Abstract
Myeloma cells express the idiotype (Id)–specific antigen that may be targeted by Id vaccination. Six patients with stage I IgG myeloma were immunized with the autologous purified M component together with the adjuvant cytokines interleukin 12 (IL-12) alone or in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF). The effect of Id vaccination on circulating clonal tumor B cells was monitored by a real-time allele-specific oligonucleotide polymerase chain reaction method. No other treatment was given. Reduction of blood tumor mass was observed in 4 of 6 patients, with one patient achieving a complete molecular remission in blood. In 3 of these 4 patients an Id-specific T-cell response was induced. In the remaining 2 patients with an unchanged level of blood tumor cells, one patient mounted a T-cell response, whereas the other did not. No significant change in the serum M protein level was noted. Id vaccination may target clonal B cells, suggesting that this strategy might be conducive to achieving tumor control. The clinical significance of these findings remains to be established.
Introduction
Although a high response rate is achieved in patients with multiple myeloma (MM) by high-dose chemotherapy, the management of patients with MM remains a problem. Complementary therapeutics are needed to cure or control the disease. The idiotype (Id) of the myeloma immunoglobulin (Ig) is regarded as a tumor-specific antigen and a target for immunotherapy.
Naturally occurring major histocompatibility complex (MHC)–restricted myeloma Id-specific T cells have been identified.1 Such T cells may target Id Ig peptide/MHC complexes on myeloma cells and might lyse tumor cells.2,3 Id vaccination may therefore be a treatment approach to induce a therapeutic Id-specific T-cell response.2, 3, 4 The concept of Id immunization in MM was introduced about 30 years ago.5 The Id seems, however, to be a weak immunogen; to evoke a strong response, adjuvant cytokines should be added. Granulocyte-macrophage colony-stimulating factor (GM-CSF) augments the functional capacity of antigen-presenting cells and interleukin 12 (IL-12) amplifies the immune response and leads to a type 1 T-cell response.4 A TH1-type cellular response seems to be of importance for tumor regression, whereas a TH2 T-cell response might provide a microenvironment conducive to disease progression.6
Id vaccination in malignant lymphomas induced Id-specific T cells and complete blood molecular remission.7 In MM, Id vaccination evoked an Id-specific T-cell response and occasionally clinical effects such as reduction of serum M component and disease stabilization.8
The clonal cell compartment in myeloma represents an ongoing differentiating cell population9 including preswitch cells.10,11 The number of preplasma cells seems to be related to disease burden and clinical outcome.12 Clonal preplasma cells express MHC class I and II molecules and plasma cells, mainly MHC class I molecules with Id Ig peptides in the groove and may thus be targeted by Id-specific T cells.
In this study, we monitored peripheral blood (PB) tumor cells by a real-time allele-specific oligonucleotide (ASO) polymerase chain reaction (PCR) method in MM patients undergoing Id vaccination and could show that immunization, using the autologous myeloma Id, induced reduction in PB tumor mass.
Study design
Patients with MM stage I and an increased serum M-component concentration were included. Ten patients were considered for the study. In 6 of 10 patients we were able to monitor the level of clonal cells using complementarity-determining region 3 (CDR3)–specific PCR assays; results from these 6 patients are included in this report. The study was approved by the Ethical Committee of the Karolinska Institute.
The M component was purified as described.4 The patients were immunized with the autologous Id together with IL-12 with or without GM-CSF as follows: (1) In the IL-12/GM-CSF group, 0.5 mg of the autologous M component in 0.5% aluminum phosphate (SBL-Vaccin, Solna, Sweden) was given intracutaneously on day 1 together with 75 μg/d GM-CSF (Leucomax; Schering-Plough, Kenilworth, NJ) intracutaneously at the same site, days 1 to 4, and 30 ng/kg IL-12 subcutaneously day 1 (Wyeth, London, United Kingdom). (2) In the IL-12 group, 0.5 mg of the M component was given intracutaneously on day 1 and 30 ng/kg IL-12 subcutaneously on day 1. Immunizations were done at weeks 0, 2, 4, 6, 8, 14, and 30.
Two methods were applied to detect Id-specific T cells: a proliferation assay (3H-thymidine incorporation) and ELISPOT (interferon-γ [IFN-γ]). The techniques have been described in detail elsewhere.4
Briefly, for the proliferation assay peripheral blood mononuclear cells (PBMCs) were stimulated with purified F(ab′)2 fragments of the autologous monoclonal IgG (1 pg/mL to 100 μg/mL) as well as with 3 allogeneic monoclonal isotype-matched IgGs from other myeloma patients and cultured for 6 days. 3H-thymidine was added during the last 18 hours. Tests were run in triplicate, and mean reactivity was calculated for each triplicate. For each testing time, the highest mean value of a triplicate obtained from the 5 different concentrations of the Id was used. A stimulation index (SI) was calculated by dividing the mean radioactivity for a triplicate of stimulated cells by that of unstimulated cells.
Briefly, for the IFN-γ ELISPOT, wells of nitrocellulose-bottomed microtiter plates were coated with a mouse monoclonal antihuman IFN-γ (Mabtech, Stockholm, Sweden) at 4°C overnight. After removal of the coating solution, the plates were washed twice in phosphate-buffered saline (PBS). Subsequently, 100-μL aliquots of freshly prepared PBMCs (106 cells/mL) were added to each well. Cells were incubated with F(ab′)2 fragments of the autologous or isotypical monoclonal IgG (1 pg/mL to 100 μg/mL) for 30 hours. To visualize spots corresponding to single IFN-γ–secreting cells, the cells were washed with PBS and the wells were incubated with a rabbit monoclonal antihuman IFN-γ at 37°C for 2 hours. After washing, biotinylated antirabbit IgG (Vector Lab, Burlingame, CA) was added, followed by avidin-biotin-peroxidase complex (ABC, Vectastin elite kit, Vector Lab) for 50 minutes. After peroxidase staining using the substrate 3-amino-9-ethyl-carbasol (Sigma, St Louis, MO), the number of spots corresponding to cells secreting IFN-γ was quantified using an automatic device (Zeiss-Kantron, Jena, Germany). The results are expressed as mean number of spots (cells) of duplicate wells after subtraction of the mean number of spots of unstimulated cells. The highest value obtained by any of the concentration is used. A patient was considered to have developed Id-specific T cells if a response was observed at 2 different time points in at least one of the 2 test (read-out) systems. To establish a cutoff level for an Id-specific T-cell response, lymphocytes of the myeloma patients were stimulated with purified irrelevant M components. An SI of equal to or more than 3.1 (1.21 + 1.88 [mean + 2 SD] of 131 control experiments) was considered a positive response. In the IFN-γ ELISPOT assay at least 10 cells/105 PBMCs was considered a positive response (1.16 + 7.76 [mean + 2 SD] of 213 control experiments). Both cutoff levels are in line with our previous results.1,4 The patients were tested at weeks 0, 4, 8 or 10, 14, or 16 and 30, respectively.
For the generation of ASO primers and probes, the VH gene sequence was determined by reverse transcription–PCR (RT-PCR) using consensus VH family primers.13 Patient-specific quantitative real-time PCR analyses were performed as previously described.14 Briefly, real-time PCRs contained a DNA sample, 10 × PCR buffer A (Tris-HCl, pH 8.4, KCl, BSA; 5 μL), MgCl2 (optimized for each ASO assay), 200 μM each deoxyadenosine triphosphate (dATP), deoxycytidine triphosphate (dCTP), deoxyguanosine triphosphate (dGTP), 400 μM deoxyuridine triphosphate (dUTP), 1.25 U AmpliTaq gold DNA polymerase, 0.5 U AmpErase uracil N-glycosylase (UNG), and concentration of ASO primer; JH primer (5′-ACCTGAGGAGACGGTGACCAG-3′) and ASO TaqMan probe were optimized for each ASO assay. For the β-actin assay, forward and reverse primer concentrations were 0.3 μM (each) and the β-actin probe concentration was 0.2 μM. Reaction volumes were adjusted to 50 μL with dH2O. For generation of a standard curve, the TaqMan DNA template reagents kit containing DNA of known concentrations was used. All PCR consumables except the oligonucleotides previously mentioned were supplied by PE Applied Biosystems (Foster City, CA). The thermal cycling conditions included 2 minutes at 50°C and 10 minutes at 95°C followed by 45 cycles of 95°C for 0.15 minutes and 58°C for 1 minute. All reactions were performed in the ABI PRISM 7700 Sequence Detector (PE Applied Biosystems). Collection and analysis of data were done with Sequence Detector Software version 1.6 (PE Applied Biosystems). Blood samples were obtained at weeks 0, 8, 14, and 30 for the assessment of the levels of clonal B cells. For each sample, 6 aliquots with approximately 1 μg DNA were subjected to analysis, 3 samples for quantitation of β-actin and 3 samples for clonal cells. Nested ASO RT-PCR including approximately 0.5 μg total RNA was performed as described previously.13
Results and discussion
For the development of an Id vaccination strategy in MM patients we are focusing on stage I based on the observation that patients with early-stage MM may naturally exhibit mainly type I Idspecific T cells, whereas in late-stage, type II Id-specific T cells seemed to prevail.1 In the present study, half of the patients had an Id-specific proliferative T-cell response before vaccination, which is in agreement with our earlier observations.15 In stage I, the general T-cell functions also seem to be well preserved (F. Mozzafari, unpublished data, January 2003).
In 8 of 10 of the stage I patients considered for the study, we were able to identify the myeloma-specific immunoglobulin heavy chain gene (IgH) DNA sequence. For these 8 patients we designed a patient-specific ASO primer and a patient-specific dual-labeled fluorogenic probe (ASO TaqMan probe) corresponding to CDR3 (Table 1). The CDR3 primers were tested on a panel of DNA from different patients, ensuring that the primers amplified only an IgH sequence from the patient of interest. The forward CDR3 primer was designed to span the V-D junction, and the CDR3 TaqMan probe was designed to span the D-J junction giving maximum specificity (Table 1).
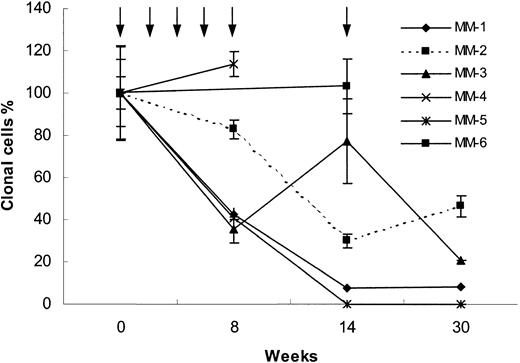
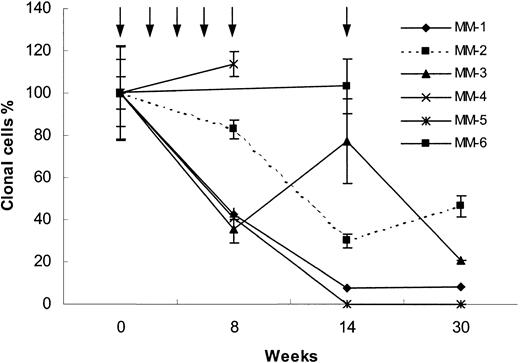
Sequential analyses of circulating blood myeloma B cells were performed (Figure 1). In 2 of 8 patients, circulating clonal tumor cells were detectable by nested ASO RT-PCR, but below reliable quantitation by real-time ASO PCR. The sensitivity of the real-time ASO PCR is in the range of 1 clonal tumor B cell in 105 lymphocytes or 5 clonal tumor B cells in 105 lymphocytes and 1 clonal tumor B cell in 106 lymphocytes for nested ASO RTPCR.12,13 Of the remaining 6 patients, 3 patients were immunized with the Id immunoglobulin together with IL-12 alone (MM-1, MM-3, and MM-6) and 3 patients received a combination of the adjuvant cytokines IL-12 and GM-CSF (MM-2, MM-4, and MM-5). No other treatment was given during the study period.
Real-time ASO PCR results. Blood clonal myeloma tumor B-cell levels measured by real-time ASO PCR (see “Study design”) in 6 MM patients immunized with the autologous Id during 30 weeks of follow-up. Arrows indicate immunization times. Three patients received IL-12 alone (MM-1, MM-3, and MM-6) and 3 patients received IL-12 plus GM-CSF (MM-2, MM-4, and MM-5) as adjuvants. The number of IgH copies detected at initiation of vaccination was set to 100%. The values during follow-up are given as percent of the initial sample. Mean ± SEM for 3 measurements are shown.
Real-time ASO PCR results. Blood clonal myeloma tumor B-cell levels measured by real-time ASO PCR (see “Study design”) in 6 MM patients immunized with the autologous Id during 30 weeks of follow-up. Arrows indicate immunization times. Three patients received IL-12 alone (MM-1, MM-3, and MM-6) and 3 patients received IL-12 plus GM-CSF (MM-2, MM-4, and MM-5) as adjuvants. The number of IgH copies detected at initiation of vaccination was set to 100%. The values during follow-up are given as percent of the initial sample. Mean ± SEM for 3 measurements are shown.
No hematologic toxicity was observed in any of the patients and no abnormalities in kidney and liver function tests were noted (data not shown). Patient MM-2 experienced transient dyspnea, weight on the chest, and shivering starting 2 hours following the sixth vaccination. The symptoms disappeared spontaneously within 1 hour. Patient MM-3 had fever (up to 38.5°C) for about 24 hours after each vaccination and a National Cancer Institute (NCI) grade 2 local skin reaction. Patient MM-4 had an NCI grade 2 local skin reaction at the first 5 vaccination times. Patient MM-5 developed at the last vaccination petechial bleedings of the lower legs considered due to capillary leakage.
One patient (MM-5) achieved a complete peripheral blood molecular remission (below 1:106, PCR negative using both ASO real-time and nested RT-PCR). Three patients had a reduction of 92%, 79%, and 54%, respectively, of the clonotypic B cells (MM-1, MM-3 and MM-2, respectively). Two patients (MM-4 and MM-6) had unchanged levels of clonal B cells. In no patient was a significant change of the serum M-component concentration observed during the study period (Table 2).
Patients MM-1, MM-2, MM-4, and MM-5 mounted an Idspecific T-cell response, whereas in patients MM-3 and MM-6 no Id-specific T-cell response was detected (Table 2). Thus, 3 patients mounting an Id-specific T-cell response had tumor cell reduction, whereas one patient developing an anti-Id cellular response showed no decrease in circulating tumor B cells. However, one patient (MM-3) had a tumor mass reduction without a detectable T-cell response. A similar phenomenon has been described in melanoma patients immunized with MAGE-3 peptides. Partial and complete remissions were obtained without detectable MAGE-3–specific T cells.16
Massaia and coworkers17 immunized 2 MM patients with minimal residual disease using the Id but were not able to detect a reduction of circulating tumor cells by PCR. The reason for the discrepancy is not clear but may be due to the small number of patients, a different vaccination schedule, and the fact that the patients probably exhibited a pronounced T-cell dysfunction due to high-dose chemotherapy. Moreover, in that study GM-CSF or IL-2 was used as an adjuvant cytokine, whereas all our patients received IL-12. It was recently shown in an animal model that immunization with myeloma cells transfected both with the GM-CSF and IL-12 genes induced proliferative as well as cytotoxic T-cell responses directed against the secreted monoclonal Ig and protected more than 90% of the animals against tumor challenge.18 It is not likely that IL-12 alone contributed to the blood tumor cell reduction observed in our patient because the adjuvant dose of IL-12 was very low; only 1% of the dose used for treatment of patients with malignancies. However, in our patients no major tumor reduction was noted, as measured by serum M-component concentration, which was stable over time in all patients, indicating that myeloma-specific T cells might only be able to eradicate a minimal tumor burden. This is the first study in myeloma showing that Id immunization is able to induce reduction at the molecular level of circulating tumor cells similar to the observation in follicular lymphomas where immunotherapy could induce molecular remission after chemotherapy induced clinical remission using Id vaccination7 as well as monoclonal antibody therapy (rituximab).19
In conclusion, Id vaccination in patients with MM may induce tumor-specific T cells that might target circulating clonal tumor B cells. Id vaccination may preferentially be applied when the tumor load is low, that is, early during the course of the disease or after chemotherapy. In the latter case measures should be taken to augment T-cell functions because otherwise an effective tumorspecific immune response may not be induced.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-06-1925.
Supported in part by the Hans Edstrand Foundation, Danish Cancer Society, Swedish Cancer Society, Cancer Society of Stockholm, Torsten and Ragnar Söderbergs Foundation, Cancer Allergy Foundation, Hedberg Foundation, International Myeloma Foundation, and Multiple Myeloma Research Foundation.
T.R. and L.H. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lone Honoré and Ingrid Eriksson for technical assistance.