Abstract
Tetrocarcin-A (TC-A), an antibiotic agent isolated from actinomycetes, has recently been described to antagonize Bcl-2 functions, thereby sensitizing tumor cells to cell death signals under control of Bcl-2. In this study, we analyzed the direct proapoptotic effect of TC-A in the B-chronic lymphocytic leukemia (B-CLL) model. We focused on the signal cascade triggered by TC-A in B-CLL cells and identified activated mitochondrial as well as endoplasmatic reticulum (ER) stress signals. The expression levels of known effector molecules mediating mitochondrial signaling, such as Bax and Bid, and the antagonistic molecule Bcl-2 did not influence sensitivity of B-CLL cells to TC-A. Furthermore, the molecular chaperone and sensor of ER stress, HSP70, though significantly up-regulated in B-CLL cells undergoing TC-A—triggered apoptosis, was ineffective to exert its anti-apoptotic function described in multiple cell death pathways. Autologous T cells of B-CLL patients were significantly less sensitive to TC-A as were also T cells from healthy donors when compared with their normal B-cell fraction. Furthermore, sensitivity of B-CLL cells to TC-A treatment in vitro was dependent neither on the expression levels of CD38—a prognostic factor for survival of B-CLL patients as well as for their response to therapy—nor on the clinical stage or pretreatment status of patients. From our data showing that TC-A induced a cell death pathway via ER stress preferentially in B cells and that it acted independently of important markers of drug sensitivity and of clinical markers, we conclude that TC-A might represent an attractive candidate drug for further evaluation in preclinical trials.
Introduction
B-chronic lymphocytic leukemia (B-CLL) is a neoplastic disease characterized by a usually slow but progressive accumulation of monoclonal B cells. The accumulation of tumor cells is mainly caused by defective cell death signaling, as opposed to increased proliferation. Defects in the apoptotic machinery not only increase spontaneous cell survival but also contribute to chemoresistance of the neoplastic clone. B-CLL cells have been reported to express high levels of the antiapoptotic protein Bcl-2, which is not due to gene rearrangement but rather to DNA hypomethylation of the bcl-2 promotor region.1 Bcl-2 and its opponent Bax2 have been shown to contribute to the chemosensitivity of B-CLL cells. Moreover, inactivation of p53 by mutation or deletion has been reported in B-CLL,3,4 becomes more frequent with disease progression, and predicts aggressive disease that will be unresponsive to alkylator or purine analog—based therapy.4
Antiapoptotic proteins not only regulate spontaneous survival of tumor cells but also play a decisive role in the initial clinical response to treatment and the development of resistance to cytotoxic drugs. Thus, the identification of new agents that activate signaling pathways bypassing the currently known essential regulators of sensitivity to spontaneous and/or chemotherapy-induced apoptosis in B-CLL is still needed.
Besides dysregulation of apoptotic pathways at the cellular level of the neoplastic clone, profound disturbances in the immune system leading to severe infections, autoimmune diseases, and frequent occurrences of secondary solid or hematopoietic neoplasias are found in B-CLL.5 A pathologic T-cell distribution together with an impairment of T-cell functions were correlated with the stage of disease6, 7, 8 and thought to be primarily responsible for the immunodeficiency and the increased frequencies of infection episodes occurring in these patients.9 The most efficient cytotoxic agents currently available such as fludarabine phosphate10 or 2-CdA 11 lead to severe and long-lasting depletion of T-cell compartments, thereby augmenting T-cell deficiencies in B-CLL patients and counteracting attempts to reconstitute an active immune surveillance. Thus, the degree of therapeutic aggression feasible under in vivo conditions remains limited, and new drugs with more selective targeting of the neoplastic clone are urgently required.
Using a T-acute lymphoblastic leukemia (T-ALL) cell line model, we previously observed the induction of apoptosis via an endoplasmatic reticulum (ER)—stress pathway by tetrocarcin-A (TC-A),12 an antibiotic and antitumor agent isolated from actinomycetes.13 Importantly, apoptosis by TC-A was found to be inhibited neither by aberrant expression/function of important regulators of death receptor— and drug-induced apoptosis (such as overexpression of Bcl-2, p53 mutation, or caspase-8 deficiency) nor by HSP70, a survival protein involved in tumorigenesis.14
These specific characteristics of TC-A signaling and the high expression level of Bcl-2 in B-CLL prompted us to test whether TC-A might represent an effective therapeutic agent for B-CLL cells fulfilling the following criteria—namely, to trigger a Bcl-2—, p53-, and caspase-8—independent apoptotic signaling cascade and to preferentially act on B-CLL tumor cells.
Patients, materials, and methods
Patients' cells
After obtaining informed patient consent, tumor cells from 48 B-CLL patients were collected in heparinized tubes during routine examinations. B-CLL was defined by clinical criteria as well as by cellular morphology and the coexpression of CD19 and CD5 in lymphocytes simultaneously displaying restriction of light-chain rearrangement. Staging was performed according to Rai classification.15 Characteristics of the patients studied are summarized in Table 1. Lymphocytes were separated by density gradient centrifugation (Lymphoprep, Nycomed, Oslo, Norway). Cells from the interface were carefully collected, washed twice, and cultured in RPMI 1640 medium (PAA Laboratories, Linz, Austria) in the presence of 10% heat-inactivated fetal calf serum (PAA Laboratories), 2 mM l-glutamine (GIBCO, Grand Island, NY), and 100 μg/mL gentamicin (GIBCO) at 37°C in a humidified atmosphere containing 5% CO2. If not used immediately, the cells were resuspended in a solution of fetal calf serum (FCS) and 5% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Vienna, Austria), frozen, and stored in liquid nitrogen. As controls, peripheral blood of age-matched healthy donors was collected, prepared, and cultured as described for blood samples of B-CLL patients.
Cell line
The B-CLL cell line EHEB was obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). The EHEB cell line was established from a patient with B-CLL and immortalized in vitro by means of Epstein-Barr virus (EBV) transformation. The cell line is characterized by an immunophenotype typical of B-CLL disease (CD5+, CD19+, light-chain restriction).16 The cell line was cultured as described for the native cell samples.
Reagents and antibodies
For this study the following reagents and antibodies were used: tetrocarcin-A (a gift from Dr M. Hara, Kyowa Hakko Kogyo, Shizuoka, Japan), fludarabine phosphate (Schering, Vienna, Austria), vincristine (Pharmacia, Vienna, Austria), mafosfamide (Asta Medica, Vienna, Austria), cisplatin (Ebewe, Unterach, Austria), dexamethasone (Ludwig Merckle, Vienna, Austria), brefeldin A, pancaspase inhibitor zVAD-fmk (both from Calbiochem, San Diego, CA), inhibitor of caspase-8—like caspases zLETD-fmk (Alexis, Läufelfingen, Switzerland); for immunoblotting: antihuman Bcl-2 (Dako, Vienna, Austria), antihuman Bax, antihuman poly(adenosine diphosphate ribose) polymerase (PARP) (both from Santa Cruz, Santa Cruz, CA), antihuman Bid, antihuman caspase-3, -7, and -9 (all from Pharmingen, San Diego, CA), antihuman caspase-8 (Upstate Biotechnologies, Lake Placid, NY), antihuman tubulin (Sigma), antihuman HSP70 (Stress Gen Biotechnologies, Szabo Scandic, Vienna, Austria); for flow cytometric analyses: R-phycoerythrin—cyanine 5 (RPE-Cy5)—conjugated mouse antihuman CD19 antibody (Becton Dickinson, San Jose, CA), RPE-Cy5—labeled anti-CD4, CD8 (Dako), fluorescein isothiocyanate (FITC)—conjugated antihuman Bcl-2 (Dako), antihuman Bax (Santa Cruz), and antihuman HSP70 (NeoMarkers, Fremont, CA).
Separation of B and T cells
Purification of B and T cells was performed by positive selection using immunomagnetic separation (Miltenyi Biotec, Bergisch-Gladbach, Germany). Briefly, the cells were labeled with monoclonal mouse antihuman CD19 antibodies or a combination of anti-CD4 and anti-CD8 antibodies, all linked to magnetic microbeads. After incubating for 30 minutes on ice, cells were washed and selected using magnetic separation columns. Isolated cells were more than 90% pure as determined by flow cytometry (FACScan, Becton Dickinson) and appeared viable as determined by their forward/scatter profile and by trypan blue exclusion.
Flow cytometric analyses
Apoptosis assay. The percentages of early and late apoptotic (aponecrotic17 ) cells in the respective lymphocytic subpopulations were determined by staining peripheral blood cells with annexin V—FITC (Alexis) and propidium iodide (PI; Sigma), according to manufacturer's instructions. Briefly, 1 × 106 cells were incubated with saturating concentrations of annexin V—FITC, PI, and the RPE-Cy5—conjugated monoclonal antibodies against CD19 or against CD4 and CD8. Cells were incubated for 20 minutes and analyzed immediately by flow cytometry.
Mitochondrial transmembrane potential (ΔΨm). For detection of the breakdown of ΔΨm during apoptosis, cells were stained with the dual-emission potential-sensitive probe JC-1 (5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzimidazolylcarbocyanine iodide; Molecular Probes, Leiden, Netherlands). JC-1 has been demonstrated to be a reliable probe for assessing mitochondrial changes during apoptosis.18 Briefly, 5 × 106 purified B cells were incubated with 10 μM JC-1 for 30 minutes at 37°Cina humidified atmosphere containing 5% CO2. The fluorescence signal intensity of FL-2 representing cells with high ΔΨm and of FL-1 representing cells with low ΔΨm was analyzed by flow cytometry immediately after staining of the cells.
Determination of protein expression. For intracellular detection of Bcl-2, Bax, Bid, and HSP70, 5 × 106 cells were fixed and permeabilized using a cell permeabilization kit (An der Grub, Kaumberg, Austria) according to manufacturer's instructions. Permeabilized cells were stained with the specific antibodies (1 μg) or the relevant control antibodies for 20 minutes at room temperature (RT) and, in the case of Bax, Bid, and HSP70 detection, subsequently with a secondary FITC-labeled antibody for 20 minutes at RT. Cells were finally stained with a specific RPE-labeled antibody for CD19, CD4, or CD8, washed, and analyzed immediately by flow cytometry.
Western blotting
For Western blot analysis, 5 × 106 cells were incubated in the absence or presence of different concentrations of TC-A with or without the pancaspase inhibitor zVAD-fmk. Following stimulation, cells were resuspended in 100 μL lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]—HCL [pH 7.5], 150 mM NaCl, 2 mM EDTA [ethylenediaminetetraacetic acid], and 1 mM EGTA [ethylene glycol tetraacetic acid] supplemented with 25 μg/mL leupeptin, 25 μg/mL aprotinin, and 1% Triton X-100) and frozen in liquid nitrogen and thawed 3 times. The samples were then cleared by centrifugation (14 000g, 30 minutes, 4°C). Equal amounts of protein were separated by sodium dodecyl sulfate—polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Millipore, Bedford, MA). Immunoblots were blocked with 5% skim milk in TRIS-buffered saline (TBS); incubated for 1 hour at RT with specific antibodies for bcl-2, bax, bid, PARP, and caspase-3, -7, -8, and -9; washed in TBS-T (0.05% Tween 20 in TBS); and further incubated with the respective horseradish peroxidase—conjugated secondary antibody (Dako). After extensive washing, blots were developed by chemiluminescence reaction and exposed to autoradiographic films (Amersham Pharmacia, Buckinghamshire, United Kingdom). After stripping, the same blots were reprobed with antitubulin antibodies to test for equal protein contents.
Statistical analysis
For statistical analyses, P values were assessed using either Mann-Whitney U, analysis of variance (ANOVA) factorial, or paired t test, as appropriate (StatView 5.1, Abacus Concepts, Berkeley, CA).
Results
TC-A induces apoptosis in B-CLL cells
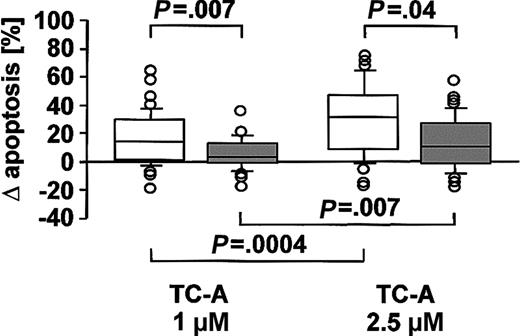
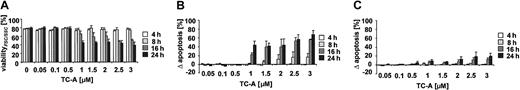
The antibiotic agent TC-A has recently been characterized by our group to possess proapoptotic activity in T-ALL tumor cells, acting via an ER-stress pathway independent of Bcl-2 overexpression.12 However, no data are currently available concerning the efficacy and mechanisms of action of this substance in other lineages of the hematopoietic system in general and of native tumor cells in particular. As presented in Figure 1, TC-A induced apoptosis in B-CLL cells while it was significantly less effective in the T cells of B-CLL patients. Apoptosis triggered by TC-A was characterized by changes in cell volume and granularity (Figure 2A) and exposure of phosphatidylserine (Figure 2B) in a dose- and time-dependent manner. In the T-cell population of B-CLL patients, the highest concentration of TC-A tested had only a minor proapoptotic effect; that is, the increase in annexin V—FITC binding was detectable in less than 20% of the T cells (Figure 2C).
The proapoptotic effect of TC-A is more pronounced in B cells than in T cells of B-CLL patients. B cells (□) and T cells (▦) of 48 B-CLL patients were left untreated or treated with 1 μM or 2.5 μM TC-A for 24 hours. Exposure of phosphatidylserine as a specific marker for apoptosis was determined by flow cytometry using the annexin V—FITC binding assay. Results are shown as the differences of TC-A—induced apoptosis minus spontaneous apoptosis. Box plots represent median values and 50% of the data, and whiskers represent the interquartile ranges.
The proapoptotic effect of TC-A is more pronounced in B cells than in T cells of B-CLL patients. B cells (□) and T cells (▦) of 48 B-CLL patients were left untreated or treated with 1 μM or 2.5 μM TC-A for 24 hours. Exposure of phosphatidylserine as a specific marker for apoptosis was determined by flow cytometry using the annexin V—FITC binding assay. Results are shown as the differences of TC-A—induced apoptosis minus spontaneous apoptosis. Box plots represent median values and 50% of the data, and whiskers represent the interquartile ranges.
Time- and dose-dependence of TC-A—induced apoptosis. Peripheral blood mononuclear cells (PBMCs) of 4 patients were left untreated or treated with TC-A at the indicated concentrations for 4 hours, 8 hours, 16 hours, or 24 hours. Subsequently, (A) changes in cell volume and granularity reflecting a decrease in viability and (B) the increase in annexin V—FITC binding was determined by flow cytometry in B-CLL cells. (C) In parallel, apoptosis of CD4+CD8+ T cells using the annexin V—FITC binding assay was determined. The mean values representing the differences between TC-A—induced apoptosis minus spontaneous apoptosis ± SEM of 4 independent experiments are shown.
Time- and dose-dependence of TC-A—induced apoptosis. Peripheral blood mononuclear cells (PBMCs) of 4 patients were left untreated or treated with TC-A at the indicated concentrations for 4 hours, 8 hours, 16 hours, or 24 hours. Subsequently, (A) changes in cell volume and granularity reflecting a decrease in viability and (B) the increase in annexin V—FITC binding was determined by flow cytometry in B-CLL cells. (C) In parallel, apoptosis of CD4+CD8+ T cells using the annexin V—FITC binding assay was determined. The mean values representing the differences between TC-A—induced apoptosis minus spontaneous apoptosis ± SEM of 4 independent experiments are shown.
Increased sensitivity to TC-A is an intrinsic feature of B cells and does not correlate with Bcl-2/Bax expression
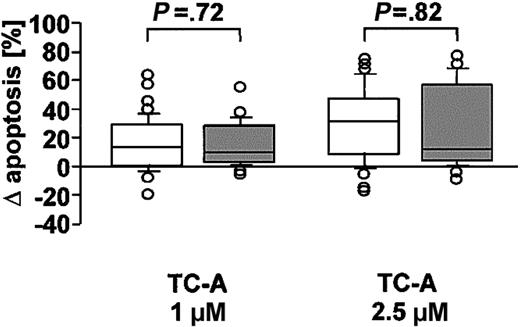
B-CLL tumor cells are characterized by a low sensitivity to drug-induced apoptosis, which is in part mediated by overexpression of Bcl-2.19 To determine whether there is a difference in the sensitivity of B-CLL cells to TC-A as compared with peripheral blood cells from healthy donors, B and T cells of 31 healthy age-matched controls were left untreated or stimulated with increasing doses of TC-A (1 or 2.5 μM) for 24 hours. As presented in Figure 3, no significant difference in the sensitivity to TC-A— induced apoptosis of B-CLL cells and normal B cells could be observed (P = .65 at 1 μM and P = .36 at 2.5 μM). In addition, the preferential sensitivity of B cells over T cells to TC-A treatment was not restricted to patients with B-CLL but also observed in healthy controls (data not shown). Apparently, the pathway of apoptosis induction under TC-A remains unaffected during the process of neoplastic transformation.
TC-A induces apoptosis in B-CLL cells and normal B cells. B-CLL cells of 48 B-CLL patients (□) and B cells of 31 healthy donors (▦) were left untreated or treated with 1 μM or 2.5 μM TC-A for 24 hours. The increase in phosphatidylserine exposure was determined by flow cytometry. Results are shown as the differences of TC-A—induced apoptosis minus spontaneous apoptosis. Box plots represent median values and 50% of the data, and whiskers represent the interquartile ranges.
TC-A induces apoptosis in B-CLL cells and normal B cells. B-CLL cells of 48 B-CLL patients (□) and B cells of 31 healthy donors (▦) were left untreated or treated with 1 μM or 2.5 μM TC-A for 24 hours. The increase in phosphatidylserine exposure was determined by flow cytometry. Results are shown as the differences of TC-A—induced apoptosis minus spontaneous apoptosis. Box plots represent median values and 50% of the data, and whiskers represent the interquartile ranges.
Also, Bcl-2 expression levels did not influence the sensitivity of B-CLL cells to TC-A. This conclusion is based on 3 observations: (1) Higher expression levels of Bcl-2 were found in B-CLL cells as compared with B cells from healthy donors (mean fluorescence intensities [MFIs] ± SD: 26.6 ± 14.9 versus 16.1 ± 4.2, P = .0002). (2) Bcl-2 expression levels in B-CLL cells did not correlate with the degree of apoptosis induction (P = .09, r = 0.17). (3) TC-A had less cytotoxic effects on T cells of B-CLL patients (Figure 1) with significantly lower Bcl-2 levels as compared with the tumor cell population (mean fluorescence intensities [MFIs] ± SD: 17.5 ± 18.3 versus 26.6 ± 14.9, P = .02). Because chemosensitivity of B-CLL cells has been shown to depend on expression levels of Bcl-2 and its opponent Bax,2,20,21 we investigated whether TC-A sensitivity correlated with basal Bax expression levels of B-CLL tumor cells. As observed for Bcl-2, no correlation of the Bax expression level (MFI ± SD: 2.7 ± 2.9) of B-CLL cells with their sensitivity to TC-A treatment could be observed (P = .36, r = 0.28).
TC-A—triggered signaling cascade in the B-CLL cell line EHEB
To characterize the signaling cascade of TC-A—induced apopotosis in B-CLL cells, we first used the B-CLL cell line EHEB as a model. Comparable with the results obtained in primary B-CLL cells (Figures 1, 2), TC-A induced cell death in EHEB cells in a dose-dependent manner (Figure 4A-B). The analysis of the mitochondrial transmembrane potential ΔΨm in EHEB cells revealed that TC-A acted on mitochondria and reduced ΔΨm (Figure 4C). We next tested whether TC-A had an effect on 2 important regulators of mitochondrial signaling, the proapoptotic Bcl-2 family member Bax and its antiapoptotic binding partner Bcl-2. Using immunoblot analyses, we observed cleavage of Bax after TC-A treatment but no changes in expression levels or molecular size of the antiapoptotic member Bcl-2 (Figure 5). Another mediator of ΔΨm breakdown is the BH3-only protein Bid.22 We failed to detect Bid expression in the EHEB cell line as well as in primary B-CLL cells (data not shown), a finding that excludes this proapoptotic protein as being necessary for efficient signal transduction in the cell death program triggered by TC-A.
TC-A—induced apoptosis in the B-CLL cell line EHEB involves mitochondrial signaling. EHEB cells were left untreated or treated with increasing concentrations of TC-A. After 24 hours, a dose-dependent decrease in viability (A), an increase in annexin V—FITC binding (B), and the loss of ΔΨm (C) were detected by flow cytometry. The mean percentages ± SEM of at least 4 independent experiments are presented.
TC-A—induced apoptosis in the B-CLL cell line EHEB involves mitochondrial signaling. EHEB cells were left untreated or treated with increasing concentrations of TC-A. After 24 hours, a dose-dependent decrease in viability (A), an increase in annexin V—FITC binding (B), and the loss of ΔΨm (C) were detected by flow cytometry. The mean percentages ± SEM of at least 4 independent experiments are presented.
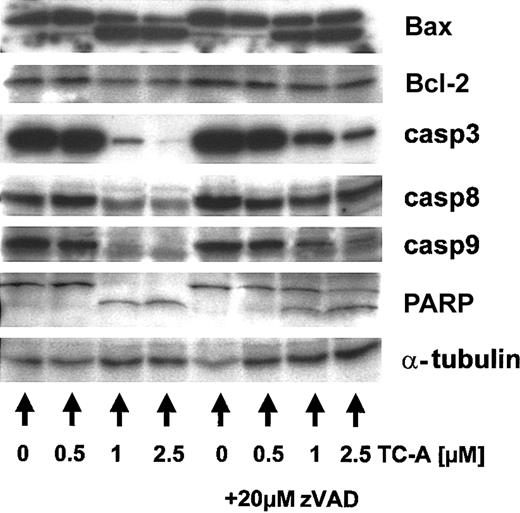
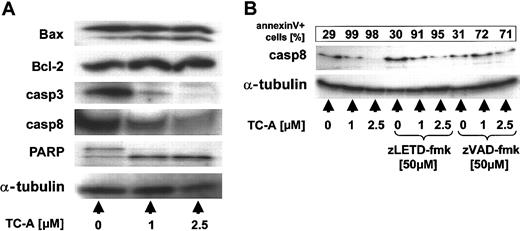
TC-A induces the processing of multiple cellular targets in B-CLL cells. EHEB cells were left untreated or treated with 0.5, 1, or 2.5 μM TC-A alone (left) or in combination with zVAD-fmk (20 μM; right) for 24 hours. Subsequently, cell lysates were prepared and immunoblot analyses for Bax, Bcl-2, caspase-3 (casp3), -8, -9, and PARP performed. As internal loading control for equal amounts of protein, α-tubulin was detected. Representative results from at least 2 independent experiments are presented.
TC-A induces the processing of multiple cellular targets in B-CLL cells. EHEB cells were left untreated or treated with 0.5, 1, or 2.5 μM TC-A alone (left) or in combination with zVAD-fmk (20 μM; right) for 24 hours. Subsequently, cell lysates were prepared and immunoblot analyses for Bax, Bcl-2, caspase-3 (casp3), -8, -9, and PARP performed. As internal loading control for equal amounts of protein, α-tubulin was detected. Representative results from at least 2 independent experiments are presented.
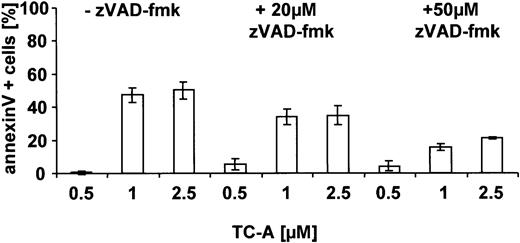
The proteolysis of Bax has been described to occur in a caspase-23 and/or a calpain-dependent manner.24 Thus, we next raised the question of whether caspases are involved in TC-A— induced apoptosis and, using immunoblotting, detected cleavage of caspase-3, -8, -9 and of PARP, a known substrate of active caspases (Figure 5). The processing of Bax, caspases, and PARP could partly be inhibited by preincubation of EHEB cells with the caspase inhibitor zVAD-fmk (20 μM), suggesting that activation of caspases occurs upstream and is involved in their cleavage (Figure 5). The contribution of caspases to TC-A—triggered cell death of B-CLL cells was further confirmed by our observation that zVAD-fmk pretreatment was capable of significantly diminishing TC-A— induced apoptosis in a dose-dependent manner (Figure 6).
TC-A—induced apoptosis in B-CLL cells proceeds via caspases. EHEB cells were left untreated or pretreated with zVAD-fmk (1 hour) before application of the indicated concentrations of TC-A. After 24 hours, apoptosis was detected using the annexin V—FITC binding assay. The mean values representing the differences between TC-A—induced apoptosis minus spontaneous apoptosis of 4 independent experiments are shown.
TC-A—induced apoptosis in B-CLL cells proceeds via caspases. EHEB cells were left untreated or pretreated with zVAD-fmk (1 hour) before application of the indicated concentrations of TC-A. After 24 hours, apoptosis was detected using the annexin V—FITC binding assay. The mean values representing the differences between TC-A—induced apoptosis minus spontaneous apoptosis of 4 independent experiments are shown.
TC-A signaling in native B-CLL cells
Our next experiments focused on the signaling pathway in native B-CLL cells. Essentially the same results as in the EHEB cell line were observed in primary tumor cells. The activation of a mitochondrial signaling pathway by TC-A was confirmed by the observation of a dose-dependent loss of ΔΨm (data not shown) and the cleavage of Bax (Figure 7A). As already observed in the cell line model, Bcl-2 expression remained unchanged (Figure 7A). TC-A treatment induced the activation of caspase-3 and -8 and resulted in the processing of PARP (Figure 7A). To determine the role of caspase-8 activation in TC-A signaling in B-CLL in more detail, we pretreated primary B-CLL cells with the specific caspase-8 inhibitor LETD-fmk. In spite of inhibiting caspase-8 cleavage (Figure 7B), this treatment was ineffective in blocking TC-A—triggered apoptosis (Figure 7B), arguing against an important role of caspase-8 in TC-A signaling and confirming our previous results in the T-ALL model.12
TC-A signaling in native B-CLL cells. (A) After purification of B-CLL cells of patients using immunomagnetic separation, cells were left untreated or treated with 1 or 2.5 μM for 24 hours. Cell lysates were prepared, subjected to immunoblotting, and analyzed for Bax, Bcl-2, caspase-3 and -8, and PARP. As internal loading control for equal amounts of protein, α-tubulin was detected. (B) B-CLL cells of patients were left untreated or pretreated with either the pancaspase inhibitor zVAD-fmk (50 μM) or an inhibitor of caspase-8—like caspases zLETD-fmk (50 μM) for 1 hour followed by the application of TC-A at the indicated concentrations, and incubations continued for 24 hours. Cell lysates were prepared, and caspase-8 was detected by immunoblotting. α-Tubulin was used as internal loading control for equal amounts of protein. In parallel, apoptosis was detected using the annexin V—FITC binding assay. The corresponding percentages of apoptotic cells are presented in the top box of the panel.
TC-A signaling in native B-CLL cells. (A) After purification of B-CLL cells of patients using immunomagnetic separation, cells were left untreated or treated with 1 or 2.5 μM for 24 hours. Cell lysates were prepared, subjected to immunoblotting, and analyzed for Bax, Bcl-2, caspase-3 and -8, and PARP. As internal loading control for equal amounts of protein, α-tubulin was detected. (B) B-CLL cells of patients were left untreated or pretreated with either the pancaspase inhibitor zVAD-fmk (50 μM) or an inhibitor of caspase-8—like caspases zLETD-fmk (50 μM) for 1 hour followed by the application of TC-A at the indicated concentrations, and incubations continued for 24 hours. Cell lysates were prepared, and caspase-8 was detected by immunoblotting. α-Tubulin was used as internal loading control for equal amounts of protein. In parallel, apoptosis was detected using the annexin V—FITC binding assay. The corresponding percentages of apoptotic cells are presented in the top box of the panel.
TC-A induces ER stress, which causes the up-regulation of HSP70
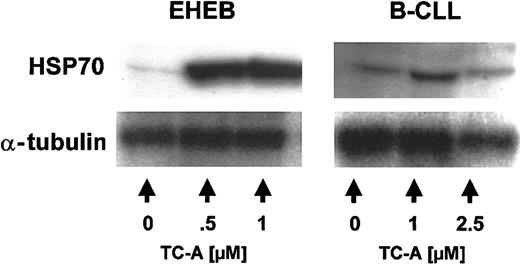
Based on our previous results in the T-ALL model,12 we next asked whether TC-A induces ER stress in B-CLL cells. Cellular stress such as heat shock resulting in disturbance of the ER machinery leads to the nearly instantaneous induction of molecular chaperones, mainly heat shock proteins (HSPs) such as HSP70.25 In agreement with the induction of ER stress, treatment of EHEB cells and B-CLL cells of patients with increasing concentrations of TC-A for 24 hours led to the up-regulation of HSP70 (Figures 8, 9).
TC-A induces the up-regulation of HSP70 in EHEB cells and native B-CLL cells. EHEB cells (left) or primary B-CLL cells (right) were left untreated or treated with 0.5 μM, 1 μM, or 2.5 μM TC-A for 24 hours. Subsequently, up-regulation of HSP70 was detected by immunoblot analysis. As internal loading control for equal amounts of protein, α-tubulin was used. One representative result from at least 2 independent experiments is shown.
TC-A induces the up-regulation of HSP70 in EHEB cells and native B-CLL cells. EHEB cells (left) or primary B-CLL cells (right) were left untreated or treated with 0.5 μM, 1 μM, or 2.5 μM TC-A for 24 hours. Subsequently, up-regulation of HSP70 was detected by immunoblot analysis. As internal loading control for equal amounts of protein, α-tubulin was used. One representative result from at least 2 independent experiments is shown.
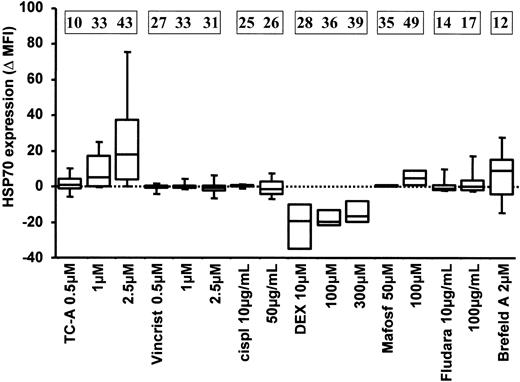
The up-regulation of HSP70 is specific for cytotoxic treatment with TC-A. B-CLL cells of 5 patients were left untreated or treated with the following cytotoxic agents: TC-A, vincristine (Vincrist), cisplatin (cispl), dexamethasone (DEX), mafosfamide (Mafosf), and fludarabine phosphate (Fludara) at the indicated concentrations. Brefeldin A (Brefeld A), a known inducer of ER stress, was used as positive control. The expression of HSP70 was determined in permeabilized cells by immunofluorescence staining followed by flow cytometric analysis. The differences of basal and TC-A—induced HSP70 expression levels are presented as mean fluorescence intensities (Δ MFI). The mean values representing the differences between TC-A—induced apoptosis minus spontaneous apoptosis of at least 4 independent experiments are presented in the boxes at the top.
The up-regulation of HSP70 is specific for cytotoxic treatment with TC-A. B-CLL cells of 5 patients were left untreated or treated with the following cytotoxic agents: TC-A, vincristine (Vincrist), cisplatin (cispl), dexamethasone (DEX), mafosfamide (Mafosf), and fludarabine phosphate (Fludara) at the indicated concentrations. Brefeldin A (Brefeld A), a known inducer of ER stress, was used as positive control. The expression of HSP70 was determined in permeabilized cells by immunofluorescence staining followed by flow cytometric analysis. The differences of basal and TC-A—induced HSP70 expression levels are presented as mean fluorescence intensities (Δ MFI). The mean values representing the differences between TC-A—induced apoptosis minus spontaneous apoptosis of at least 4 independent experiments are presented in the boxes at the top.
Cytotoxic drugs in use for therapy of B-CLL, such as vincristine, cisplatin, dexamethasone, cyclophosphamide analogs, and fludarabine phosphate, have been shown to induce apoptosis in B-CLL cells in vitro. To analyze whether these cytotoxic agents also induce ER stress in B-CLL cells, we used HSP70 up-regulation as an indicator. Brefeldin A, a known inducer of ER stress, served as a positive control.26 As seen in Figure 9, massive up-regulation of HSP70 was induced by TC-A in primary B-CLL cells. Mafosfamide, a synthetic analog of cyclophosphamide, the activity of which does not rely on biotransformation, induced a moderate increase in HSP70 at high doses. The lack of up-regulation of HSP70 observed following treatment with the conventional cytotoxic drugs was not due to intrinsic insensitivity of B-CLL cells, because parallel analyses revealed induction of apoptosis following treatment with these agents (Figure 9).
Sensitivity to TC-A treatment does not correlate with clinical chemosensitivity, CD38 expression, clinical stage, or pretreatment status
Of the patients included in this study, 23 had received at least 1 prior treatment (Table 1). Of these treated patients, 19 failed to respond to therapy according the National Canter Institute (NCI) Working Group guidelines.15 In addition, subgroup analysis of our in vitro studies comparing the efficacy of TC-A and fludarabine phosphate (one of the most efficient cytotoxic agents used in the therapy of B-CLL patients) revealed that B-CLL cells refractory to in vitro treatment with fludarabine phosphate (n = 6, percentage of apoptosis ± SEM: untreated, 18% ± 2.6% versus fludarabine-treated, 25% ± 4.1%) were still sensitive to TC-A (percentage of apoptosis ± SEM: untreated, 18% ± 2.6% versus TC-A—treated, 62% ± 9.7%). We believe that the observation that B-CLL cells from these therapy-refractory patients were still sensitive to TC-A needs to be followed up.
In a recently published study, CD38 expression in B-CLL has been found to increase with the progression of the disease and was identified as a prognostic factor for the response to fludarabine phosphate treatment as well as for progression-free and overall survival.27 We therefore examined basal CD38 expression levels in B-CLL cells of patients (n = 31) and correlated them with their sensitivity to TC-A. No correlation of sensitivity to TC-A treatment with the percentage of CD38+ cells (1 μM TC-A: r = 0.27, P = .37; 2.5 μM TC-A: r = 0.32, P = .21) or with the clinical stage according to the modified RAI system (low versus intermediate at 1 μM TC-A: P = .887; at 2.5 μM TC-A: P = .792; intermediate versus high at 1 μM TC-A: P = .254; at 2.5 μM TC-A: P = .973) could be observed. Furthermore, subgroup analysis revealed that sensitivity of tumor cells to TC-A did not depend on pretreatment status of patients (at 1 μM: P = .94; at 2.5 μmol: P = .31).
Discussion
In B-CLL tumor cells, TC-A induced a novel apoptotic pathway triggered by ER stress involving mitochondrial signaling and proceeding via caspases. Sensitivity of tumor cells did not correlate with expression levels of Bcl-2 and Bax, was further independent of caspase-8 and Bid, and could not be abrogated by up-regulation of the survival protein HSP70. Tumor cells from patients with high expression of CD38, a prognostic factor for response to therapy, displayed equal sensitivity to B-CLL cells with low basal CD38 expression levels, and sensitivity was not diminished in B-CLL cells from patients with advanced disease. Of importance, the B-cell fraction displayed a significantly higher sensitivity to TC-A than autologous T cells (Figures 1, 2).
In B-CLL, the accumulation of the tumor cell clone is accompanied by an increase in absolute T-cell numbers, and the ratio between CD4+ and CD8+ T cells is often inverted7 and T-cell functions are disturbed.6,8 This T-cell immunodeficiency can even become aggravated during cytotoxic therapy in particular with purine analogs. Decreases in CD4+ cell counts have been associated with an increased risk of major and/or minor infections.10 In the present study, we provide evidence that T cells of B-CLL patients as well as of healthy donors are less sensitive to TC-A treatment than the neoplastic and normal B-cell fractions. A preferential cytotoxic action on B-CLL cells has also been observed for other conventional drugs, for example, for chlorambucil by Thomas and coworkers.28 However, it cannot be established from our study whether T cells surviving TC-A treatment are still functional. The clarification of whether the degree of T-cell preservation observed under TC-A is sufficient for clinical protection against infections and/or tumor regression will be the focus of our future studies.
The induction of cell death in target cells is the main mode of action of cytotoxic agents and, thus, the efficacy of cytotoxic therapy is primarily affected by expression levels and/or the mutational status of proteins involved in cell death pathways, such as Bcl-2 family members,29 the p53 tumor suppressor gene,30,31 inducer32 or effector caspases,33 and survival proteins such as heat shock proteins.34 The identification of new therapeutic agents that overcome such molecular signaling blockades are therefore urgent. The observation that Bcl-2 is overexpressed in most B-CLL cells1,35 (reviewed by Marschitz et al21 ) has led to the suggestion that disturbances in the balance between proapoptotic and antiapoptotic Bcl-2 family proteins may be pivotal in determining the susceptibility of cells to apoptotic signals.36,37 In accordance with this hypothesis, Bcl-2/Bax ratios determined responsiveness of B-CLL cells in vitro and/or in vivo to treatment with prednisolone,38 chlorambucil,19,36 or fludarabine.20,39 Our data demonstrate that neither the Bcl-2 nor Bax status of B-CLL cells predicted their sensitivity to TC-A treatment and thus should not hamper a potential clinical application of TC-A in B-CLL.
Our analyses of caspases involved in TC-A—induced apoptosis in primary B-CLL cells demonstrated the activation of caspase-3 (Figures 5 and 7) and caspase-8 (Figure 7). An important role of caspases in TC-A—triggered cell death can be derived from our data that the broad caspase inhibitor zVAD-fmk partially blocked this cell death program (Figure 6). Functional activation of caspase-8 in drug-induced apoptosis has been demonstrated in B-CLL.40,41 In line with these data, caspase-8 defects are relevant for development of drug resistance as recently demonstrated in the neuroblastoma cell line model42 and in a broader spectrum of tumors.32 In TC-A—induced apoptosis, caspase-8 does not seem not to be the essential inducer caspase because LETD-fmk, the specific inhibitor for caspase-8, did not protect B-CLL cells from apoptosis (Figure 7B). That caspase-8 is unimportant for the ER-stress apoptotic pathway was also confirmed by our previous results in the T-ALL model12 and by a recent study of Rao and coworkers in murine fibroblasts.43 In contrast to a specific signal for caspase-8, we failed to detect Bid protein expression or cleavage in all primary B-CLL cell samples analyzed (I.T., unpublished observation, November 2002), which could hamper the activation of the mitochondrial apoptotic pathway in B-CLL cells. Whether lack of Bid expression reduces sensitivity of B-CLL cells to other cytotoxic treatment utilizing a caspase-8—dependent cell death pathway and whether this could be overcome by treatment of cells with TC-A remains to be determined.
Our analyses revealed that TC-A treatment of B-CLL cells activated the so-called ER-stress pathway (reviewed by Welihinda et al44 ), leading to the up-regulation of HSP70, a known molecular chaperone with antiapoptotic function.45 The increase of HSP70 expression levels was found to occur to a comparable extent in neoplastic B cells (Figures 8, 9) and T cells (data not shown) of B-CLL patients undergoing apoptosis. The finding of an inhibitory role of HSP70 in the formation and activation of the apoptosome45, 46, 47 has initiated the analysis of its involvement in tumor cell survival. These studies revealed an important role of HSP70 for the basal survival of human cancer cells of various origins as well as for their protection against drug-induced apoptosis.45,48, 49, 50 Our data revealed that the degree of HSP70 expression as a biologic marker of ER stress was positively correlated with the percentage of dying cells, indicating that HSP70 was unsuccessful in rescuing cells from TC-A—mediated death.
Apart from its role as a modifier of apoptosis, HSP70 can augment a tumor-specific immune response by stimulating the release of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) from monocytes or dendritic cells51 via binding to CD91, the common HSP receptor.52 More specifically, HSP70 in complex with tumor-derived antigens promotes their uptake and cross-presentation to immunocompetent T cells, an effect currently utilized in certain tumor vaccination protocols.53 It can be speculated from our data that the deficient immune system of B-CLL patients might benefit from TC-A by the rather low degree of T-cell suppression (Figures 1, 2) and, simultaneously, by its potential local immunostimulatory effects involving HSPs. These issues will be addressed in future studies.
Recently, a new protease named caspase-12 has been identified in the murine system.54 This caspase is associated with the ER and acts as initiator caspase during ER-stress—mediated apoptosis.54 A human functional homolog to murine caspase-12 is still lacking, because the human caspase-12 gene contains frame-shift mutations at least at one allele, which changes the open reading frame and produces a truncated form of human caspase-12, presumably lacking proteolytic activity.55,56 Whether a functional homolog of murine caspase-12 is involved in TC-A—induced ER stress in the human system can only be decided after its identification.
B-CLL follows heterogeneous clinical courses, and several clinical and biologic parameters have been identified that predict the response to therapy and the outcome of patients. Response to chemotherapeutic agents including fludarabine phosphate is usually more frequent in early stages of disease.57 A high CD38 expression level on B-CLL cells has previously been shown to be an independent prognostic factor,27 occurring more often in advanced stages of disease.58 Furthermore, CD38 expression seems to serve as a predictor of failure of fludarabine treatment.58 It was therefore proposed that the evaluation of CD38 expression levels should be used for identifying a cohort of patients requiring novel chemotherapeutic approaches.58 Our analyses revealed that unfavorable stage of disease and high CD38 expression levels are not predictive for a TC-A—insensitive phenotype of B-CLL cells, so that TC-A might represent such a novel approach.
In conclusion, the present study indicates that TC-A represents a potential cytotoxic agent for the treatment of B-CLL. It may be particularly useful in patients who are resistant to standard chemotherapy because it appears to activate a novel cell death pathway independent of the canonical regulators of apoptosis. Furthermore, its partial T-cell sparing effect and its potential local immunostimulatory effects seem attractive for these patients suffering from a severe immunodeficiency.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-08-2501.
Supported by a grant of the Academy of Sciences (G.A.; DOC-14/2001), the Austrian Science Foundation (I.T.; T95-PAT, P16153), the Austrian National Bank (R.G.; ÖNB-8222), the Tyrolean Cancer Aid, the Austrian Cancer Aid, and the Province of Tyrol (R.G.). Also supported by the Verein für Klinische Malignom- und Zytokinforschung (G.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Ms Rajam Csordas-Iyer for critical reading and editorial assistance.