Abstract
Mutations at nucleotides 654, 705, or 745 in intron 2 of the human β-globin gene activate aberrant 3′ and 5′ splice sites within the intron and prevent correct splicing of β-globin pre-mRNA, resulting in inhibition of β-globin synthesis and in consequence β-thalassemia. Transfection of HeLa cells expressing the 3 thalassemic mutants with modified U7 snRNA (U7.623), containing a sequence antisense to a region between the aberrant splice sites, reduced the incorrect splicing of pre-mRNA and led to increased levels of the correctly spliced β-globin mRNA and protein. A lentiviral vector carrying the U7.623 gene was effective in restoration of correct splicing in the model cell lines for at least 6 months. Importantly, the therapeutic value of this system was demonstrated in hematopoietic stem cells and erythroid progenitor cells from a patient with IVS2-745/IVS2-1 thalassemia. Twelve days after transduction of the patient cells with the U7.623 lentiviral vector, the levels of correctly spliced β-globin mRNA and hemoglobin A were approximately 25-fold over background. These results should be regarded as a proof of principle for lentiviral vector–based gene therapy for β-thalassemia.
Introduction
β-Thalassemia, a hereditary anemia caused by defects in the β-globin gene, is one of the most common genetic disorders.1 Current treatment consists of regular, lifelong blood transfusions combined with iron chelation therapy.2 The only cure, bone marrow transplantation, is limited by the scarcity of suitable donors and facilities and the high cost of the procedure, and even when available, poses significant risk.3,4 The lack of correct β-globin expression in β-thalassemia can be partially compensated for by using hydroxyurea or butyric acid to induce the expression of fetal hemoglobin (Hb F). However, these experimental treatments have not yet become an effective clinical treatment.5 Gene replacement therapy for β-thalassemia is a difficult task6; although the β-globin gene is small, its expression is tightly controlled by a large locus control region (LCR).7 Despite unquestionable success with gene replacement in mouse models of β-thalassemia,8,9 alternative methods of therapy, such as gene repair, need to be explored.10 Although defective β-globin gene expression and β-globin deficiency can be attributed to almost 200 thalassemic mutations, only 10 mutations are responsible for the majority of cases worldwide. Among those, some of the most frequent cause aberrant splicing of intron 1 (IVS1-110, IVS1-6, IVS1-5) or intron 2 (IVS2-654, IVS2-745) of the human β-globin gene.11 These mutations activate aberrant splice sites, leading to incorrectly spliced mRNAs, improper translation, and a deficiency in β-globin that ultimately results in β-thalassemia. The intron 2 mutations that are the focus of this report, IVS2-654 (C>T), IVS2-745 (C>G), and the rare IVS2-705 (T>G), create aberrant 5′ splice sites and activate the same cryptic 3′ splice site within the intron, leading to inclusion of the intron fragment in the spliced mRNA (Figure 1A).12-14 Work in this laboratory has shown that blocking the aberrant splice sites with antisense oligonucleotides forces the splicing machinery to reselect the existing correct splice sites, restoring the correct splicing pattern.15-17 Because the oligonucleotides do not remove the mutation from the gene but instead repair the defective pre-mRNA, they would require periodic administration throughout the lifetime of the thalassemic patient. Long-term or permanent expression of antisense sequences targeted to the aberrant splice sites in thalassemic pre-mRNA would improve this approach.
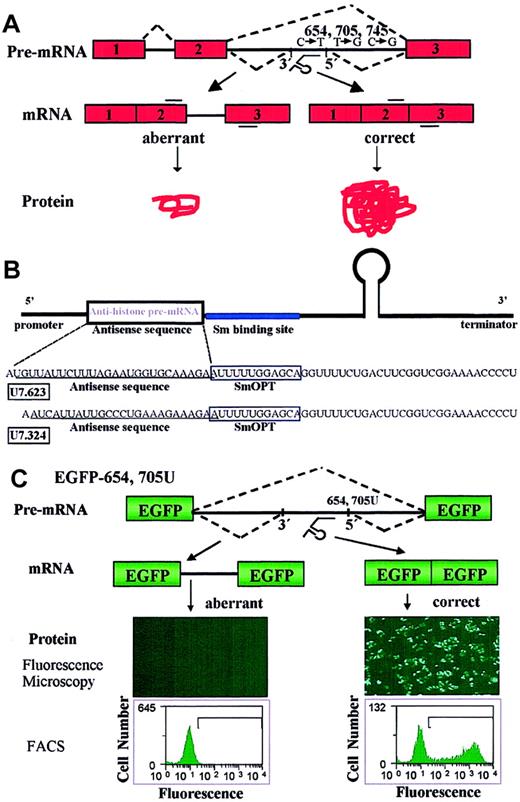
Correction of aberrant splicing by modified U7 snRNAs.
(A) Correction of splicing of β-globin pre-mRNA by modified snRNAs. Boxes indicate exons; lines, introns; short bars above and below RNA, primers used in PCR and RT-PCR analysis. The dashed lines represent correct and aberrant splicing pathways. The modified U7 snRNA targeted to the 623 sequence (U7.623, see “Materials and methods”) is depicted under the pre-mRNA. (B) Structure of modified U7 snRNA constructs. Wild-type U7 snRNA includes a stem-loop structure, the U7-specific Sm sequence, and a sequence antisense to the 3′ end of histone pre-mRNA. The promoter and 3′ terminator regions are indicated. In modified U7 snRNAs, the Sm and antisense sequences were replaced with the spliceosomal Sm sequence SmOPT55 and with antisense sequences targeted to the β-globin pre-mRNA (see “Materials and methods”). The SmOPT site is boxed and the antisense sequences are underlined. (C) Splicing modification antisense assay. Cells lines were created stably expressing the enhanced green fluorescence (EGFP) gene in which the coding sequence was interrupted by the IVS2-654 or IVS2-705U β-globin intron 2 (see “Results”). Proper splicing and translation of EGFP-654 or EGFP-705U elicited by modified antisense U7 snRNA and visualized by either fluorescence microscopy (middle panels; magnification × 4) or flow cytometry (bottom panels) provide a positive readout for antisense activity. In this and subsequent figures histograms plot EGFP fluorescence intensity versus cell number.
Correction of aberrant splicing by modified U7 snRNAs.
(A) Correction of splicing of β-globin pre-mRNA by modified snRNAs. Boxes indicate exons; lines, introns; short bars above and below RNA, primers used in PCR and RT-PCR analysis. The dashed lines represent correct and aberrant splicing pathways. The modified U7 snRNA targeted to the 623 sequence (U7.623, see “Materials and methods”) is depicted under the pre-mRNA. (B) Structure of modified U7 snRNA constructs. Wild-type U7 snRNA includes a stem-loop structure, the U7-specific Sm sequence, and a sequence antisense to the 3′ end of histone pre-mRNA. The promoter and 3′ terminator regions are indicated. In modified U7 snRNAs, the Sm and antisense sequences were replaced with the spliceosomal Sm sequence SmOPT55 and with antisense sequences targeted to the β-globin pre-mRNA (see “Materials and methods”). The SmOPT site is boxed and the antisense sequences are underlined. (C) Splicing modification antisense assay. Cells lines were created stably expressing the enhanced green fluorescence (EGFP) gene in which the coding sequence was interrupted by the IVS2-654 or IVS2-705U β-globin intron 2 (see “Results”). Proper splicing and translation of EGFP-654 or EGFP-705U elicited by modified antisense U7 snRNA and visualized by either fluorescence microscopy (middle panels; magnification × 4) or flow cytometry (bottom panels) provide a positive readout for antisense activity. In this and subsequent figures histograms plot EGFP fluorescence intensity versus cell number.
Using HeLa cell lines that model expression of thalassemic β-globin genes, it was found that U7 and U1 snRNAs modified to contain sequences antisense to the 3′ cryptic splice site in the β-thalassemic pre-mRNA led to stable reduction of the incorrect splicing of pre-mRNA and to increased levels of the correctly spliced mRNA and β-globin protein.18-20 The snRNAs were selected as antisense vectors for modification of splicing because they are expressed at high levels in a cell cycle–independent fashion and are concentrated in the nucleus, the site of splicing. Furthermore, their small size, secondary structure, and tight interactions with common Sm and other small nuclear ribonucleoprotein (snRNP)–specific proteins render them resistant to nuclease degradation.21
To lay a foundation for in vivo studies, we chose to incorporate U7 constructs into integrating viral vectors, which can confer permanent correction of the splicing defects in the ultimate target, the hematopoietic stem cell (HSC). In this respect, the lentiviral vectors appeared to be the obvious choice (for a review, see Kafri22). The ability of these vectors to efficiently transduce human and nonhuman HSCs without cytokine stimulation has been demonstrated by a number of research groups.23,24Lentiviral vector–transduced HSCs engrafted efficiently into irradiated host bone marrow and maintained long-term transgene expression in all hematopoietic lineages following primary and secondary transplantation.25,26 More importantly, lentiviral vectors have been used successfully to correct genetic deficiencies by gene replacement in cellular models of Fanconi anemia group C27 and chronic granulomatous disease (CGD),28 as well as in a mouse model of β-thalassemia.8 9
In this report, a modified U7 snRNA, either in the context of a plasmid or a lentiviral vector, was used to correct aberrant splicing of thalassemic pre-mRNA. This U7 snRNA was targeted not to the aberrant splice sites but to a recently identified splicing enhancer sequence located between them.29 This retargeting enhanced the antisense effects of the snRNA. Transfection with U7 constructs as well as transduction with a U7 lentiviral vector of HeLa cells modeling IVS2-654, IVS2-705, and IVS2-745 thalassemic splicing mutations led to effective pre-mRNA repair and restoration of expression of full-length β-globin protein. More importantly, significant correction of β-globin pre-mRNA splicing and restoration of expression of hemoglobin A was also effected by transduction of erythroid progenitor cells from patients carrying the IVS2-745 mutation.
Materials and methods
Cell lines
HeLa cell lines IVS2-654, IVS2-705, and IVS2-745 expressed human thalassemic β-globin genes.16,30 HeLa cell lines EGFP-654 and EGFP-705U expressed a construct in which the coding region of enhanced green fluorescent protein (EGFP) was interrupted by the mutated β-globin intron.31 All cell lines were grown at 37°C, 5% CO2 in minimal essential medium modified for suspension cells (S-MEM) supplemented with 5% fetal calf sera, 5% horse sera, 50 μg/mL gentamicin, and 200 μg/mL kanamycin.
Erythroid progenitor cells
Blood samples were obtained from a patient with IVS2-745/IVS2-1 β-thalassemia with informed consent as required by Italian and US regulations. Following the manufacturer's protocols, total mononuclear cells were isolated by Ficoll gradient (lymphocyte separation medium, ICN/Cappel, Aurora, OH), purified from the remaining red blood cells with ammonium chloride solution (Stem Cell Technologies, Vancouver, BC, Canada), and washed twice with Iscove modified Dulbecco medium (IMDM; Gibco, Grand Island NY) supplemented with 2% fetal bovine serum (FBS). The cells were suspended in the above medium, 30% FBS, 1% bovine serum albumin (BSA; Stem Cell Technologies), 100 μM 2-mercaptoethanol, 2 mM l-glutamine, 100 U/mL penicillin-streptomycin, 3 U/mL recombinant human epoetin-α (Epo; Amgen, Thousand Oaks, CA), and 25 ng/mL recombinant mouse stem cell factor (SCF; R & D Systems, Minneapolis, MN), and cultured at 4 × 106 cells/mL in 2-cm2 wells. After transduction with the lentiviral vector the mononuclear cells were mixed with methylcellulose medium M4434 (Stem Cell Technologies) to 1.3 × 106cells/mL and cultured in 35-mm plates for the subsequent 10 days.
Recombinant plasmid constructs
All the modified U7 snRNA genes contained their respective promoter and terminator sequences (Figure 1A). The modified U7.324 construct, in which the natural 18-nucleotide sequence complementary to the 3′ processing site of histone pre-mRNA was replaced with a 24-nucleotide sequence (AUCAUUAUUGCCCUGAAAGAAAGA) antisense to the 3′ cryptic splice site activated in intron 2 of IVS2-654 mutant β-globin gene, was described previously.18 In U7.623, this antisense sequence was replaced with a 25-nucleotide sequence (UGUUAUUCUUUAGAAUGGUGCAAAG) antisense to position 623 of intron 2 of β-globin pre-mRNA.
pTKU7 plasmids, the lentivirus-derived constructs, carried the modified U7 inserts between the central polypurine track and the downstream long terminal repeat (LTR) of the pTK134 plasmid (T.K., unpublished plasmid; Figure 4). The forward orientation U7 lentivirus-derived plasmid (pTKU7.324) was constructed by inserting the 605 bp Ecl136/XbaI fragment, containing the entire modified U7 coding region of the snRNA, intoEco47/XbaI-cleaved pTK134. The reverse orientation U7 lentivirus-derived plasmids (pTKU7.324r, pTKU7.623r) were constructed by inserting the 765 bpPvuII/BamHI fragment intoBamHI/HpaI-cleaved pTK134.
Transfections
For all transfection experiments, HeLa cells were plated 24 hours before treatment at 0.8 × 105 cells/mL in 2-cm2 wells. The cells were treated for 72 hours with plasmid (0.05, 0.1, 0.25, 0.5, 1 μg/mL) complexed with 2.5 μg/mL Lipofectamine 2000, as suggested by the supplier (Invitrogen, Carlsbad, CA).
Production and assays of viral vector
The lentiviral vector was produced by a transient 3-plasmid transfection as described.32,33 Briefly, 7.0 × 106 human kidney 293T cells were transfected by calcium phosphate precipitation with 5 μg of the pMDG envelope plasmid and 15 and 10 μg of the packaging (ΔNRF)34 and vector plasmids, respectively. After 62 hours the conditioned medium was harvested, centrifuged at low speed, and filtered through a 0.45-μm (pore size) filter. Vector titers were determined by p24gag enzyme-linked immunosorbent assay (ELISA). Further vector concentration was achieved by ultracentrifugation at 50 000g for 2 hours.
Lentiviral vector transduction
HeLa cells were transduced with lentiviral vector particles containing 100 ng p24gag 24 hours after the cells had been seeded at 2 × 105 cells/2.5 mL in 9.6-cm2wells. Cells were split on days 3, 7, 10, and 12. On day 12 a cell sample was removed for analysis. Mononuclear cells from peripheral blood were seeded 4.0 × 106 cells/mL in 2-cm2 wells and transduced for 5 hours on days 1 and 2 with lentiviral vector particles containing 8 μg p24gag in serum-free medium (SFM) composed of IMDM supplemented with 10% BIT 9500 serum substitute (Stem Cell Technologies). Following transduction, the SFM was supplemented with the culture media described above and replated in methylcellulose as described above.
FACS and fluorescence microscopy
For fluorescence-activated cell sorter analysis (FACS), transfected HeLa EGFP-654 and EGFP-705U cells were trypsinized and resuspended in media, whereas transduced cells were trypsinized and fixed with 4% paraformaldehyde, washed, and resuspended in media. Approximately 104 cells were subjected to analysis in a Becton Dickinson FACScan (San Jose, CA). Gating of side versus forward scatter allowed the exclusion of dead or abnormal cells from analysis. The remaining cells were used to generate semilog single variety histograms (EGFP intensity versus cell number). Total mean fluorescence of untreated samples was set to 101, and the brightest 2.5% of that sample constituted background fluorescence. Treated samples were analyzed in terms of the percentage of cells fluorescing above background and mean fluorescence intensity of cell populations. Fluorescence index (FI), that is, mean fluorescence intensity × percentage of cells fluorescing above background, was calculated. For fluorescence microscopy, bright-field and UV images were taken with an inverted Olympus microscope. Images were digitized and processed with Adobe Photoshop software.
Isolation and analysis of β-globin mRNA
Total cellular RNA was isolated using TRI-Reagent (Molecular Research Center, Cincinnati, OH) and 10 to 200 ng was subjected to analysis by reverse transcription–polymerase chain reaction (RT-PCR) using rTth DNA polymerase (Perkin-Elmer, Norwalk, CT) with 0.2 μCi (0.0074 MBq) [α-32P] deoxyadenosine triphosphate (dATP) per sample at 18 cycles. Correction of human IVS2-654, IVS2-705, and IVS2-745 β-globin pre-mRNA splicing was detected with forward and reverse primers spanning positions 21-43 of exon 2 and positions 6-28 of exon 3, respectively, in β-globin mRNA (Figure 1B). The RT-PCR products were separated by electrophoresis on nondenaturing 8% polyacrylamide gel and detected by autoradiography. No product was detectable without the reverse transcription step.
Immunoblot analysis of hemoglobin
The cultured mononuclear cells (1.5 × 106) were washed twice with phosphate-buffered saline (PBS), suspended in 20 μL hemolysate reagent, and centrifuged at 14 000 rpm to remove the cell membranes. Approximately 3 μL supernatant was applied on Titan III-H cellulose acetate strips (76 × 60 mm) alongside with standard hemoglobins. Electrophoresis was performed with Supre-Heme buffer, pH 8.2, at 350 V for 35 minutes. The electrophoresis protocol and the materials were from Helena Laboratories (Beaumont, TX). Protein bands were visualized by staining the cellulose acetate strip with 0.5% Ponceau S and destaining with 5% acetic acid. The strip was blocked for 1 hour in 5% solution of fat-free dry milk in PBS containing 0.1% Tween 20 and the hemoglobins were detected with polyclonal affinity-purified chicken antihuman hemoglobin IgG as primary antibody and rabbit antichicken horseradish peroxidase (HRP)–conjugated IgG as secondary antibody (Accurate, Westbury, NY), both at 1000-fold dilution in the blocking solution. The blots were also developed with an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ) and fluorography.
Results
Modification of EGFP-654 and EGFP-705U splicing by U7.623 snRNA
A 4-bp insertion centered at position 623 of intron 2 of the β-globin gene was found to prevent aberrant and restore correct splicing of IVS2-654 pre-mRNA. Presumably, this insertion disrupted a sequence that in the context of the thalassemic mutation acted as a splicing enhancer and promoted the inclusion of the exonlike sequence contained between the aberrant 3′ and 5′ splice sites (Figure1A).29 Moreover, oligonucleotides antisense to the IVS2-623 region corrected splicing of IVS2-654 pre-mRNA approximately 50% more effectively than those targeted against the aberrant 3′ and 5′ splice sites (data not shown). These results prompted the generation of a U7 snRNA targeted against position 623 of the β-globin pre-mRNA (U7.623, Figure 1B). U7 snRNA has been used previously as an effective antisense carrier by replacing the 18-nucleotide antihistone pre-mRNA sequence with a 24-nucleotide sequence antisense to the cryptic 3′ splice site.18
Initially, correction of aberrant splicing by U7.623 snRNA was tested in a recently developed assay that uses EGFP as a read-out. In this assay the coding region of EGFP is interrupted by intron 2 of the β-globin gene, containing either the -654 or -705 mutation (EGFP-654 and EGFP-705U, respectively). In the latter construct, IVS2-705U, the splice site was converted to consensus sequence (Figure1C).31 As in IVS2-654 and IVS2-705 thalassemic pre-mRNAs, these mutations cause the inclusion of part of the intron into the EGFP mRNA, preventing its translation into a functional protein. Correction of pre-mRNA splicing by antisense molecules results in up-regulation of EGFP expression visualized by either fluorescence microscopy or FACS analysis (Figure 1C). FACS analysis of HeLa EGFP-705U cells following transient transfection with U7.623 and U7.324 plasmids showed a dose-dependent correction of pre-mRNA splicing (Figure2A). The highest levels of correction were achieved at 1 μg plasmid DNA/0.8 × 105 cells. Neither of the plasmids caused any obvious cytotoxicity because there was no detectable change in the morphology or growth rate of transfected cells (data not shown).
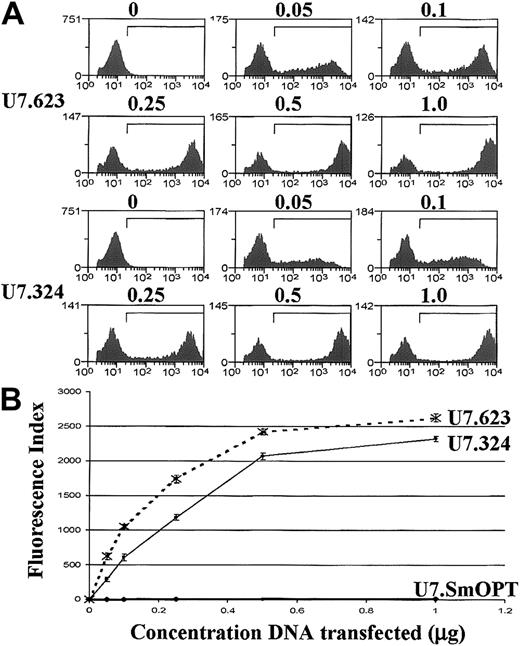
Correction of aberrant splicing by modified U7 snRNAs in EGFP-705U HeLa cells.
(A) FACS analysis of HeLa EGFP-705U cells transiently transfected with 0.05 to 1.0 μg U7 snRNA plasmids (indicated on the left). (B) Cells transfected with U7.623, U7.324, and control U7.SmOPT (3 independent experiments) were analyzed in terms of FI (percentage of cells scoring above the background threshold × mean fluorescence intensity of that cell subpopulation).
Correction of aberrant splicing by modified U7 snRNAs in EGFP-705U HeLa cells.
(A) FACS analysis of HeLa EGFP-705U cells transiently transfected with 0.05 to 1.0 μg U7 snRNA plasmids (indicated on the left). (B) Cells transfected with U7.623, U7.324, and control U7.SmOPT (3 independent experiments) were analyzed in terms of FI (percentage of cells scoring above the background threshold × mean fluorescence intensity of that cell subpopulation).
Quantitative analysis of FACS data from 3 independent experiments showed that the U7.623 construct was more effective than the previously tested U7.324. The effects of the 2 constructs were sequence specific because a U7 snRNA lacking the antisense sequence (U7SmOPT) had little effect (Figure 2B and Table 1). The U7.623 construct was also more effective than the U7.324 snRNA when transfected in the EGFP-654 HeLa cells. Nevertheless, in these cells the level of correction by all 3 constructs was lower than that seen in the transfected EGFP-705U cell line (Table 1), indicating that EGFP-654 pre-mRNA might be less susceptible to modification of splicing by antisense sequences than its 705U counterpart (see below and “Discussion”).30
β-Globin IVS2-654, IVS2-705, and IVS2-745 pre-mRNAs as targets
The antisense activity of the U7.623 snRNA was also tested in HeLa β-globin IVS2 mutant cells (Figure 3), which more closely model the mutations found in β-thalassemic patients. In these cells, the correction of aberrant splicing was assessed by RT-PCR of total RNA from transfected cells, using primers that flank intron 2 of the β-globin gene (Figure 1A). To increase sensitivity as well as to ascertain linear response and quantifiable ratio of PCR products obtained from aberrant and corrected β-globin mRNAs, RT-PCR was carried out at low cycles and with [α-32P]dATP. No product was detectable without the reverse transcription step (not shown; see “Materials and Methods” and Lacerra et al17 for more details).
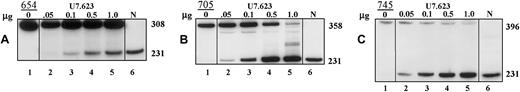
Correction of aberrant splicing by U7.623 snRNA in β-globin IVS2 mutant cells.
Total RNA from HeLa β-globin IVS2 mutant cells transiently transfected with 0.05 to 1.0 μg (indicated at the top) U7.623 snRNA plasmid was analyzed by RT-PCR. (A) β-Globin IVS2-654, (B) β-globin IVS2-705, and (C) β-globin IVS2-745 HeLa cells. Lane 1, untreated cells; lanes 2 to 5, cells transfected with U7.623 plasmid; lane 6, (N) RNA from normal human blood. The sizes (in nucleotides) of PCR bands representing aberrantly (308, 358, and 396) and correctly (231) spliced mRNAs are indicated on the right. Similar designations were used in Figures 5, 6C, and 7A.
Correction of aberrant splicing by U7.623 snRNA in β-globin IVS2 mutant cells.
Total RNA from HeLa β-globin IVS2 mutant cells transiently transfected with 0.05 to 1.0 μg (indicated at the top) U7.623 snRNA plasmid was analyzed by RT-PCR. (A) β-Globin IVS2-654, (B) β-globin IVS2-705, and (C) β-globin IVS2-745 HeLa cells. Lane 1, untreated cells; lanes 2 to 5, cells transfected with U7.623 plasmid; lane 6, (N) RNA from normal human blood. The sizes (in nucleotides) of PCR bands representing aberrantly (308, 358, and 396) and correctly (231) spliced mRNAs are indicated on the right. Similar designations were used in Figures 5, 6C, and 7A.
U7.623 snRNA corrected splicing of all 3 thalassemic pre-mRNAs in a dose-dependent fashion. As seen in EGFP cells, the β-globin IVS2-654 pre-mRNA was more resistant to splicing correction by antisense molecules than the remaining 2 mutants.
Even though in IVS2-654 cells correctly spliced β-globin mRNA was detectable at 0.1 μg transfected plasmid (Figure 3A, lane 3), its level was only slightly increased at 1 μg transfected DNA (lane 5). In contrast, the same concentrations of U7.623 construct elicited much higher levels of correction in IVS2-705 (nearly complete at 1 μg DNA, Figure 3B, lane 5) and IVS2-745 cells (Figure 3C, lane 5).
Correction of aberrant splicing by U7 lentiviral plasmids
Figure 4 shows the structure of 3 U7 antisense lentiviral constructs in which the snRNA genes were inserted into the pTK134 plasmid (T.K., unpublished plasmid). The inserts were composed of the modified U7.324 and U7.623 genes and included the flanking promoter and terminator sequences. The U7.324 was inserted in forward and reverse orientations with respect to the viral LTRs (pTKU7.324 and pTKU7.324r), and the U7.623 only in reverse orientation (pTKU7.623r; see “Materials and methods”). The plasmids were transiently transfected into EGFP-654 and EGFP-705U HeLa cells and the effects quantitated by FACS analysis as described above.
Lentiviral vector design.
The modified U7 snRNA genes were inserted between the central polypurine track of HIV-1 (cPPT) and the downstream long terminal repeat (LTR) of the pTK134 plasmid (“Materials and methods”) in forward (pTKU7.324) or reverse (pTKU7.324r, pTKU7.623r) orientations. Transcription of the full-length vector RNA was driven by human cytomegalovirus (CMV) promoter. The vector also contains a packaging signal (ψ), the Rev response element (RRE), a sequence containing the woodchuck hepatitis virus posttranscriptional regulatory element (PRE), and a self-inactivating (SIN) deletion in the U3 region of the downstream LTR.
Lentiviral vector design.
The modified U7 snRNA genes were inserted between the central polypurine track of HIV-1 (cPPT) and the downstream long terminal repeat (LTR) of the pTK134 plasmid (“Materials and methods”) in forward (pTKU7.324) or reverse (pTKU7.324r, pTKU7.623r) orientations. Transcription of the full-length vector RNA was driven by human cytomegalovirus (CMV) promoter. The vector also contains a packaging signal (ψ), the Rev response element (RRE), a sequence containing the woodchuck hepatitis virus posttranscriptional regulatory element (PRE), and a self-inactivating (SIN) deletion in the U3 region of the downstream LTR.
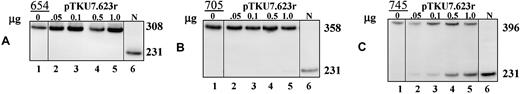
Table 1 shows that transfection of HeLa EGFP IVS2-654 and IVS2-705U cells with 0.25 μg pTKU7.324 yielded fluorescence indices of 18 ± 11 and 19 ± 12, respectively, which were 44- and 62-fold lower than those obtained in analogous experiments with the parent U7.324. To test if this loss of activity was caused by occlusion of U7 promoter by RNA polymerase II, which would elongate the full-length transcript from the upstream LTR, the U7.324 was inserted in reverse orientation (pTKU7.324r). This change in construction seemed promising because other studies have shown effective expression of antisense constructs inserted in the viral vectors in reverse orientation.35-37 Transfection of the cells with pTKU7.324r increased correction about 11-fold higher for EGFP-654 and 19-fold for EGFP-705U cells (FIs, 194 ± 56 and 360 ± 58, respectively). The U7 construct in reverse orientation targeted to the 623 sequence was even more effective, reaching FIs of 349 ± 58 and 576 ± 60. Note that even the latter construct was 3 times less effective than its parent plasmid in both cell lines. The most effective lentiviral construct, pTKU7.623r, was tested in HeLa cell lines expressing IVS2-654, IVS2-705, and IVS2-745 β-globin genes (Figure 5). In these cells the effects of pTKU7.623r were significantly lower than in EGFP cells; the IVS2-654 cells were unaffected by the treatment (Figure 5A) and only minimal correction was detectable in IVS2-705 cells transfected with 1 μg pTKU7.623r DNA (Figure 5B). Importantly, significant, dose-dependent correction was detected in IVS2-745–treated cells (Figure 5C). This result indicated that pTKU7.623r was not only expressed but also functioned properly and underscored the difference in susceptibility of IVS2-654, IVS2-705, and IVS2-745 pre-mRNAs.
Correction of aberrant splicing by lentiviral plasmids in β-globin IVS2 mutant HeLa cells.
Total RNA from HeLa β-globin IVS2 mutant cells transiently transfected with 0.05 to 1.0 μg pTKU7.623r lentiviral construct was analyzed by RT-PCR. (A) β-Globin IVS2-654, (B) β-globin IVS2-705, and (C) β-globin IVS2-745 cells. Lane 1, untreated cells; lanes 2 to 5, cells transfected with pTKU6.623r; lane 6, (N) RNA from normal human blood.
Correction of aberrant splicing by lentiviral plasmids in β-globin IVS2 mutant HeLa cells.
Total RNA from HeLa β-globin IVS2 mutant cells transiently transfected with 0.05 to 1.0 μg pTKU7.623r lentiviral construct was analyzed by RT-PCR. (A) β-Globin IVS2-654, (B) β-globin IVS2-705, and (C) β-globin IVS2-745 cells. Lane 1, untreated cells; lanes 2 to 5, cells transfected with pTKU6.623r; lane 6, (N) RNA from normal human blood.
Correction of pre-mRNA splicing in HeLa cells transduced with U7.623 lentiviral vector
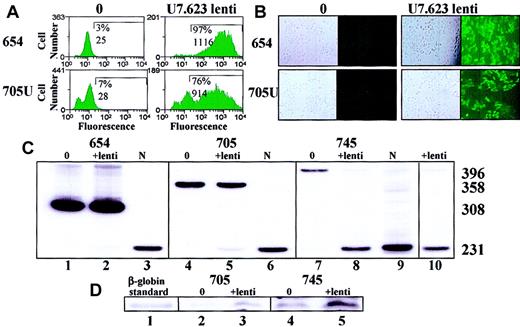
The pTKU7.623r construct was used to produce a lentiviral vector (“Materials and methods”), which was tested in EGFP-654, EGFP-705U, β-globin IVS2-654, IVS2-705, and IVS2-745 cells. Note that in the context of the self-inactivating lentiviral vector, in which the endogenous promoter is inactivated after one round of reverse transcription, the problem of transcriptional interference should be eliminated. Of the HeLa EGFP-654 cells transduced with the lentiviral vector, 97% fluoresced with a mean fluorescence intensity of 1116, resulting in an FI of 1086 (Figure 6A). Transduction of EGFP-705U cells was less efficient (76%), with a lower mean fluorescence of 914, resulting in an FI of 694. For both cell lines the FI of nontransduced cells was negligible. Fluorescence microscopy corroborated the results of FACS analysis (Figure6B).
Correction of aberrant splicing of HeLa EGFP and β-globin IVS2 mutants with U7 lentiviral vector.
(A) FACS analysis. EGFP-654 and EGFP-705U cells were transduced with U7.623 lentiviral vector and subjected to flow cytometry analysis after 2 weeks of transduction. (B) Fluorescence microscopy of identically treated cells. Phase contrast (left) and UV (right) images are shown. Magnification × 10. (C) RT-PCR of total RNA from HeLa β-globin IVS2-654 (lane 2), IVS2-705 (lane 5), and IVS2-745 (lane 8) cells transduced with U7.623 lentiviral vector. Untreated IVS2-654 (lane 1), IVS2-705 (lane 4), and IVS2-745 (lane 7) cells served as negative controls. Lanes 3, 6, and 9 show RNA from normal blood. Lane 10 shows IVS2-745 cells transduced with U7.623 lentiviral vector analyzed at 6 months. (D) Immunoblot analysis of total protein from HeLa IVS2-705 and IVS2-745 cells transduced as in panel C. The blots were probed with polyclonal anti–β-globin antibody. Lane 1, β-globin marker; lanes 2 and 4, untreated cells; lanes 3 and 5, lentiviral vector–transduced cells.
Correction of aberrant splicing of HeLa EGFP and β-globin IVS2 mutants with U7 lentiviral vector.
(A) FACS analysis. EGFP-654 and EGFP-705U cells were transduced with U7.623 lentiviral vector and subjected to flow cytometry analysis after 2 weeks of transduction. (B) Fluorescence microscopy of identically treated cells. Phase contrast (left) and UV (right) images are shown. Magnification × 10. (C) RT-PCR of total RNA from HeLa β-globin IVS2-654 (lane 2), IVS2-705 (lane 5), and IVS2-745 (lane 8) cells transduced with U7.623 lentiviral vector. Untreated IVS2-654 (lane 1), IVS2-705 (lane 4), and IVS2-745 (lane 7) cells served as negative controls. Lanes 3, 6, and 9 show RNA from normal blood. Lane 10 shows IVS2-745 cells transduced with U7.623 lentiviral vector analyzed at 6 months. (D) Immunoblot analysis of total protein from HeLa IVS2-705 and IVS2-745 cells transduced as in panel C. The blots were probed with polyclonal anti–β-globin antibody. Lane 1, β-globin marker; lanes 2 and 4, untreated cells; lanes 3 and 5, lentiviral vector–transduced cells.
Transduction of HeLa cell lines expressing thalassemic pre-mRNAs showed (Figure 6C) that the U7 lentiviral vector resulted in an increase in correctly spliced products to about 3% of total in IVS2-654 cells (lane 2), 17% in IVS2-705 (lane 5), and essentially complete in IVS2-745 (lane 8). The IVS2-745 cell line transduced with the lentiviral vector was maintained in continuous culture for 6 months. Importantly, and as expected with the genome integrated viral sequences, the RT-PCR analysis of the RNA after this period showed the level of correction equal to that seen 2 weeks after transduction (compare lanes 8 and 10). Similar results were seen with lentiviral vector transduction of K562 cells stably expressing the β-globin IVS2 mutants (data not shown). The RT-PCR results were also corroborated by the analysis of cellular protein by immunoblotting with a polyclonal antibody to human hemoglobin (Figure 6D). The results show that in IVS2-705 and IVS2-745 HeLa cells transduced with the U7.623 lentiviral vector the newly generated correctly spliced β-globin mRNA was translated into full-length, functional β-globin protein.
Correction of IVS2-745 splicing in erythroid progenitor cells by U7.623 lentiviral vector
The U7.623 lentiviral vector was applied to a therapeutically relevant target, the erythroid progenitor cells from a patient with IVS2-745/IVS2-1 thalassemia. Based on the results shown in Figure 6, this mutation was expected to be the easiest to correct, allowing the proof of principle for antisense RNA treatment of thalassemia to be established. Furthermore, the splicing of IVS2-745 pre-mRNA in human erythroid progenitor cells has been repaired by treatment of the cells with oligonucleotides targeted to the aberrant 3′ splice site.17
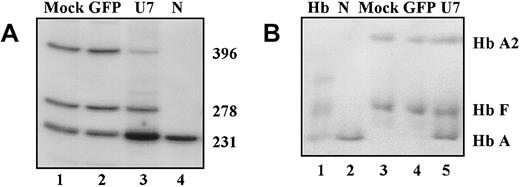
The mononuclear cells were isolated from the patient's blood and cultured in the presence of Epo and SCF, conditions that promote erythroid differentiation of stem cells and early progenitors.17 The cells were transduced with U7.623 lentiviral vector on days 1 and 2 of culture and subsequently cultured in methylcellulose medium containing growth factors (“Materials and methods”) for 12 days; at this time total RNA was analyzed by RT-PCR (Figure 7A). As expected, in cells transduced with a control viral vector in which the U7.623 gene was replaced with the GFP gene (Figure 7A, lane 2), there was no increase in the amount of correctly spliced β-globin mRNA over the existing background in mock-transduced cells (lane 1). In contrast, in cells transduced with U7.623 lentiviral vector (lane 3) the level of the aberrant band was diminished and that of the correct band greatly increased, demonstrating significant correction of splicing of IVS2-745 pre-mRNA. In addition to 396-nucleotide and 231-nucleotide products, representing the aberrantly and correctly spliced β-globin mRNAs, a 278-nucleotide product was also generated by RT-PCR. The corresponding RNA is spliced via a cryptic splice site activated by the IVS2-1 mutation and located 47 nucleotides downstream.12Quantitative analysis of the ratio of correct to aberrant RNAs showed that lentiviral vector–mediated antisense delivery led to a 23-fold increase in the production of correctly spliced β-globin mRNA.
U7.623 lentiviral vector–induced β-globin pre-mRNA repair in erythroid progenitors from patient with IVS2-745/IVS2-1 thalassemia.
(A)RT-PCR. On days 1 and 2 of culture, the cells were transduced for 5 hours with either no virus (lane 1), mock, GFP lentiviral vector (lane 2), or U7.623 lentiviral vector (lane 3). Lane 4 is RNA from normal blood. (B) Immunodetection of hemolysates separated by electrophoresis on cellulose acetate with antihuman hemoglobin antibody (“Materials and methods”). Lane 1, hemoglobin standards; lane 2, (N) normal blood; lane 3, mock-transduced cells; lane 4, cells transduced with GFP lentiviral vector; lane 5, cells transduced with U7.623 lentiviral vector.
U7.623 lentiviral vector–induced β-globin pre-mRNA repair in erythroid progenitors from patient with IVS2-745/IVS2-1 thalassemia.
(A)RT-PCR. On days 1 and 2 of culture, the cells were transduced for 5 hours with either no virus (lane 1), mock, GFP lentiviral vector (lane 2), or U7.623 lentiviral vector (lane 3). Lane 4 is RNA from normal blood. (B) Immunodetection of hemolysates separated by electrophoresis on cellulose acetate with antihuman hemoglobin antibody (“Materials and methods”). Lane 1, hemoglobin standards; lane 2, (N) normal blood; lane 3, mock-transduced cells; lane 4, cells transduced with GFP lentiviral vector; lane 5, cells transduced with U7.623 lentiviral vector.
To test for hemoglobin synthesis, lysates of lentiviral vector–transduced cells at day 12 of culture were separated by electrophoresis on cellulose acetate and probed with polyclonal antihemoglobin antibody. This analysis showed significant levels of newly generated hemoglobin A (Hb A; Figure 7B). Quantitation of the ratio of Hb A bands to Hb F (used as an internal control) showed that the amount of Hb A in the patient cells transduced with the U7.623 lentiviral vector increased about 25-fold over that in cells transduced with the control GFP viral vector. This result is consistent with the increase of β-globin mRNA detected by RT-PCR and also shows that the antisense treatment of progenitor cells not only repaired β-globin pre-mRNA splicing but also restored proper expression of the Hb A protein.
Discussion
Previously, viral vector transfer of antisense RNAs has been used for down-regulation of gene expression (for a review, see Sazani et al38). Here we demonstrate that this strategy can be used to successfully restore expression of a gene inactivated by a splicing mutation. This novel application of the lentiviral vector–mediated antisense approach was achieved not only in cell line models but also in the clinically relevant erythroid progenitor cells from a patient with IVS2-745/IVS2-1 thalassemia.
The fact that U7.623 snRNA (targeted downstream of the cryptic 3′ splice site) is more effective in the correction of aberrant splicing than U7.324 targeted against the splice site itself implies the presence of a splicing enhancer at the target site.29Splicing enhancers function as binding sites for SR proteins, a family of serine/arginine-rich essential splicing factors involved in regulation of alternative splicing (for a review, see Blencowe39). Presumably, antisense RNAs targeted against the 623 region prevented the binding of SR proteins and induced skipping of the aberrant pseudoexon.40
The modified U7 snRNAs were most efficient in the correction of splicing of IVS2-745 pre-mRNA, followed by IVS2-705 and IVS2-654. Similar results have also been observed in correction of splicing of these pre-mRNAs by antisense oligonucleotides targeted to the aberrant 3′ and 5′ splice sites.30 Here, these observations are extended to antisense RNA that is targeted between the splice sites and is expressed intracellularly. One concludes that the strength of the aberrant 5′ splice sites and the distance between the 3′ and 5′ splice sites flanking the internal exons determine the differences in correction achieved in the 3 mutants. Because the HeLa IVS2-654 cell line was relatively resistant to correction by the lentiviral vector, these experiments were not attempted in the IVS2-654 β-thalassemic mouse model.41 The resistance of this splice site to correction most likely reflects the nature of the interactions of the spliceosomes with the pre-mRNAs, including the interactions of the splicing factors that bridge the exon.30
Previous experiments with erythroid mononuclear cultures have established that in the presence of Epo and SCF β-globin pre-mRNAs and mRNAs did not appear until days 4 to 5 of culture.17 Thus, the lentiviral vector added to the cultured mononuclear cells at days 1 and 2 must have transduced HSCs or very early erythroid progenitor cells or both, which were not yet engaged in the expression of β-globin gene. Because maximum correction of aberrant β-globin splicing by antisense oligonucleotides was found at day 12 to 15 of culture,17,42 it is likely that the very early, multipotent erythroid progenitor cells, which produce in about 2 weeks large numbers of highly multicellular erythroid burst-forming units (BFU-Es),43 are responsible for the bulk of the repair response elicited by the U7.623 virus. This does not exclude the possibility that a fraction of the response was generated in the ultimate target of the antisense approach, HSCs. Although these cells are found in only 1/10 000 to 1/100 000 bone marrow cells and even less frequently in the peripheral blood, the latter material has been used for stem cell transplantation in thalassemia.44 Clearly additional studies are required to determine the ability of thalassemic patients' mobilized CD34+ HSCs to engraft and differentiate into Hb A–producing erythroid cells following transduction with lentiviral vectors carrying the modified U7snRNA expression cassettes.
An important advantage of our antisense approach is that the correction occurs in the β-globin pre-mRNA transcribed from the β-globin gene in its natural chromosomal environment and properly controlled by the native locus control region. Therefore, the expression of β-globin cannot exceed the wild-type levels, precluding the possibility of overexpression of β-globin mRNA, and offering an attractive alternative to gene replacement as a treatment for hemoglobinopathies. Furthermore, complete splicing correction is not necessary. Even if only 10% levels of correction were achieved in thalassemic patients, this would lead to a clinically relevant outcome.7
The effects of the U7.623 snRNAs expressed by the lentiviral vector are expected to be limited only to cells that express the target sequence, that is, the erythroid precursor and progenitor cells. This is because hybridization of the U7.623 snRNA to other RNAs will occur with a number of mismatches, rendering the molecules ineffective.16 The fact that cell growth of cultured mononuclear cells was unaffected by lentiviral vector transduction shows that U7.623 RNA did not effect widespread inhibition of gene expression. Likewise, antisense oligonucleotides have been found relatively nontoxic in clinical trials and as a marketed drug.45
It was estimated that 15% of point mutations contributing to genetic diseases cause aberrant splicing.46 Recent results, which take into account not only genomic sequence but also RNA expression and splicing patterns, indicate that the percentage of splicing defects may be much higher. For example, when analyzed at the RNA level, 50% of mutations in the ataxia-telangiectasia and neurofibromatosis type 1 genes resulted in defective splicing.47,48 Thus, the antisense repair of defective pre-mRNAs can be applied to other disorders in addition to thalassemia syndromes. In fact, correction of pre-mRNA splicing by antisense oligonucleotides was investigated in the context of cystic fibrosis,49 Duchenne muscular dystrophy,50,51Alzheimerlike frontotemporal dementia and Parkinsonism associated with chromosome 17 (FTDP-17) syndrome,52 and spinal muscular atrophy.53 This method was also used for modification of alternative splicing by targeting a cancer-related splice variant of bcl-x pre-mRNA.20,54 56 The approach described here provides a way to effect permanent correction of the aberrant splicing that gives rise to disease by incorporating these and other antisense sequences into viral vectors.
We are grateful to the patients and their parents for donating blood samples. We thank Elizabeth Smith for technical assistance and Thipparat Suwanmanee for guidance in erythroid cell culture.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-06-1869.
Supported in part by grant HL-51940, National Heart, Lung, and Blood Institute, National Institutes of Health (R.K.) and grant DK-58702, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (T.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ryszard Kole, University of North Carolina, Lineberger Comprehensive Cancer Center, CB no. 7295, Chapel Hill, NC 27599; e-mail: kole@med.unc.edu, or Tal Kafri, University of North Carolina, Gene Therapy Center, Thurston-Bowles, CB no. 7352, Chapel Hill, NC 27599; e-mail: kafri@med.unc.edu.