Increased angiogenesis is a feature of many solid tumors but also has been observed in hematological malignancies, including chronic myeloid leukemia (CML), where vessel density is increased approximately 2-fold over normal controls.1 Elevated plasma concentrations of vascular endothelial growth factor (VEGF) also were demonstrated,2 and high bone marrow VEGF levels may be associated with a poor prognosis.3 A recent study showed that Bcr-Abl tyrosine kinase activity induces VEGF via a pathway that involves phosphatidyl inositol 3 kinase and mTOR.4 These findings were corroborated by data that showed dose-dependent down-regulation of VEGF in BCR-ABL–positive cell lines upon treatment with imatinib.5 These results suggest that imatinib treatment of CML patients may normalize bone marrow vascularity, but no data presently are available.
We studied blood vessel density in 18 CML patients in first chronic phase prior to imatinib therapy. All patients were treated within multicenter trials,6,7 where bone marrow biopsies for follow-up were optional. Of the patients, 12 patients were newly diagnosed, while 2 were cytogenetically resistant to and 4 intolerant of interferon-alpha. All patients achieved or maintained complete hematological remission on imatinib. Follow-up biopsies after 3, 6, and 12 months of imatinib therapy are available for 12, 16, and 7 patients, respectively. Serving as controls were 19 biopsies without pathological findings. After decalcification, blood vessels in paraffin-embedded sections were stained with anti-CD34 monoclonal antibody (Immunotech, Marseille, France). Values represent the median number of blood vessels in 5 randomly chosen fields (magnification, 200-fold). The Mann-Whitney U test was used to compare median blood vessel numbers between groups of patients and the Wilcoxon test to compare relative changes over time (relative blood vessel density in percent of initial values).
Compared to normal controls (median, 4; range, 0-15), blood vessel density was significantly increased in the CML patients (median, 13; range, 1-74, P = .001), confirming earlier studies.1,2 After 3 months on imatinib, the median blood vessel density had decreased to 7 (range, 1-19) (P = .036) vessels per field and the median relative density to 56% (range, 17-200) (P = .002) of the initial values (Table 1). After 6 months (Figure 1), there was a further reduction to a median of 4 (range, 0-13) vessels per field (P = .001 for comparison with initial values) or 22% (range, 0-250) of initial values (P = .001). If only patients with markedly increased median vessel density (> 8 per field) prior to imatinib (which eliminates potential mistakes as a result of small numbers) are considered, then 11 of 11 patients evaluable at 6 months showed a reduction to a median of 22% (range, 6-72) of initial values. Few patients had biopsies at 12 months. However, there was a significant increase in vessel density at 12 months in 2 patients (nos. 5, 9), without evidence for a cytogenetic or hematological relapse. Cytogenetic response and reduction of vascularity were generally not correlated. Although 2 patients (nos. 8, 13) were 100% Ph positive at 3 months, their vessel density was reduced to 24% and 17%, respectively. By contrast, in 2 other patients (nos. 7, 12), blood vessel density at 3 months had not decreased (136% and 100% of initial values, respectively), but both had entered major cytogenetic response (9% and 25% Ph-positive metaphases, respectively). The finding of a vascular response in the absence of a cytogenetic response can be explained, since down-regulation of VEGF expression by imatinib may occur in the absence of cell death. In addition, vascular effects of imatinib that are mediated by inhibition of the platelet-derived growth factor receptor8 may play a role. By contrast, the occurrence of a cytogenetic response without vascular response is puzzling. Both patients subsequently achieved complete cytogenetic response at 12 months, but no biopsies are available from this date.
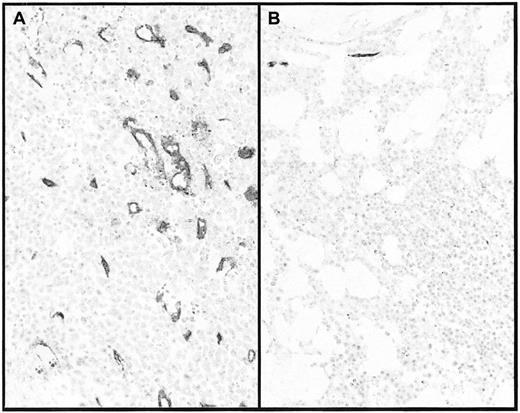
Blood vessels before and after imatinib treatment. Blood vessels in patient no. 2 prior to (A) and after (B) 6 months on imatinib. After decalcification, paraffin-embedded sections were stained with anti-CD34 monoclonal antibody. Note also the marked reduction in cellularity. Magnification × 200.
Blood vessels before and after imatinib treatment. Blood vessels in patient no. 2 prior to (A) and after (B) 6 months on imatinib. After decalcification, paraffin-embedded sections were stained with anti-CD34 monoclonal antibody. Note also the marked reduction in cellularity. Magnification × 200.
In conclusion, imatinib normalizes bone marrow vascularity in most CML patients in chronic phase, without clear correlation to cytogenetic response. Studies in larger cohorts of patients are needed to clarify whether failure to normalize vascularity on imatinib has prognostic significance. Furthermore, there may be a therapeutic role for angiogenesis inhibitors in CML: a complete cytogenetic response was seen in a CML patient with myeloid blast crisis treated with a monoclonal antibody against VEGF (Bevacizumab) in combination with chemotherapy,9 and disease stabilization was observed in several blast crisis patients treated with SU5416, a combined VEGF receptor and KIT inhibitor.10 Given the notorious refractoriness of blast crisis, these results are encouraging enough to justify further studies of angiogenesis inhibitors in CML. Combining imatinib with such agents may hold particular promise for patients with advanced disease or for those who fail to normalize bone marrow vascularity with imatinib alone.