Abstract
Conventional therapy for childhood acute lymphoblastic leukemia (ALL) includes prednisone and oral 6-mercaptopurine. Prior observations suggested potential advantages for dexamethasone over prednisone and for intravenous (IV) over oral 6-mercaptopurine, which remain to be validated. We report the results of a randomized trial of more than 1000 subjects that examined the efficacy of dexamethasone and IV 6-mercaptopurine. Children with National Cancer Institute standard-risk ALL were randomly assigned in a 2 × 2 factorial design to receive dexamethasone (6 mg/m2/d) for 28 days in induction, plus taper, compared with prednisone (40 mg/m2/d). The second randomized assignment was for daily oral or weekly IV 6-mercaptopurine during consolidation. During maintenance, 5 days of the randomized steroid was given monthly, at the same dose, and all patients received daily oral 6-mercaptopurine. During delayed intensification, all patients received a dexamethasone dosage of 10 mg/m2/d for 21 days, with taper. Intrathecal (IT) methotrexate was the sole central nervous system–directed therapy. Patients randomly assigned to receive dexamethasone had a 6-year isolated central nervous system–relapse rate of 3.7% ± 0.8%, compared with 7.1% ± 1.1% for prednisone (P = .01). There was also a trend toward fewer isolated bone marrow relapses with dexamethasone. The 6-year event-free survival (EFS) was 85% ± 2% for dexamethasone and 77% ± 2% for prednisone (P = .002). EFS was similar with oral or IV 6-mercaptopurine; however, patients assigned to IV 6-mercaptopurine had decreased survival after relapse.
Introduction
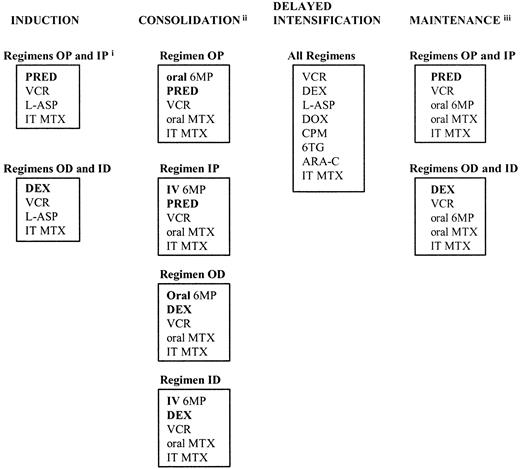
According to current National Cancer Institute (NCI) criteria,1 children older than 1 year and younger than 10 years who have white blood cell (WBC) counts lower than 50 × 109/L are considered at standard risk. In this report, we detail treatment outcomes for standard-risk acute lymphoblastic leukemia (ALL) patients treated on the Children's Cancer Group (CCG)-1922 trial. All patients received a 3-drug induction phase and experienced a 3-month consolidation phase with 6-mercaptopurine (6-MP), methotrexate, vincristine and steroid; a 2-month delayed intensification (DI) phase2; and a 20- or 32-month maintenance phase for girls and boys, respectively. The details of therapy are depicted in Figure 1.
CCG 1922 schema.
(i) Abbreviations: OP, oral mercaptopurine/prednisone; IP, intravenous mercaptopurine/prednisone; OD, oral mercaptopurine/dexamethasone; ID, intravenous mercaptopurine/dexamethasone; PRED, prednisone; VCR, vincristine; L-ASP, L-asparaginase; IT, intrathecal; MTX, methotrexate; DEX, dexamethasone; 6MP, mercaptopurine; IV, intravenous; DOX, doxorubicin; CPM, cyclophosphamide; 6TG, thioguanine; ARA-C, cytarabine. (ii) Patients with central nervous system leukemia at diagnosis received 2400 cGy cranial radiation and 600 cGy spinal radiation therapy; patients with testicular disease at diagnosis received 2400 cGy bilateral testicular radiation therapy. (iii) Cycles of maintenance therapy continued for 20 months (girls) or 32 months (boys) from the start of maintenance therapy. Boldface signifies differences in therapy.
CCG 1922 schema.
(i) Abbreviations: OP, oral mercaptopurine/prednisone; IP, intravenous mercaptopurine/prednisone; OD, oral mercaptopurine/dexamethasone; ID, intravenous mercaptopurine/dexamethasone; PRED, prednisone; VCR, vincristine; L-ASP, L-asparaginase; IT, intrathecal; MTX, methotrexate; DEX, dexamethasone; 6MP, mercaptopurine; IV, intravenous; DOX, doxorubicin; CPM, cyclophosphamide; 6TG, thioguanine; ARA-C, cytarabine. (ii) Patients with central nervous system leukemia at diagnosis received 2400 cGy cranial radiation and 600 cGy spinal radiation therapy; patients with testicular disease at diagnosis received 2400 cGy bilateral testicular radiation therapy. (iii) Cycles of maintenance therapy continued for 20 months (girls) or 32 months (boys) from the start of maintenance therapy. Boldface signifies differences in therapy.
In CCG-1922, 2 hypotheses were tested. The first hypothesis was that dexamethasone will be superior to prednisone in preventing central nervous system (CNS) relapse and provide better event-free survival (EFS). Dexamethasone provides better CNS penetration than prednisone.3 The superior cytotoxicity of dexamethasone is not explained fully by the conventional 6:1 to 7:1 ratio of glucocorticoid activity.4 Although overall EFS was similar, Cancer and Leukemia Group B (CALGB) found that children randomly assigned to dexamethasone had a lower CNS relapse rate than those assigned to prednisone.5 The Dutch ALL Study VI and Dana Farber Consortium replaced prednisone with dexamethasone and found better outcomes than a historical control.6-9 The second hypothesis was that weekly intravenous (IV) 6-MP will provide higher intracellular accumulation of thioguanine nucleotides, resulting in better EFS than daily oral 6-MP. Poor outcome has been linked to a lesser accumulation of intracellular thioguanine nucleotides.10,11 Intravenous administration of 6-MP provides better bioavailability and less interpatient variability.12-14 Preliminary results from a feasibility trial showed a striking benefit from IV 6-MP.15 16 Thus, patients on CCG-1922 were assigned randomly to receive dexamethasone or prednisone during induction, consolidation, and maintenance and daily oral or weekly IV 6-mercaptopurine during consolidation. All patients received dexamethasone during delayed intensification and daily oral 6-mercaptopurine during maintenance.
Patients, materials, and methods
Patients
CCG-1922 opened in March 1993 and closed in August 1995. Eligible patients included those who were 1 to less than 10 years of age with WBC counts lower than 50 × 109/L. Patients with lymphoma syndrome or French-American-British (FAB) L3 lymphoblasts were excluded. Lymphoma syndrome is defined as the presence of one of the following clinical features: massive lymphadenopathy; massive splenomegaly; large mediastinal mass, or one of the following laboratory features: WBC counts higher than 50 × 109/L; hemoglobin (Hgb) levels higher than 100 gm/L; more than 25% CD2-positive blasts.
During the first 6 months of the CCG-1922 study, a subset of standard-risk patients (aged 1 to less than 2 years with WBC counts lower than 50 × 109/L; aged 2 to less than 10 years with WBC counts of 10 × 109 to less than 50 × 109/L; and boys aged 2 to less than 10 years with WBC counts less than 10 × 109/L and platelet counts less than 100 × 109/L) was enrolled in the CCG-1891 study for patients with intermediate-risk ALL until accrual goals were met.17 After the closure of CCG-1891 and for the remainder of the study period, all NCI-defined standard-risk patients were enrolled in CCG-1922, excluding those with lymphoma syndrome. Diagnosis of ALL was based on morphologic, biochemical, and flow cytometric features of leukemic cells, including lymphoblast morphology on Wright-Giemsa–stained bone marrow smears, negative staining for myeloperoxidase, and reactivity with monoclonal antibodies to B-lineage–associated or T-lineage–associated lymphoid differentiation antigens, as described before.18
Treatment protocol
The National Cancer Institute and local institutional review boards approved the protocol. Written informed consent was obtained from guardians or patients, as per National Institutes of Health (NIH) guidelines. Patients were assigned randomly at diagnosis in a 2 × 2 factorial design to one of 4 treatment arms shown in Figure 1 (Regimen OP: daily oral mercaptopurine and prednisone; Regimen IP: weekly IV mercaptopurine and prednisone; Regimen OD: daily oral mercaptopurine and dexamethasone; Regimen ID: weekly IV mercaptopurine and dexamethasone). Details of therapy are listed in Table1. Patients were required to have M1 (< 5% blasts) or M2 (5%-25% blasts) marrow status by the end of induction. Patients with a M2 day-28 marrow required a M1 marrow status by day 14 of consolidation therapy to continue on study. Girls were treated for approximately 26 months and boys for 38 months.
Therapy modifications for toxicity
Treatment was modified to adjust for morbidity and to optimize delivery. Chemotherapy was not interrupted unless hepatic transaminases (alanine aminotransferase [ALT] or aspartate aminotransferase [AST]) levels were higher than 1000 units/L on 2 determinations at least 1 week apart or the total bilirubin level was higher than 0.02 g/L. Maintenance chemotherapy was not interrupted unless the absolute neutrophil count fell to lower than 750 × 106/L or the platelet count to lower than 75 × 109/L. Therapy was not increased above prescribed doses for patients with persistent elevations of absolute neutrophil count.
Toxicity grading
To evaluate better the morbidity anticipated from the experimental interventions, specific toxicity questions were added to the data forms 14 months after initiation of the study. The specific toxicities evaluated included avascular necrosis, clinically significant infections determined by treating physician, grade 3 or 4 pancreatitis, steroid-associated proximal myopathy, any CNS toxicity, and hematuria or renal stones.19 The percentage of patients by phase for whom these data were available was 69% for induction; 79% for consolidation; 87% for delayed intensification; and 93+% for maintenance.
Karyotype analysis
Leukemic karyotypes were accepted after central review of studies performed at the local institutions.20
Statistical methods
Sample size and power calculations were based on a proportional hazard assumption for the treatment regimen, with few treatment failures assumed after 5 years of follow-up. The study used a 2 × 2 factorial design for examining effects of (1) oral versus IV 6MP in the consolidation phase, and (2) dexamethasone versus prednisone in induction, consolidation, and maintenance. An accrual of 1050 randomized patients was planned to have in excess of 80% power (2-sided log-rank test) for detecting a change in 5-year EFS from an assumed 80% baseline to 87.5%, representing a relative risk (RR) of 0.598 for the better regimen. The study also had power above 80% (2-sided Gray statistic) for detecting a change in the incidence of CNS relapse from 10% to 5%, representing an RR of 0.487 for the better regimen. The study was not designed to detect significant differences in bone marrow relapses.
Assignments were randomized at study entry. Data were analyzed in July 2001 using a January 2001 cutoff. The primary end point used for life table comparisons of treatment regimen outcomes and prognostic factor effects was EFS, which was defined as the time to first occurrence of any one of the following events: induction death, no response to induction therapy, relapse after remission at any site, death in remission, or second malignant neoplasm. A secondary end point of interest was incidence of isolated CNS relapse as the initial event. Analysis of CNS incidence was done using a cumulative incidence function.21 Selected comparisons of overall survival outcomes also are provided. Comparison of treatment regimen outcomes was done with the intent-to-treat approach. EFS and survival life table estimates were done with the Kaplan-Meier (KM) method,22and these estimates are provided at 6 years of follow-up. The standard deviation of the KM estimate was calculated using the Peto variance formula.23 Relative hazard rates were estimated by the log-rank observed divided by expected (O/E) method. Chi-square tests for homogeneity of distributions were used in some comparisons (similarity of patient characteristics, patterns of outcome events, etc). Multivariate analysis of prognostic factors was done with the Cox proportional hazards model.24
Results
Patient characteristics
Among 1080 patients entered on study, 19 were deemed ineligible because of lack of adequate consent or incorrect diagnosis, and one was not assigned randomly. Among the 1060 remaining patients, 530 were assigned randomly to dexamethasone and 530 to prednisone. Canadian institutions that participated in this study were unable to administer IV 6-mercaptopurine on an outpatient basis, and all 30 patients treated at Canadian institutions were not assigned randomly with respect to the 6-MP question. Among 1030 patients assigned randomly with respect to the 6-MP route, 514 were assigned to receive daily oral administration and 516 were randomized to receive weekly IV administration. Presenting characteristics were not significantly different in the treatment groups and are detailed in Table2.
Treatment outcome
Blast percentage in marrow was determined on day 7 of induction therapy. Overall, 53% of all patients achieved M1 marrow status (fewer than 5% blasts); 25% of patients had M2 marrow status (5%-25% blasts); and 22% had M3 status (more than 25% blasts). There was no difference in day-7 marrow response or induction end marrow status by randomized steroid. At the end of the induction phase, 99% of patients had M1 marrow status, 10 had M2 marrow status, and one had M3 marrow status. The 6-year EFS rate for the entire cohort of patients treated on CCG-1922 was 81% ± 2% with a 6-year survival rate of 92% ± 1%.
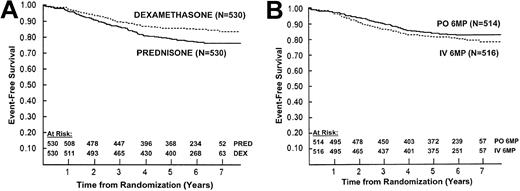
Outcome was significantly improved for patients assigned to dexamethasone (6-year EFS = 85% ± 2%) compared with that of patients assigned to prednisone (6-year EFS = 77% ± 2%;P = .002; RR = 0.65; Figure2A). Survival was similar between patients treated with dexamethasone and prednisone, with 6-year estimates of 93% ± 1% and 91% ± 1%, respectively (P = .17; RR = 0.74). For the randomized comparison of daily oral and weekly IV 6-MP, 6-year EFS was 82% ± 2% and 80% ± 2%, respectively (P = .2; RR = 0.83; Figure2B). Survival was worse for patients treated with IV versus oral 6-MP, with 6-year estimates of 90% ± 1% and 94% ± 1%, respectively (P = .02; RR = 1.7).
EFS by randomized treatment.
(A) Event-free survival (EFS) by randomized steroid. The 6-year EFS ± standard error is 85% ± 2% in patients randomized to dexamethasone and 77% ± 2% in patients randomized to prednisone (P = .002). (B) Event-free survival (EFS) by 6-mercaptopurine route randomization during consolidation. The 6-year EFS ± standard error is 82% ± 2% in patients randomized to daily oral (PO) 6-mercaptopurine and 80% ± 2% in patients randomized to weekly intravenous (IV) 6-mercaptopurine in consolidation (P = .2). All patients received oral 6-mercaptopurine during maintenance.
EFS by randomized treatment.
(A) Event-free survival (EFS) by randomized steroid. The 6-year EFS ± standard error is 85% ± 2% in patients randomized to dexamethasone and 77% ± 2% in patients randomized to prednisone (P = .002). (B) Event-free survival (EFS) by 6-mercaptopurine route randomization during consolidation. The 6-year EFS ± standard error is 82% ± 2% in patients randomized to daily oral (PO) 6-mercaptopurine and 80% ± 2% in patients randomized to weekly intravenous (IV) 6-mercaptopurine in consolidation (P = .2). All patients received oral 6-mercaptopurine during maintenance.
We found no evidence of an interaction between the 2 assigned treatment comparisons. As such there was no difference in EFS between patients assigned to receive oral or IV 6-MP within the dexamethasone or prednisone subsets. Likewise, the significant improvement in EFS for the overall cohort of patients assigned to receive dexamethasone compared with those assigned to receive prednisone was preserved among subsets of patients treated with daily oral or weekly IV 6-MP. The survival advantage observed among the overall cohort of patients treated with oral 6-MP was preserved among patients who received dexamethasone or prednisone. Also, survival for patients who received dexamethasone or prednisone was similar within the subsets of patients treated with oral or intravenous 6-MP. The 6-year EFS by regimen was OD (oral mercaptopurine/dexamethasone) 86% ± 2%; ID (intravenous mercaptopurine/dexamethasone) 84% ± 2%; OP (oral mercaptopurine/prednisone) 78% ± 3%; and IP (intravenous mercaptopurine/prednisone) 77% ± 3% (P = .01).
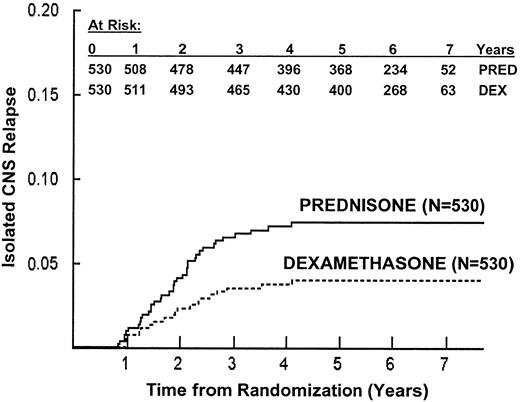
The primary reason for treatment failure was marrow relapse. Table3 lists first events by treatment regimen. As hypothesized, the cumulative incidence of isolated CNS relapse was lower for dexamethasone patients than for prednisone patients, with 6-year cumulative estimates of 3.7% ± 0.8% and 7.1% ± 1.1%, respectively (P = .01; Figure3). In addition, dexamethasone patients had a trend toward fewer marrow relapses, with 6-year estimates of 7.9% ± 1.3% and 11.1% ± 1.5%, respectively (P = .08). The cumulative incidence of isolated CNS or bone marrow relapse was similar for patients who received oral or IV 6-MP (data not shown). Thus far, 3 second malignancies have been reported (Table 3).
Isolated central nervous system (CNS) relapse by randomized steroid.
The 6-year risk of isolated CNS relapse ± SE is 3.7% ± 0.8% in patients randomized to receive dexamethasone, and 7.1% ± 1.1% in patients randomized to receive prednisone (P = .01).
Isolated central nervous system (CNS) relapse by randomized steroid.
The 6-year risk of isolated CNS relapse ± SE is 3.7% ± 0.8% in patients randomized to receive dexamethasone, and 7.1% ± 1.1% in patients randomized to receive prednisone (P = .01).
Prognostic factors
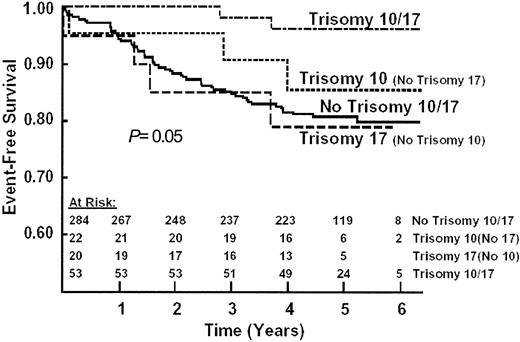
Univariate analysis of a large number of clinical and laboratory presenting features found adverse prognostic significance of age, spleen enlargement, hypodiploidy, and low hyperdiploidy. Children in the 2-to-5-year age range had only about half as many events as those in the 1-year age group or the older than 6 years age group (RR = 0.51 and 0.56, respectively, P = .0001). The WBC count had a minimal prognostic effect (P = .20) in the restricted WBC eligibility range for this study. Trisomy 10 and double trisomies 10 and 17 had favorable prognostic significance (P = .03 and .007, respectively) in 379 patients who had adequate banded karyotype analysis. Outcomes were similar for patients with or without acceptable karyotypes. The 6-year EFS results were 92% ± 5% for trisomy 10 and 81% ± 3% for those without trisomy 10 independent of other trisomy status (log-rank,P = .03). For the analysis of combined trisomy 10/17 status (P = .03), the 6-year results were 96% ± 4% for combined trisomy 10/17 and 81% ± 3% for those not having both trisomy 10 and trisomy 17 (P = .007) (Figure4).
EFS by trisomy status.
The 6-year EFS ± standard deviation was 93% ± 6% for both trisomies; 85% ± 17% for trisomy 10 without trisomy 17; 79% ± 18% for trisomy 17 without trisomy 10; and 80% ± 4% for no trisomy 10 or 17 (log rank P = .09 for difference among the 4 groups; P = .02 for both trisomies versus all others).
EFS by trisomy status.
The 6-year EFS ± standard deviation was 93% ± 6% for both trisomies; 85% ± 17% for trisomy 10 without trisomy 17; 79% ± 18% for trisomy 17 without trisomy 10; and 80% ± 4% for no trisomy 10 or 17 (log rank P = .09 for difference among the 4 groups; P = .02 for both trisomies versus all others).
The favorable prognostic significance of trisomy 10 was observed among dexamethasone and IV 6-MP patients (P = .03 and .03, respectively), but not in those assigned to oral 6-MP or prednisone (P = .19 and .47, respectively). None of the 29 patients with trisomy 10 who were assigned to dexamethasone have relapsed.
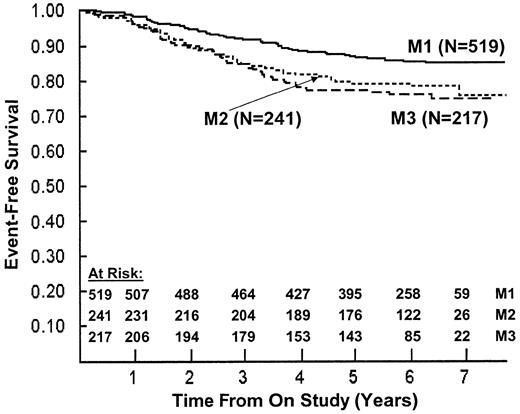
Marrow response on day 7 of therapy also was a significant prognostic factor (P = .002; Figure 5). We found worse outcomes for patients with M2 (RR = 1.59) or M3 (RR = 1.82) versus M1 marrow status. Similar trends were observed in the IV 6-MP, prednisone, and dexamethasone subsets but not in the oral 6-MP subset. Dexamethasone was superior to prednisone for patients with day 7 M1 marrow status (6-year EFS estimates of 89% ± 2% versus 82% ± 3%), M2 marrow status (6-year EFS estimates of 83% ± 4% versus 77% ± 4%), and M3 marrow status (6-year EFS estimates of 82% ± 4% versus 71% ± 4%). Outcomes were similar within day-7 marrow status subsets for oral and IV 6-MP patients.
EFS by day 7 bone marrow response.
The 6-year EFS ± SD was 85% ± 2% for M1 (fewer than 5% blasts, solid line); 79% ± 3% for M2 (5%-25% blasts, dotted line); and 76% ± 3% for M3 (fewer than 25% blasts, dashed line) (P = .002).
EFS by day 7 bone marrow response.
The 6-year EFS ± SD was 85% ± 2% for M1 (fewer than 5% blasts, solid line); 79% ± 3% for M2 (5%-25% blasts, dotted line); and 76% ± 3% for M3 (fewer than 25% blasts, dashed line) (P = .002).
Toxicities
Infectious complications and deaths.
Patients assigned to dexamethasone or prednisone had identical incidence of bacteremia during induction (13%). Fungal infections were found in 6 dexamethasone patients and 10 prednisone patients. Viral infections were found in 11 dexamethasone patients and 14 prednisone patients. Incidence of fever and neutropenia and duration of hospital stay were similar between steroid groups, as were supportive care interventions. Six patients died from infections during induction or the month after (0.6%): 2 prednisone patients (Staphylococcus aureus, varicella) and 4 dexamethasone patients (adult respiratory distress syndrome in 3, 2 of whom also had alpha-streptococcal sepsis, and parainfluenza pneumonia in one). During delayed intensification, when all patients receive dexamethasone, 5 patients died from infections: 4 prednisone-assigned patients and one dexamethasone-assigned patient. Infectious deaths during maintenance included 2 patients in the prednisone group (Pseudomonas; alpha-streptococcus) and one in the dexamethasone group (varicella).
Steroid myopathy.
Reversible proximal myopathy was seen during induction, early consolidation, and delayed intensification, but not in maintenance. No patient had grade 4 toxicity (ie, respiratory embarrassment). Incidence of grades 1 to 3 steroid myopathy during induction and early consolidation was 1.5% in prednisone patients and 6.3% in dexamethasone patients (P < .0001 by chi-square). Grade 3 weakness (ie, inability to ambulate) was seen in 22 dexamethasone patients (4.1%) and only 2 prednisone patients (0.3%;P < .0001 by chi-square). Grade 3 myopathy was more common in younger children and boys. During delayed intensification, when all patients received dexamethasone, incidence of myopathy was 0.7% and was the same for prednisone- and dexamethasone-assigned patients. Weakness was reversible when the steroid was discontinued, and none recurred during monthly 5-day steroid pulses in maintenance.
Pancreatitis.
Incidence of grade 3 or 4 amylase elevation in induction was 1.3% (7 of 528) in dexamethasone patients and 0.4% (2 of 529) in prednisone patients (P = .09). Symptomatic pancreatitis was reported in 5 dexamethasone patients and one prednisone patient. All recovered without sequelae.
Isolated hepatic transaminase elevations.
The incidence of grade 3 or 4 ALT elevations was similar among prednisone and dexamethasone patients. During consolidation, elevations were more common among daily oral 6-MP patients (19%; 104/537) than among weekly IV 6-MP patients (13%; 64/500;P = .003).
Hyperglycemia.
Patients who received dexamethasone had a significantly higher incidence of reversible grade 3 or 4 hyperglycemia (26/528; 5%) versus those who received prednisone (8/529; 1.5%; P = .001). Data on specific use of insulin for hyperglycemia was not collected.
Avascular osteonecrosis.
Two prednisone patients and one dexamethasone patient were diagnosed with avascular necrosis of the ankles early in maintenance therapy.
Neuropsychiatric effects.
Two patients had consistent dysesthesia and agitation with each 5-day course of dexamethasone in maintenance. These symptoms were unresponsive to sedatives and to reduction of steroid dosage by 50%. Both patients were switched to prednisone with no or less intense reactions. Four patients were switched from dexamethasone to prednisone at their parents' requests because of similar reactions.
Other toxicity.
There were no apparent differences in incidence of other rare toxicities by assigned treatment, including seizures, CNS hemorrhage, thrombotic stroke, hematuria, and renal stones. Incidence and severity of other toxicities noted on the CCG Toxicity Rating Scale were not different by steroid or 6-MP route randomization.
Discussion
Dexamethasone versus prednisone
Against a background of 11-drug therapy, replacement of prednisone with dexamethasone at conventional equivalence provided a 34% reduction in risk of any relapse for children with standard-risk ALL. Corticosteroids are one of the mainstays in therapy for acute lymphoblastic leukemia.4,25 Attempts to delay corticosteroids until after induction have resulted in inferior results.26 The Berlin-Frankfurt-Münster (BFM) group, the Children's Cancer Group, and United Kingdom Acute Lymphoblastic Leukemia (UKALL) have used dexamethasone as part of successful postinduction intensification strategies.27-30 In the current randomized comparison, we observed a statistically significant and clinically important decrease in rate of isolated CNS relapses and an increase in EFS with dexamethasone, as hypothesized. The study was not designed with a sample sufficient to determine a statistically significant reduction in bone marrow relapses. However, the absolute reduction in bone marrow relapses with dexamethasone was identical to the reduction in CNS relapses, in the range of 3% to 4%. The benefit of dexamethasone was apparent for oral and IV 6-MP patients and for day-7 M1, M2, and M3 marrow status patients.
Although no differences were found in duration of hospitalization or supportive care interventions, the substitution was not truly isotoxic. Published equivalency tables suggest that dexamethasone is approximately 7-fold more potent than prednisone. We chose a dose of 6 mg/m2/d to replace 40 mg/m2/d of prednisone. However, in vitro assays of phytohemagglutinin-induced lymphocyte proliferation suppression have shown dexamethasone to be 10-fold more active than prednisolone.31 In vitro assays of cytotoxicity have shown dexamethasone to be 6 to 16 times more potent against ALL blast cells.32,33 In vivo, dexamethasone has a longer biologic half-life.34Thus, the superior results that we have obtained with dexamethasone may follow, in part, from the fact that 6 mg/m2/d of dexamethasone might be biologically equivalent to at least 100 mg/m2/d of prednisone. We observed a higher incidence of reversible corticosteroid-induced side effects (hyperglycemia and myopathy) in dexamethasone patients. Our studies have shown clearly that dexamethasone at a dose of 6 mg/m2 was superior to prednisone at 40 mg/m2.
Steroid-related complications
Infectious complications.
Hurwitz et al described an increased incidence of gram-negative bacteremia and induction death in a group of patients who received dexamethasone during induction compared with historical controls who received prednisone.35 In contrast, we did not see any differences in infectious complications during induction or any other phase of therapy. Hurwitz et al used doxorubicin during induction, unlike this trial, which might have deepened neutropenia and delayed neutrophil recovery. In addition, dexamethasone may blunt the febrile response to infection, delaying recognition of fever and initiation of antibiotic therapy, thus increasing risk of death. Although CCG and others have used dexamethasone with anthracycline in delayed intensification for more than 20 years, we concur with Hurwitz et al's caution about prolonged exposure to dexamethasone in conjunction with myelosuppressive chemotherapy.
Skeletal complications.
Avascular necrosis of bone is a known complication of corticosteroids. Mattano et al36 found an incidence of 14% in patients older than 10 years versus 1% in younger patients. Factors increasing risk include being older than 10, female, and white, as well as the number of dexamethasone-containing delayed intensification courses.36 Others have suggested that avascular necrosis may be related to the combination of agents used in delayed intensification and not to dexamethasone alone.37 All patients in the current study were younger than 10 years at diagnosis, and only 3 were diagnosed with symptomatic avascular osteonecrosis, all after receiving dexamethasone in the delayed intensification phase. However, there was no difference in this rare occurrence by randomized steroid received.
Patients who receive therapy for ALL are at risk of other skeletal abnormalities including osteopenia, osteoporosis, and symptomatic and asymptomatic fractures. Disease status and use of intensive corticosteroids may contribute to that risk.38 Atkinson et al found increased subclinical fractures in patients who received dexamethasone during induction versus historical controls who received prednisone and otherwise identical therapy.38 Strauss et al also found increased bony morbidity, including osteonecrosis, when dexamethasone was substituted for prednisone.39 In the current study, clinical fractures were not reported for any patient. We did not screen for subclinical fractures.
Steroid myopathy.
Proximal myopathy is a complication of corticosteroid therapy.40 Vincristine, usually given with corticosteroids during ALL therapy, also may cause or exacerbate neuropathy.40 Myopathy may be more prevalent with dexamethasone than with other corticosteroids.41 Patients assigned to dexamethasone had significantly greater incidence of proximal muscle weakness during or immediately after induction therapy in which they received 28 days of corticosteroid, followed by a taper. Male sex and younger age were risk factors for development of more severe weakness. Weekly vincristine was given during induction. It may be difficult to clinically differentiate corticosteroid myopathy from vincristine neuropathy, so it is possible that those patients may have had weakness from both effects. In most affected patients, ambulation was impaired. However, all patients recovered completely without recurrence during corticosteroid pulses in maintenance.
Neuropsychiatric effects.
Agitation and even frank psychosis are known complications of corticosteroid use.42,43 The mechanism is multifactorial because of effects on levels of biogenic amines, pro-opiomelanocortin (POMC)–related peptides and somatostatin, and on brain electrophysiology.44 Risk depends upon corticosteroid dose, potency, and individual susceptibility. Although a rare occurrence, 2 patients in the current study were unable to tolerate dexamethasone pulses in maintenance because of severe agitation, and 4 others were switched to prednisone at their parents' requests. In some cases, we found that concomitant sedatives and potassium supplements may decrease agitation level. Otherwise, a change to prednisone reduced or eliminated symptoms.
Waber et al linked dexamethasone with cognitive dysfunction after comparing 2 cohorts of children who received one drug or the other.45 Methodologic considerations led Loring and Meador to state in an accompanying editorial that “The results do not answer the question regarding the presence of differential long-term cognitive side effects of dexamethasone versus prednisone.”46 (p195) The dexamethasone and prednisone patients were populations of convenience. In the report by Waber et al,45 70% of the dexamethasone patients received prophylactic cranial radiation, as did half the prednisone patients. The dexamethasone patients were younger at diagnosis and fewer had parents who had gone to college. In the current study, no patient received prophylactic cranial radiation. Craniospinal irradiation was reserved for the less than 1% with overt CNS leukemia at diagnosis. Dexamethasone doses differed from doses in the current study. Neuropsychologic testing in a cohort of long-term survivors of this trial would address the question most productively.
Intravenous 6-mercaptopurine
Daily oral 6-mercaptopurine is a mainstay in the treatment of childhood ALL.47 In vitro studies also have shown correlation between blast sensitivity to thiopurines and risk of relapse.48 Some have shown a link between intracellular accumulation of thioguanine nucleotides and outcome. Others have linked more myelosuppression during maintenance therapy with daily oral 6-MP and weekly oral methotrexate with better EFS, which suggests that biologic activity of these drugs has an important bearing on outcome.11,49-51 More recently, against a background of more effective therapy, Relling et al52 found that prolonged interruptions of oral 6-MP and methotrexate are linked to lesser dose intensity and inferior outcome. Van Eys and coworkers were unable to improve outcome by escalating the dose of oral 6-MP to modest levels of leukopenia.53
The bioavailability of oral 6-MP is poor, and its interpatient variability is wide. Intravenous 6-MP has better bioavailability and less interpatient variability.47,54 The Pediatric Oncology Group (POG) reported favorable outcomes with alternating weeks of IV 6-MP (1000 mg/m2 over 6 hours) and oral 6-MP (50 mg/m2/d × 7 days) in conjunction with IV methotrexate for early intensification in standard-risk patients.15,16,55 However, recently published randomized trials showed no difference or worse outcomes for patients treated with IV versus oral 6-MP.56 In the current trial, we found no difference in EFS. We did observe a significantly improved overall survival for oral 6-MP patients because of an increased risk of death after relapse in IV 6-MP patients.57 Daily use may be superior to more intermittent IV bolus use because of the antiangiogenic properties of 6-MP metabolites.58
Weekly IV 6-MP does not improve outcome despite reports of superior bioavailability in the literature. In fact, IV 6-MP patients have unexplained shorter survival after relapse than oral 6-MP patients. In contrast, dexamethasone significantly improves outcomes of children with standard-risk ALL by decreasing relapses without causing undo short-term toxicity. This improvement was apparent even though all patients received dexamethasone during delayed intensification. In this standard-risk population younger than 10 years who received no anthracycline during induction, neither infectious complications nor avascular necrosis of bone were problematic. For older patients at higher risk of avascular necrosis, and for patients who receive anthracycline in induction, dexamethasone should be introduced only in the context of a clinical trial where benefits and harms may be assessed accurately.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-08-2454.
Supported in part by research grants including Children's Cancer Group Chairman's Grant No. CA-13539 from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce C. Bostrom, c/o Children's Oncology Group, Attn Shaun Mason, PO Box 60012, Arcadia, CA 91066-6012; e-mail: bostrom@childrenshc.org; cc:smason@childrensoncologygroup.org.