-related gene transcripts in CNL neutrophils.
The expression of cIAP1, cIAP2, XIAP, Mcl-1, A1, Bcl-XL, and Bcl-2 mRNA in HL-60 cells, CNL neutrophils, and normal neutrophils was analyzed by semiquantitative RT-PCR. As internal controls, β-actin and 18S rRNA were used. The results shown are representative of 3 independent experiments.
Expression of apoptosis-related gene transcripts in CNL neutrophils.
The expression of cIAP1, cIAP2, XIAP, Mcl-1, A1, Bcl-XL, and Bcl-2 mRNA in HL-60 cells, CNL neutrophils, and normal neutrophils was analyzed by semiquantitative RT-PCR. As internal controls, β-actin and 18S rRNA were used. The results shown are representative of 3 independent experiments.
Overexpression of cIAP2 mRNA in CNL neutrophils.
The expression of cIAP1 and cIAP2 mRNA in HL-60 cells, normal neutrophils (Neut), monocytes (Mono), lymphocytes (Lym), and CML and CNL neutrophils was analyzed by real-time PCR. As an internal control, 18S rRNA was used. The ratios of cIAP1 to 18S rRNA and cIAP2 to 18S rRNA were calculated for each cell type, and the data are expressed as the values relative to those of HL-60 cells for proper comparison. The experiments were performed on samples from individual donors, and the number of donors (n) is indicated. In this experiment, 15 healthy donors were used for the analysis of normal neutrophils.
Overexpression of cIAP2 mRNA in CNL neutrophils.
The expression of cIAP1 and cIAP2 mRNA in HL-60 cells, normal neutrophils (Neut), monocytes (Mono), lymphocytes (Lym), and CML and CNL neutrophils was analyzed by real-time PCR. As an internal control, 18S rRNA was used. The ratios of cIAP1 to 18S rRNA and cIAP2 to 18S rRNA were calculated for each cell type, and the data are expressed as the values relative to those of HL-60 cells for proper comparison. The experiments were performed on samples from individual donors, and the number of donors (n) is indicated. In this experiment, 15 healthy donors were used for the analysis of normal neutrophils.
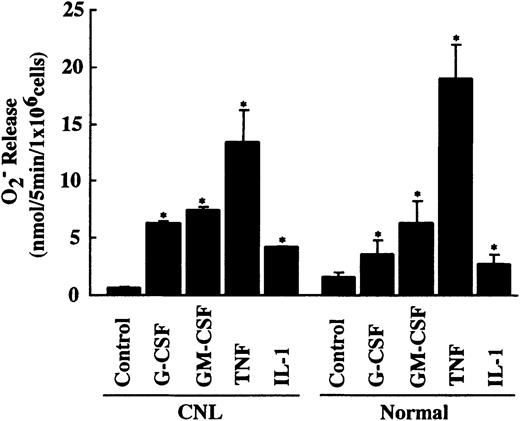
Overexpression of cIAP2 mRNA in neutrophils from this CNL patient might be caused by in vivo activation by inflammatory cytokines such as G-CSF. However, this possibility is unlikely because the patient was afebrile and the serum concentrations of G-CSF and GM-CSF were below the detectable levels when examined. To obtain additional evidence indicating that neutrophils from this patient were not activated in vivo by inflammatory cytokines, we measured FMLP-induced O release using the same neutrophil preparation as that used for the real-time PCR analysis. If neutrophils were activated or primed in vivo by inflammatory cytokines, it would be expected that these primed neutrophils would show increased release of O in response to FMLP stimulation and decreased responsiveness to further in vitro priming by inflammatory cytokines.30,31 As shown in Figure10, CNL neutrophils released a normal amount of O in response to FMLP stimulation and showed normal responsiveness to in vitro priming by G-CSF, GM-CSF, TNF, and IL-1β. These findings indicate that CNL neutrophils are unlikely to be activated or primed in vivo by inflammatory cytokines. These characteristics in CNL neutrophils also contrast with those observed in CML neutrophils, which show increased O release in response to FMLP stimulation and decreased responsiveness to in vitro priming by inflammatory cytokines.32 Further analysis of neutrophils from this CNL patient could not be performed because of sudden onset of fatal infection.
Priming effect of cytokines on FMLP-induced O release in CNL and normal neutrophils.
CNL and normal neutrophils (1 × 106/mL) were preincubated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), TNF (100 U/mL), or IL-1β (300 U/mL) for 10 minutes at 37°C before FMLP (10−7 M) was added. The data for CNL neutrophils are expressed as means ± standard deviations of a single experiment done in triplicate, and the data for normal neutrophils are expressed as means ± standard deviations of 5 experiments. *Significantly increased as compared with control cells (P < .01).
Priming effect of cytokines on FMLP-induced O release in CNL and normal neutrophils.
CNL and normal neutrophils (1 × 106/mL) were preincubated with G-CSF (50 ng/mL), GM-CSF (5 ng/mL), TNF (100 U/mL), or IL-1β (300 U/mL) for 10 minutes at 37°C before FMLP (10−7 M) was added. The data for CNL neutrophils are expressed as means ± standard deviations of a single experiment done in triplicate, and the data for normal neutrophils are expressed as means ± standard deviations of 5 experiments. *Significantly increased as compared with control cells (P < .01).
Discussion
The present experiments show that human neutrophils express members of the IAP family, including XIAP, cIAP1, and cIAP2. Among these members, the expression of cIAP2 mRNA and protein was selectively up-regulated by stimulation with G-CSF, but not with GM-CSF. G-CSF–induced up-regulation of cIAP2 protein was also observed in vivo; that is, the elevated level of cIAP2 protein was detected in peripheral blood neutrophils from donors receiving G-CSF administration. G-CSF–induced up-regulation of cIAP2 mRNA and protein, phosphorylation of STAT3, and antiapoptosis were all inhibited by AG490. These findings support the concept that cIAP2 expression is regulated by G-CSF through activation of the JAK2-STAT3 pathway, and up-regulation of cIAP2 protein plays an important role in the G-CSF–induced antiapoptotic effect on human neutrophils. This concept is also consistent with our recent observations that protein synthesis is absolutely required for the G-CSF–induced antiapoptotic effect on human neutrophils.1
It has been reported that cIAP2 is inducible by a variety of NF-κB–inducing stimuli, including TNF, IL-1β, CD40, lipopolysaccharide, and etoposide, in multiple cell lines, whereas induction of cIAP1 and XIAP appears to be cell type– and stimulus-dependent.33,34 In fact, we observed that TNF induced cIAP2 mRNA expression in neutrophils with the same potency as G-CSF (data not shown), a finding consistent with the report that NF-κB is activated in neutrophils stimulated by TNF.35In contrast, it has been reported that NF-κB is not activated in neutrophils stimulated by G-CSF or GM-CSF.35 The results presented here suggest that G-CSF up-regulates cIAP2 through, at least in part, activation of the JAK2-STAT3 pathway. It has been reported that p38 is involved in CD40-induced cIAP2 expression in dendritic cells, but not in B cells.36 In addition, it has been demonstrated that survivin expression in normal human CD34+cells and myeloid leukemia cells is increased by stimulation with hematopoietic growth factors, such as thrombopoietin, Flt3 ligand, stem cell factor, G-CSF, and GM-CSF; and MEK or PI3K may be involved in GM-CSF–induced up-regulation of survivin.18,19 The expression of XIAP and cIAP1 in granulosa cells is also induced by stimulation with gonadotropin.37 These findings suggest that the expression of the IAP family members may be regulated by different mechanisms, depending on the cells and the stimuli used. The differential regulation of cIAP2 by G-CSF and GM-CSF may be ascribed to the qualitative and/or quantitative differences in the signaling pathways activated by G-CSF and GM-CSF stimulation. In fact, ERK and p38 are differentially activated by these cytokines,26 and neutrophil alkaline phosphatase transcript is induced by G-CSF, but not by GM-CSF, stimulation,38 even though G-CSF and GM-CSF share several signaling pathways. It is likely that the expression of genes is variously affected by the qualitative and/or quantitative differences in the signaling molecules activated at the same time.
The level of Mcl-1 protein was not consistently increased in peripheral blood neutrophils isolated from donors receiving G-CSF administration or in neutrophils cultivated with G-CSF in vitro (data not shown). These findings suggest that Mcl-1 is unlikely to play a primary role in G-CSF–mediated regulation of neutrophil survival. The elevated level of cIAP2 protein observed in neutrophils from donors receiving G-CSF administration is consonant with the fact that neutrophil survival is prolonged after G-CSF administration39 and indicates a close relationship between G-CSF–induced antiapoptosis and cIAP2 protein, but not Mcl-1 protein. The gradual increase in the level of cIAP2 protein in vivo over the span of 4 days might reflect the effect of G-CSF on immature myeloid cells. These findings are also in agreement with a recent study by Maianski et al,11which showed no correlation between the expression of Bcl-2–related proteins (Mcl-1, Bcl-XL, Bak, and Bax) and neutrophil survival.
It is of interest that cIAP1 and cIAP2 proteins are differentially expressed in neutrophils, monocytes, and lymphocytes, suggesting a cell type–specific role of these molecules. Strong expression of cIAP1 protein in monocytes suggests that cIAP1 as well as cIAP2 plays an important role in regulation of monocyte survival. The IAP family proteins have been shown to suppress apoptosis induced by a variety of stimuli, including TNF, Fas, etoposide, and growth factor withdrawal.15 These proteins have been shown to inhibit active caspase-3 and caspase-7 directly and to inhibit activation of pro–caspase-9.15 Activation of caspase-3 and caspase-8 has been demonstrated in human neutrophils undergoing apoptosis.40 Furthermore, it has been reported that cIAP1 and cIAP2 are recruited to TNF receptors by binding to TNF receptor–associated factor-1 (TRAF-1)/TRAF-2 heterocomplexes and function to suppress caspase-8 activation.34 These findings, taken together, suggest that cIAP2 up-regulated by G-CSF may contribute to the G-CSF–induced antiapoptotic effect on human neutrophils by inhibiting caspase-8 activation and/or caspase-3 activity. This concept is consonant with our recent study showing that G-CSF may prolong neutrophil survival by inhibiting activation of the caspase cascade.1 It has been reported recently that spontaneous neutrophil apoptosis is associated with the translocation of Bax to mitochondria, and that this can be prevented by G-CSF.11 A possible relationship between cIAP2 and the translocation of Bax remains to be determined.
CNL is a rare form of a clonal myeloproliferative disorder characterized by sustained neutrophilia.23 CNL neutrophils were found to exhibit prolonged survival.29 This characteristic contrasts well with that of CML neutrophils, the survival of which was essentially similar to that of normal neutrophils. These findings imply that the underlying mechanism for sustained neutrophilia in CNL may be different from that in CML. Among the apoptosis-related gene transcripts examined, cIAP2 mRNA was markedly overexpressed in neutrophils from a patient with CNL. These findings suggest that sustained neutrophilia in some cases of CNL may be partly caused by prolonged neutrophil survival, which may result from the overexpression of cIAP2. It remains to be determined why cIAP2 mRNA was overexpressed in this patient. When examined, the patient was afebrile and the serum concentrations of G-CSF and GM-CSF were below the detectable levels. Furthermore, the study with neutrophil functions indicated that neutrophils are unlikely to be activated or primed in vivo by inflammatory cytokines. These findings may exclude the possibility that cIAP2 overexpression is induced by inflammatory cytokines such as G-CSF and TNF, and rather suggest that it may be caused by intrinsic dysregulation of cIAP2 expression. The present experiment and previous reports33,34 raise the possibility that the overexpression of cIAP2 could be induced by constitutive activation of STAT3 or NF-κB. In this regard, it is of interest that NF-κB has been reported recently to be constitutively activated in primitive human AML cells.41 Further studies with many cases are required to establish the possible pathogenetic role of dysregulation of cIAP2 expression in CNL and the underlying mechanisms for the overexpression of cIAP2.
Prepublished online as Blood First Edition Paper, October 3, 2002; DOI 10.1182/blood-2002-05-1505.
Supported by a Grant-in-Aid for Scientific Research, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Seiichi Kitagawa, Department of Physiology, Osaka City University Medical School, Asahi-machi, Abeno-ku, Osaka 545-8585, Japan; e-mail:kitagawas@med.osaka-cu.ac.jp.