Abstract
The novel prodrug of butyric acid, pivaloyloxymethyl butyrate (AN-9), a histone deacetylase inhibitor, shows great promise as an effective and relatively nontoxic anticancer agent for solid malignancies. However, little is known about its effects on hematopoietic malignancies. In this study, we show that 21 primary samples of acute leukemia were sensitive to the antiproliferative effects of AN-9, with a 50% inhibitory concentration (IC50) of 45.8 ± 4.1 μM. In colony-forming assays, primary T-cell acute lymphoblastic leukemia (T-ALL) cells were 3-fold more sensitive to AN-9 than the normal hematopoietic progenitors, erythroid burst-forming units and granulocyte/monocyte colony-forming units. AN-9 induced apoptosis in the T-ALL cell line CEM. A common problem with cancer is chemoresistance, which is often typical of relapsed cancers. Remarkably, a T-ALL sample at diagnosis and an acute myeloid leukemia sample at relapse that were resistant to doxorubicin in vitro were sensitive to AN-9, with an IC50 of 50 μM for both samples. More strikingly, samples from 2 infants with t(4;11) ALL obtained at diagnosis and relapse each were the most sensitive to AN-9, with IC50values of 25 μM and 17 μM, respectively. Furthermore, a doxorubicin-resistant clone of HL60, HL60/ADR, obtained by the transfection of the MDR-1 gene, was equally sensitive to AN-9 cytotoxicity as the parental cells. AN-9 induced the expression of p21 in an infant leukemia sample with 11q23 rearrangement, but not in T- or B-precursor ALL. Collectively, our results suggest that AN-9 is a selective agent for hematopoietic malignancies that can circumvent the mechanisms of chemoresistance limiting most conventional chemotherapy.
Introduction
The leukemias account for the largest number of cases of childhood cancer. Acute lymphoblastic leukemia (ALL) represents approximately three fourths, whereas acute myeloid leukemia (AML) represents one sixth of the cases. Despite recent advances in the treatment of childhood cancers, leukemias remain the primary cause of cancer-related mortality among children in the United States. Although ALL and AML are potentially curable diseases, with a 5-year survival of 70% for ALL and 40% for AML, once leukemia recurs, the outcome is dismal. Therefore, there is a need for novel antileukemia agents, especially those that are effective for relapsed leukemias resistant to existing chemotherapeutic agents.
Histone acetylation plays a key role in the regulation of transcription by modulating chromatin structure.1-3 In general, histone acetylation is associated with activation of transcription, whereas histone deacetylation is associated with repression of transcription. It has recently been established that many malignancies, particularly leukemias, are associated with aberrant recruitment of histone deacetylases (HDACs) or with mutations in histone acetyl transferases such as EP300.4-6 For example, several investigators reported that the oncoprotein promyelocytic leukemia/retinoic acid receptor–α, generated as a result of a translocation in acute promyelocytic leukemia (APL), suppressed transcription by recruiting HDACs and thus interfering with normal cell growth and differentiation.7-9 Furthermore, resistance to the differentiating actions of all-trans retinoic acid in APL-derived cells could be overcome by cotreatment with the HDAC inhibitor sodium phenyl butyrate.10 There is in fact an increasing body of evidence that HDAC inhibitors are effective therapeutic agents for a variety of cancers that are refractory to conventional anticancer agents.11 12
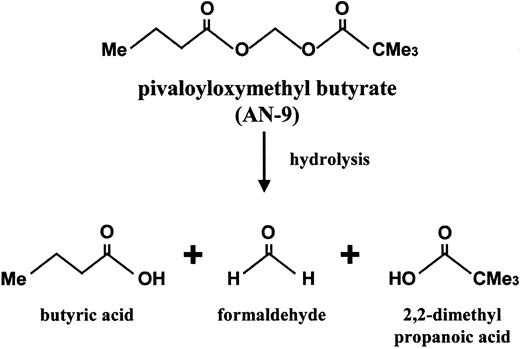
Pivaloyloxymethyl butyrate (AN-9), a butyric acid prodrug, is a relatively new member of an established family of acyloxyalkyl ester prodrugs of carboxylic acids that undergo rapid hydrolysis. Upon hydrolysis, the resulting products are butyric acid, pivalic acids, and formaldehyde (Figure 1). The anticancer effect of AN-9 is assumed to stem primarily from the release of butyric acid, an HDAC inhibitor.13 It is important to note, however, that AN-9 is 10-fold more potent than butyric acid in vitro and has anticancer effects in vivo not observed with butyric acid even at 10-fold higher concentrations.14 The increased potency of AN-9 over butyric acid is most likely due to its increased permeability across cell membranes, allowing for efficient delivery of butyric acid to subcellular targets.15 AN-9 inhibits cell proliferation and soft agar colony formation of a variety of cancer cell lines and primary human solid tumor cells, including those of colon, breast, ovary, lung, kidney, and bladder.13,16,17In addition to being an antiproliferative agent, AN-9 has been shown to induce differentiation and apoptosis in HL60 cells.18 Earlier studies in mouse tumor models have demonstrated that AN-9 is an effective antitumor agent and displays low toxicity.19 Currently, AN-9 is being studied in a phase II clinical trial for non–small cell lung cancer. To date, all in vitro and in vivo studies of AN-9 have been confined to solid tumors, with the exception of a murine monocytic leukemia and the HL60 cell line. In this study, we investigated the in vitro therapeutic efficacy of AN-9 in primary human acute leukemias, including doxorubicin-resistant and/or clinically refractory acute leukemias. Our findings demonstrate the selective toxicity of AN-9 to acute leukemias, including drug-resistant relapsed leukemias, and thus provide the rationale for the initiation of clinical trials of AN-9 in relapsed acute leukemias.
Structure of AN-9 and the products released upon metabolic hydrolysis.
Structure of AN-9 and the products released upon metabolic hydrolysis.
Patients, materials, and methods
Antibodies and reagents
AN-9 was prepared as described previously and determined to be 99% pure by nuclear magnetic resonance spectroscopy.13doxorubicin was obtained from Bedford Laboratories (Bedford, OH). The p21 and p27 antibodies were purchased from Pharmingen (San Diego, CA), and the β-actin antibody was from Sigma (St Louis, MO). Ficoll-Hypaque was purchased from Pharmacia (Piscataway, NJ).
Cell lines
The leukemia cell lines HL60 and HL60/ADR, derived by transfection of HL60 with the mdr1 gene, were generously provided by Dr Michael Kelner.20,21 The T-cell acute lymphoblastic leukemia (T-ALL) cell line CEM was obtained from American Type Culture Collection (Rockville, MD). The neuroblastoma cell lines Be2c and Be2c/ADR, derived by selection in doxorubicin-containing media, were generously provided by J. Biedler.22
Patient population and isolation of primary leukemia cells
Heparinized bone marrow or peripheral blood samples were obtained at diagnosis and relapse from patients with AML or B-precursor ALL at the University of California in San Diego. T-ALL samples were obtained from patients enrolled in Pediatric Oncology Group protocols 9900 (ALL Biology Study) and 9673 (Relapsed T-ALL Study) and were shipped overnight to the University of California San Diego for biology studies. Bone marrow cells obtained from patients in complete remission who underwent diagnostic bone marrow examination were used as normal controls. Only excess samples obtained for clinical purposes were analyzed. These samples were collected under a protocol approved by the Institutional Review Board. Mononuclear cells were isolated by density gradient centrifugation through Ficoll-Hypaque (specific gravity 1.077 g/mL) at 400g for 30 minutes, followed by 2 washes in RPMI 1640 (Ammersham Biosciences, Uppsala, Sweden). The content of lymphoblasts, as determined by Wright stain, was generally 80% or higher. Leukemia cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM glutamine, and 1% penicillin/streptomycin (complete medium).
3H-thymidine incorporation assay
Primary leukemia and normal bone marrow cells were plated in complete RPMI at 75 × 103 cells per well in a 96-well plate. Leukemia and neuroblastoma cell lines were plated at 10 and 5 × 103 cells per well, respectively. Cells were treated with increasing concentrations of AN-9 or with 0.1% dimethyl sulfoxide (DMSO; control) for 3 days. Cells were then pulsed with3H-thymidine at 1.6 μCi/well (0.0592 MBq) for 6 hours. Incorporation of 3H-thymidine was determined in a scintillation counter (Beckman Coulter, Fullerton, CA) after the cells were washed and deposited onto glass microfiber filters using cell harvester M-24 (Brandel, Gaithersburg, MD).
Colony-formation assays
Primary T-ALL and normal bone marrow cells were plated at 5 × 105 per 35-mm dish in methylcellulose medium with increasing concentrations of AN-9 for 14 days. An erythroid burst-forming unit (BFU-E) was identified as a large aggregate of more than 64 hemoglobinized cells or as clusters of 3 or more subcolonies consisting of 8 or more hemoglobinized cells per subcolony. The granulocyte/monocyte colony-forming unit (CFU-GM) was enumerated as a group of more than 50 granulocytic/monocytic translucent cells. T-ALL colonies were identified as clusters of more than 50 cells.
Cytotoxicity assays
Primary leukemia and CEM cells were plated in complete RPMI at 75 × 103 cells per well and 10 × 103 cells per well (96-well plate), respectively. Cells were treated with increasing concentrations of AN-9 or 0.1% DMSO for 3 days. The number of viable cells was determined by trypan blue exclusion.
Apoptosis assays
CEM cells were plated at 1.2 × 106 cells per well (6-well plate) in complete RPMI and treated with 0.1% DMSO or 60 and 75 μM AN-9 for 20 and 45 hours. Cells were then visualized and photographed using a Nikon Eclipse TE-300 inverted microscope and the SPOT camera (SPOT Diagnostic Instruments, Sterling Heights, MI). Alternatively, cells were stained with Alexa Fluor 488 annexin V and propidium iodide using the Vybrant Apoptosis Assay Kit (Molecular Probes, Eugene, OR) and then analyzed by flow cytometry using a FACScan flow cytometer and CellQuest 3.2.1 software (Becton Dickinson).
Western blot analysis
Primary leukemia cells (10-20 × 106) were treated with 10, 25, 50, and 100 μM AN-9 for 24 and 48 hours. As a control, cells were treated with 0.1% DMSO. Protein was extracted with 30 μL sodium dodecyl sulfate (SDS) lysis buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 0.1% bromophenol blue, 10% glycerol, 5 mM dithiothreitol). Samples were heated in boiling water for 10 minutes, and then the lysate was passed through a 28-gauge needle to shear chromosomal DNA. Supernatants were then collected after spinning in a microfuge at 13 000 rpm for 10 minutes at 4°C. Proteins were resolved on an 8% SDS-polyacrylamide gel and transferred to Immobilon-P nylon membranes (Millipore, Bedford, MA). Membranes were probed with p21 and p27 antibodies at 0.5 μg/mL. Membranes were also probed with a β-actin antibody at 2 μg/mL as a control for protein loading. Antibody-bound proteins were detected by chemifluorescence and analyzed using the Storm 840 Phosphorimager (Molecular Dynamics).
Results
Primary ALL cells are more sensitive than normal bone marrow cells to AN-9
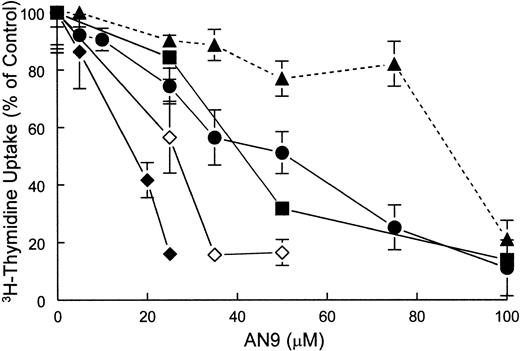
The effect of AN-9 on DNA synthesis in both primary ALL and normal bone marrow was examined by 3H-thymidine incorporation assays. The results demonstrated that ALL cells were more sensitive than normal bone marrow cells to inhibition of 3H-thymidine uptake by AN-9 (Figure 2).
Inhibition of 3H-thymidine incorporation by AN-9 in primary ALL cells and normal bone marrow cells.
Normal bone marrow cells and primary ALL cells were treated with increasing concentrations of AN-9 for 3 days. Cells were then pulsed with 3H-thymidine for 6 hours. ● indicates T-ALL; ▪, B-precursor ALL; ♦, relapsed infant ALL; ⋄, diagnosed infant ALL; ▴, normal bone marrow. All experimental conditions were performed in triplicate. Error bars represent standard error of the mean for data obtained with normal bone marrow (n = 6) and primary T-ALL (n = 16). For data obtained with infant ALL (n = 1) and for a representative B-precursor ALL (n = 1), error bars represent standard deviation.
Inhibition of 3H-thymidine incorporation by AN-9 in primary ALL cells and normal bone marrow cells.
Normal bone marrow cells and primary ALL cells were treated with increasing concentrations of AN-9 for 3 days. Cells were then pulsed with 3H-thymidine for 6 hours. ● indicates T-ALL; ▪, B-precursor ALL; ♦, relapsed infant ALL; ⋄, diagnosed infant ALL; ▴, normal bone marrow. All experimental conditions were performed in triplicate. Error bars represent standard error of the mean for data obtained with normal bone marrow (n = 6) and primary T-ALL (n = 16). For data obtained with infant ALL (n = 1) and for a representative B-precursor ALL (n = 1), error bars represent standard deviation.
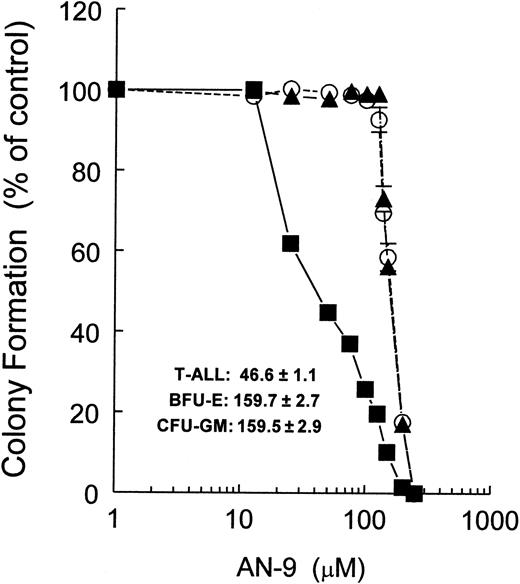
The mean 50% inhibitory concentration (IC50) of AN-9 in 6 normal bone marrow cell samples was 87 ± 5 μM. In contrast, the mean AN-9 IC50 in 15 primary T-ALL samples was 50 ± 5 μM, and that in 2 B-precursor ALL samples was 44 μM and 54 μM (data not shown). The finding that both T- and B-cell ALL have similar sensitivity to AN-9 suggests that the effects of AN-9 are not lineage specific. Remarkably, however, primary leukemias obtained from 2 infants with an 11q23 rearrangement were the most sensitive to AN-9. One of these samples was obtained from a patient who suffered from leukemia relapse shortly after bone marrow transplantation and became refractory to reinduction chemotherapy. On 2 separate occasions during his relapse, the IC50 of AN-9 was only 17 μM (Figure 2) and 13 μM (data not shown), respectively. The second infant ALL sample, collected at the time of diagnosis, had an AN-9 IC50 of 25 μM (Figure 2). To confirm the results obtained by 3H-thymidine incorporation on the selectivity of AN-9 for leukemia, we performed colony-formation assays using normal bone marrow cells and primary T-ALL cells. The mean IC50 of AN-9 in 6 primary T-ALL samples was 46.6 ± 1.1 μM, and those of 5 normal hematopoietic progenitors of BFU-E and CFU-GM were 159.7 ± 2.7 μM and 159.5 ± 2.9 μM, respectively (Figure3). The difference in the AN-9 IC50 between T-ALL and normal hematopoietic progenitors was highly significant, with P < .001. Thus, the results of colony-formation assays confirm the selectivity of AN-9 for ALL rather than normal bone marrow cells.
Inhibition of colony formation by AN-9 of primary T-ALL and normal hematopoietic progenitors.
Primary T-ALL (n = 6) and normal bone marrow cells (n = 5) were cultured in methylcellulose medium and increasing concentrations of AN-9 for 14 days. The BFU-E was identified as a large aggregate of more than 64 hemoglobinized cells, or as clusters of 3 or more subcolonies consisting of 8 or more hemoglobinized cells per subcolony. The CFU-GM was enumerated as a group of more than 50 granulocytic/monocytic translucent cells. ▪ indicates T-ALL; ▴, CFU-GM; ○, BFU-E. Error bars represent standard error of the mean.
Inhibition of colony formation by AN-9 of primary T-ALL and normal hematopoietic progenitors.
Primary T-ALL (n = 6) and normal bone marrow cells (n = 5) were cultured in methylcellulose medium and increasing concentrations of AN-9 for 14 days. The BFU-E was identified as a large aggregate of more than 64 hemoglobinized cells, or as clusters of 3 or more subcolonies consisting of 8 or more hemoglobinized cells per subcolony. The CFU-GM was enumerated as a group of more than 50 granulocytic/monocytic translucent cells. ▪ indicates T-ALL; ▴, CFU-GM; ○, BFU-E. Error bars represent standard error of the mean.
AN-9 is cytotoxic to ALL
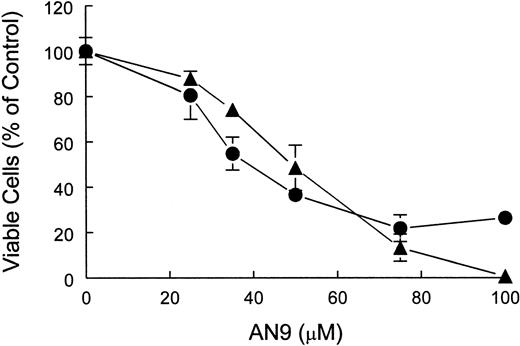
To distinguish between cytostatic and cytotoxic effects of AN-9, we performed cell-viability assays using both a T-ALL cell line, CEM, as well as primary T-ALL cells. The results of trypan blue exclusion assays demonstrated that AN-9 was cytotoxic to CEM and primary T-ALL in a dose-dependent manner (Figure 4).
Cytotoxicity of AN-9 in CEM and primary T-ALL cells.
CEM and T-ALL cells were treated with increasing concentrations of AN-9 for 3 and 5 days, respectively. Viable cells were then determined by trypan blue exclusion. ● indicates primary T-ALL; ▴, CEM. Results obtained with primary T-ALL are representative of 2 independent experiments with 2 different patient samples. Data obtained with CEM cells are an average of 2 independent experiments. All experimental conditions were performed in triplicate. Error bars represent standard deviation.
Cytotoxicity of AN-9 in CEM and primary T-ALL cells.
CEM and T-ALL cells were treated with increasing concentrations of AN-9 for 3 and 5 days, respectively. Viable cells were then determined by trypan blue exclusion. ● indicates primary T-ALL; ▴, CEM. Results obtained with primary T-ALL are representative of 2 independent experiments with 2 different patient samples. Data obtained with CEM cells are an average of 2 independent experiments. All experimental conditions were performed in triplicate. Error bars represent standard deviation.
The IC50 of AN-9 was 47 μM in CEM and 41 μM in primary T-ALL after 3 and 5 days of AN-9 treatment, respectively (Figure 4). At 75 μM AN-9, cytotoxic effects on CEM were evident as early as after 24 hours of incubation (data not shown).
Induction of apoptosis by AN-9
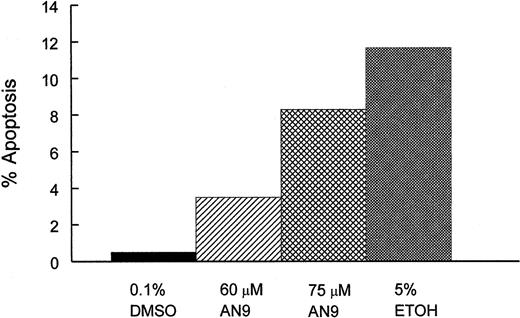
To determine whether cytotoxicity of AN-9 involved the induction of apoptosis, we treated CEM cells with 0.1% DMSO (negative control), 60 or 75 μM AN-9, or with 5% ethanol (positive control) for 20 and 45 hours, and then examined them by flow cytometry after staining with Alexa Fluor 488 annexin V and propidium iodide. As shown in Figure 5, AN-9 induced apoptosis in CEM cells in a dose-dependent manner. For instance, treatment with 60 μM and 75 μM AN-9 for 45 hours resulted in 3.5% and 8.3% apoptotic cells, respectively. Percentage apoptosis in negative control cells was 0.5%, and that in the positive control cells was 10%. Late-stage apoptosis or necrosis was also evident after treatment with 60 and 75 μM AN-9 (data not shown).
Induction of apoptosis by AN-9 in CEM cells.
CEM cells were treated with 60 or 75 μM AN-9 for 45 hours. Positive and negative control cells were treated with 5% ethanol and 0.1% DMSO, respectively. Cells were then stained with Alexa Fluor 488 annexin V and propidium iodide using the Vybrant Apoptosis Kit (Molecular Probes). Apoptotic cells were then determined by flow cytometry. Results are representative of 3 independent experiments.
Induction of apoptosis by AN-9 in CEM cells.
CEM cells were treated with 60 or 75 μM AN-9 for 45 hours. Positive and negative control cells were treated with 5% ethanol and 0.1% DMSO, respectively. Cells were then stained with Alexa Fluor 488 annexin V and propidium iodide using the Vybrant Apoptosis Kit (Molecular Probes). Apoptotic cells were then determined by flow cytometry. Results are representative of 3 independent experiments.
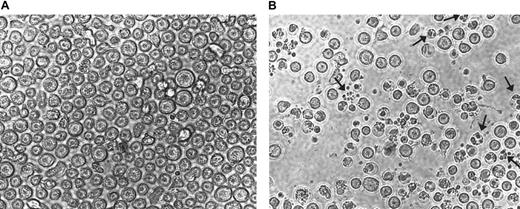
To further confirm the results presented in Figure 5, CEM cells treated with 75 μM AN-9 for 20 hours were photographed in a 12-well plate in the absence of cell manipulations to avoid the loss of fragile apoptotic cells. Results of a representative experiment as presented in Figure 6B revealed numerous cells with fragmented nuclei as well as clusters of membrane-bound bodies (see arrows), which are characteristic of apoptosis.
Morphology of CEM cells treated with AN-9.
CEM cells were treated with either 75 μM AN-9 (A) or with 0.1% DMSO (B; control) for 20 hours and then photographed using the SPOT camera. Magnification was ×10 for both samples. Arrows indicate fragmented nuclei and clusters of membrane-bound bodies characteristic of apoptosis.
Morphology of CEM cells treated with AN-9.
CEM cells were treated with either 75 μM AN-9 (A) or with 0.1% DMSO (B; control) for 20 hours and then photographed using the SPOT camera. Magnification was ×10 for both samples. Arrows indicate fragmented nuclei and clusters of membrane-bound bodies characteristic of apoptosis.
These findings demonstrate that apoptosis occurs within 20 hours and appears to be more extensive than the flow cytometry results indicate because no apoptosis was evident by flow cytometry at 20 hours (data not shown). This result is most likely due to the loss of apoptotic cells during the staining procedure before flow cytometry.
AN-9 induces p21 in infant ALL, but not in T-ALL or B-precursor ALL
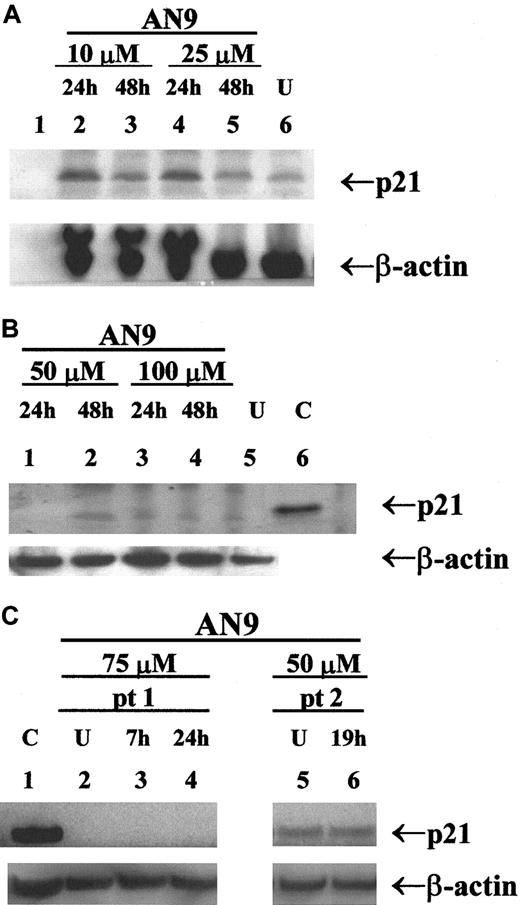
Previous studies have reported the induction of p21 by butyrate and its correlation with cell-cycle arrest and apoptosis.23 24 To determine whether p21 is involved in AN-9 action, we examined the expression of p21 in response to AN-9 by Western blot analysis. As shown in Figure7A, p21 was induced in a primary infant ALL sample within 24 hours of AN-9 treatment, and the levels declined by 48 hours, indicating a transient induction. In contrast, as presented in a representative experiment (Figure 7), induction of p21 by AN-9 was not observed in any of the 2 B-precursor ALL (Figure 7B) or the 4 primary T-ALL (Figure 7C, data shown for one patient) cells examined, even at AN-9 concentrations that effectively arrest DNA synthesis in ALL (Figure 2). The effects of AN-9 on the levels of p27, a homolog of p21, were also examined. The levels of p27 were not induced by AN-9 in B-precursor ALL or T-ALL (data not shown). These results suggest that the effects of AN-9 on T-ALL are independent of p21 or p27.
Western blot analysis of p21 in primary ALL.
Protein was extracted by the SDS/boiling method from (A) infant ALL; (B) B-precursor ALL; and (C) T-ALL cells treated with AN-9 as indicated. SJ SA-1 osteosarcoma cells, known to express p21, were used as the positive control. Western blot analysis was performed using a p21 antibody at 0.5 μg/mL. Equal amounts of protein in each lane were verified by probing the blot with a β-actin antibody at 2 μg/mL. U indicates untreated (0.1% DMSO); C, positive control for p21; pt, patient. AN-9 IC50 in the infant ALL was 13 μM in a 3H-thymidine uptake assay.
Western blot analysis of p21 in primary ALL.
Protein was extracted by the SDS/boiling method from (A) infant ALL; (B) B-precursor ALL; and (C) T-ALL cells treated with AN-9 as indicated. SJ SA-1 osteosarcoma cells, known to express p21, were used as the positive control. Western blot analysis was performed using a p21 antibody at 0.5 μg/mL. Equal amounts of protein in each lane were verified by probing the blot with a β-actin antibody at 2 μg/mL. U indicates untreated (0.1% DMSO); C, positive control for p21; pt, patient. AN-9 IC50 in the infant ALL was 13 μM in a 3H-thymidine uptake assay.
Drug-resistant primary leukemia and cancer cell lines are sensitive to AN-9
The finding that clinically refractory infant ALL cells are sensitive to AN-9 prompted us to determine whether AN-9 is cytotoxic to other drug-resistant malignant cells. The effect of AN-9 was examined in a doxorubicin-resistant clone of the myeloid leukemia cell line HL60, HL60/ADR, obtained by the transfection of the MDR-1gene; and a doxorubicin-resistant neuroblastoma cell line, Be2c/ADR, induced by repeated exposure of Be2c to doxorubicin. The doxorubicin-sensitive (IC50 = 0.002 μM) and doxorubicin-resistant HL60 cells (IC50 > 2.0 μM) were equally sensitive to AN-9, with IC50 values of 52.0 ± 5.6 μM and 57.3 ± 10.7 μM, respectively (Table 1).
Similarly, the doxorubicin-sensitive (IC50 = 0.007 μM) and -resistant neuroblastoma cell lines (IC50 > 2.0 μM) were equally sensitive to AN-9, with IC50 values of 69.4 ± 8.4 μM and 70.7 ± 6.2 μM, respectively (Table1). More important, a doxorubicin-resistant primary T-ALL and a relapsed AML that was refractory to chemotherapy were sensitive to the cytotoxicity of AN-9, each with an IC50 of 50 μM (Table1), an IC50 similar to that obtained in doxorubicin-sensitive primary ALL (Figure 2). Taken together, these findings, in addition to the finding of heightened sensitivity to AN-9 of relapsed infant leukemia (Figure 2), suggest that AN-9 may be a promising agent for acute leukemias, even in cases of relapsed or refractory leukemias.
Discussion
In the present study of 21 primary samples of acute leukemias, we demonstrated that AN-9 has antiproliferative and cytotoxic effects on leukemia cells. AN-9 arrested DNA synthesis in both T-ALL and B-precursor ALL at similar concentrations, indicating that its effects on leukemia cells were not lineage specific. More important, leukemia cells were 2- and 3-fold more sensitive than normal hematopoietic progenitors to the antiproliferative effects of AN-9 in3H-thymidine incorporation and colony-formation assays, respectively. These findings indicate that AN-9 is less toxic to normal hematopoietic progenitors and thus has selectivity for leukemia cells. In line with these results, earlier studies in a mouse tumor model as well as a phase I clinical trial of AN-9 conducted in adults with solid tumors have shown that AN-9 has efficacy and is relatively nontoxic.19,25 Notably, in vitro data from solid tumors13,16,17 and Table 1 indicate that the IC50 of AN-9 is about 2-fold higher than that observed by us in leukemia, suggesting that leukemia cells are particularly sensitive to the cytotoxicity of AN-9. Collectively, our results indicate that AN-9 is a selective anticancer agent and may have an advantage over standard chemotherapeutic agents such as daunorubicin and doxorubicin, which are equally toxic to leukemia cells and hematopoietic progenitors in vitro.26-28
The anticancer effects of several HDAC inhibitors, including butyrate, were found to be dependent on their ability to inhibit HDACs and correlated with their capability to modulate cell-cycle and apoptosis-regulatory genes.12,23,24 A recent study reported that induction of p21 expression by butyrate was required for its effect in arresting cell growth in colon carcinoma cells.23 However, another study reported that butyrate arrested cell growth in 3T3 fibroblasts derived from p21knockout mice, indicating that p21 was not required for the antiproliferative effects of butyrate.29 Interestingly, p21 induction by AN-9 in this study was observed only in an infant ALL sample that was the most sensitive to AN-9 cytotoxicity. The levels of p21 were not induced by AN-9 in any of the primary T-ALL or B-precursor ALL cells examined, even at AN-9 concentrations that completely inhibited DNA synthesis. Thus, it appears that AN-9 may exert its antiproliferative effects through different pathways depending on the cell type.
Our results demonstrate that in addition to being an antiproliferative agent, AN-9 induces apoptosis, as observed in CEM cells. Zimra et al18 previously reported that AN-9 induced apoptosis in HL60 cells and that this was accompanied by the reduction ofBcl-2 expression. However, another study reported that butyrate activated caspase 3 and induced apoptosis in lymphoid and colorectal cancer cells that was dependent on the inhibition of HDACs but independent of changes in the levels of Bcl-2 andBax.30 As these authors suggested, it is possible that other members of the Bcl-2/Bax family may influence butyrate-induced apoptosis. Alternatively, apoptosis could be induced through the death receptors, including CD95, tumor necrosis factor (TNF), and the receptor for the TNF-related apoptosis-inducing ligand. Although the hybrid polar HDAC inhibitor, M-carboxycinnamic acid bishydroxamide, was found to induce CD95/CD95 ligand expression, butyrate did not alter the expression of either the ligand or receptor, but did alter cell sensitivity to CD95-mediated apoptosis.31 The repression ofBcl-218 and the induction of Baxexpression (A.R., unpublished observations, 1997) by AN-9 suggest that the intrinsic pathway of apoptosis that does not involve the death receptors may be the pathway responsible for AN-9–induced apoptosis. Because the precise mechanisms underlying the effects of HDAC inhibitors remain unknown, future work is aimed at further examining the pathways and key components involved in AN-9–induced growth arrest and apoptosis in ALL.
Unfortunately, as with many cancers, relapses are common in ALL and are associated with multidrug resistance. In this study, AN-9 was effective in inhibiting the growth of HL60 myeloid and neuroblastoma clones that harbored multiple genetic alterations32 and displayed a multidrug-resistant phenotype.20-22 Moreover, AN-9 was equally effective in arresting proliferation of all the primary leukemia cells tested, including a doxorubicin-resistant T-ALL, a clinically refractory relapsed AML, and a relapsed infant ALL characterized by an 11q23 rearrangement and a very poor prognosis. Thus, our finding that AN-9 arrested the growth of these different cancer cell types indicates that AN-9 is a unique agent with potential advantage over standard chemotherapeutic agents. Similar observations have been reported using other HDAC inhibitors.10-12 For instance, the HDAC inhibitor MS-27-275 strongly inhibited the growth of solid tumor implants in nude mice that did not respond to the standard chemotherapeutic agent 5-fluorouracil.11 Furthermore, the more familiar HDAC inhibitor trichostatin A was shown to inhibit proliferation and induce apoptosis in gastric and oral carcinoma cell lines resistant to 9-cis–retinoic acid and interferon-β.12 Another advantage of AN-9 is its ability to synergize with anthracyclines such as daunomycin, commonly used in the treatment of leukemia patients.19,33The molecular basis for the synergistic actions of doxorubicin and AN-9 was reported to be the ability of formaldehyde, released upon cellular hydrolysis of AN-9, to facilitate the formation of DNA adducts with doxorubicin.34 35 Concurrently, butyrate, also released upon cellular hydrolysis, inhibits HDACs, resulting in histone hyperacetylation and relaxation of the chromatin structure, allowing greater accessibility of DNA for the formation of doxorubicin–DNA adducts. The synergistic action of AN-9 with doxorubicin would allow the use of significantly lower doses of these agents in treatment and consequently would greatly improve the therapeutic index.
Thus, AN-9, an HDAC inhibitor with low toxicity and selectivity toward cancer cells, may provide a novel therapeutic strategy for cancers refractory to traditional antitumor agents. The present study provides strong evidence to warrant clinical trials in relapsed ALL using AN-9 as a single agent or in combination with standard chemotherapeutic agents.
We gratefully acknowledge the investigators from the Pediatric Oncology Group for providing T-ALL samples. We also thank Louis Bridgeman, Ruby Gribi, and Dr Sigrun Gebauer for technical assistance. We are very grateful to Dr Denis Sasaki for assistance with flow cytometry.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-02-0567.
Supported by grants from the Leukemia & Lymphoma Society 6125 (to A.L.Y.) and 6226 (to J.Y.), CA79951 (to J.Y.), The Cindy Matters Fund (to A.L.Y.), and in part by grant MO1 RR00827 from the General Clinical Research Center program. In addition, it is supported by grant 542/0 (to A.R.) from the Israel Science Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alice L.Yu, Division of Pediatric Hematology/Oncology, University of California San Diego Medical Center, 200 West Arbor Dr, San Diego, CA 92103-8447.