Dendritic cells (DCs) initiate and direct immune responses. Recent studies have defined different DC populations, therefore we undertook this study comparing 2 types of myeloid DCs: blood CD11c+DCs and in vitro monocyte-derived DCs (Mo-DCs), which are both candidates as cellular adjuvants for cancer immunotherapy. Blood CD11c+ DCs were prepared by cell sorting from peripheral blood mononuclear cells cultured overnight in RPMI 1640 medium supplemented with autologous or pooled AB serum. Mo-DCs were prepared in the same medium using granulocyte macrophage–colony-stimulating factor (GM-CSF)/interleukin 4 (IL-4) and differentiated/activated with lipopolysaccharide or monocyte-conditioned medium (ActMo-DCs). Morphologically, differences between the DC preparations were noted both at a light and and electron microscopic level. Blood CD11c+ DCs expressed similar levels of HLA-DR, CD40, CD86, and CD83 as Mo-DCs. CD209 was present on Mo-DCs but not on blood CD11c+ DCs. Blood CD11c+ DCs generated a lower proliferative mixed leukocyte response (MLR) than Mo-DCs. Blood CD11c+ DCs loaded with 0.1 μg/mL tetanus toxoid (TT)–generated greater T lymphocyte proliferative responses than did Mo-DCs or ActMo-DCs, but when loaded with higher TT concentrations no difference in T lymphocyte proliferative response was observed. Keyhole limpet hemocyanin (KLH)–loaded blood CD11c+ DCs generated greater T lymphocyte proliferative responses than Mo-DCs or ActMo-DCs. Allogeneic MLR- or KLH-specific responses induced by blood CD11c+ DCs generated more Th1 effectors than the responses induced by Mo-DCs or ActMo-DCs. These data establish several differences in the properties of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs, which suggest that blood DCs merit further consideration as DC preparations for clinical programs are evolved.
Introduction
Dendritic cells (DCs) have been identified as very effective antigen- presenting cells (APCs) with the apparently unique ability to prime naive T lymphocytes to novel antigens.1,2 Thus, DCs are key cells for developing vaccination strategies, including anticancer responses.3
It has been accepted that DCs are produced in the bone marrow and migrate via the blood stream into virtually all tissues in the body.1 These surveillance DCs capture antigen when appropriate and migrate to draining lymph nodes. The DCs process and present the antigen as major histocompatibility complex (MHC)–peptide complexes to antigen-specific naive T lymphocytes. The DCs responsible for these processes are thought to be myeloid DCs, but recent analyses have defined an additional blood “lymphoid” DC population.4,5 Subsets of monocytes including CD16+CD14+,6 and CD2+CD14+,7 cells have also been identified as potential DC precursors. This new, and in some cases, conflicting, information regarding DC ontogeny makes it essential to clarify basic DC hematopoeitic development and also to define the functional properties of the different DC preparations proposed as immunotherapeutic cellular adjuvants.3
DCs in the peripheral blood are identified within the HLA-DR+, lineage (CD3, CD14, CD19, CD56) negative (Lin−) blood mononuclear cell population. The precursors for the peripheral epithelial (CD1ahi) and dermal (CD1alo) DCs8 are identified within myeloid blood CD11c+ DCs.9 The mix of lineage antibodies and the procedure used in purification of these cells influences the constitution of the myeloid blood DCs and new reagents now make it possible to define further subsets.10Certainly, CD14+ monocytes incubated with granulocyte macrophage–colony-stimulating factor (GM-CSF)/interleukin 4 (IL-4) and subsequently differentiated/activated with tumor necrosis factor alpha (TNF-α) or other agents develop into monocyte-derived DCs (Mo-DCs) with many characteristics similar to the myeloid blood CD11c+ DCs.11-14 Studies in mice indicate that monocytes may differentiate into DCs in vivo.15 However, we have noted that stress-induced changes in human myeloid blood CD11c+ DCs and CD14+ monocyte counts peak independently.16 We have hypothesized that Mo-DCs may represent an inflammatory boost pathway for APCs, whereas the blood CD11c+ DCs represent the preformed surveillance DCs.1 On the other hand, the “lymphoid” blood DC population distinguished as CD11c−CD123hi has different morphologic, phenotypic, and functional properties,4,5,17 and has been suggested to link innate and adaptive immune responses.18
Human DC preparations derived from CD34+ stem cells, adult blood, cord blood, peripheral tissues (eg, epidermal, dermal DCs) and lymphoid tissues (eg, thymus, tonsil, lymph node, spleen) have been described. Early clinical studies investigating the feasibility and efficacy of DC-based immunotherapy protocols used minimally purified blood DCs.19 Subsequently, Mo-DCs have been used more extensively,3,14 mainly because of the relative abundance and accessibility of CD14+ monocytes in peripheral blood. However, the use of different starting monocyte preparations, serum supplements, cytokines, and differentiating/activating agents has contributed to marked heterogeneity in opinion as to the optimal phenotype and functional characteristics of Mo-DCs for immunotherapy.20 A recent report suggests that Mo-DCs generated with GM-CSF/IL-4 may reduce rather than enhance immune responses.21 Accumulating data suggest that the quality of immune responses may also be affected by the route of administration of DCs.22
An alternative to manufacturing in vitro–derived Mo-DCs is to make use of the preformed, in vivo–generated, myeloid blood CD11c+DCs. Myeloid blood CD11c+ DCs are differentiated/activated in culture but this requires only a brief period of culture. During this they up-regulate MHC class I and II molecules, costimulatory molecules, and acquire characteristic DC differentiation/activation markers such as CD83, CMRF-44, and CMRF-56.23,24 Early clinical trial data suggest that relatively low numbers of blood DCs administered intravenously will induce an effective immune response that produces clinical benefit.19,22,25 The ability to count blood DCs and to obtain these cells via a limited pheresis procedure51 and even to purify them by monoclonal antibody (moAb)–positive selection52 makes blood DCs a rational alternative source for clinical studies.
As discussed here, both types of myeloid DCs have merit and each has been used for clinical trials, but surprisingly, no formal comparison between them has been reported. This paper describes the preclinical evaluation of blood CD11c+ DCs and Mo-DCs prepared from the same donors. To ensure a meaningful comparison, both blood CD11c+ DCs and Mo-DCs are prepared by similar technologies.
Patients, materials, and methods
Human subjects
Blood was obtained from volunteer donors, according to ethical committee guidelines, with appropriate informed consent. Volunteers vaccinated within the past 3 years were used for the tetanus toxoid (TT) presentation studies.
Monoclonal antibodies
The following moAbs were produced from hybridomas maintained in the laboratory: CMRF44 (IgM), CMRF50 (control IgM), CD14 (CMRF31, IgG2a). CD1a (Na1/34, IgG2a) was provided by Prof A. J. McMichael (Institute of Molecular Medicine, Oxford, England). CD19 (FMC63, IgG1) was provided by Prof H. Zola (Flinders Medical Center, Adelaide, Australia). CD16 (HuNK2, IgG2a) was provided by Prof I. F. C. McKenzie (Austin Research Institute, Melbourne, Australia). The moAbs CD3 (OKT3, IgG2a), CD8 (OKT8, IgG2a), CD45RO (UCHL1, IgG2a), HLA-DR (L243, IgG2a), CD11b (OKM1, IgG1), prepared in our laboratory from hybridomas, were obtained from American Type culture collection (ATCC, Manassas, VA).
The following reagents were purchased: fluorescein isothiocyanate (FITC)–conjugated IgG1 control, CD3 (SK7, IgG1), CD8 (OKT8, IgG1), CD4 (SK3, IgG1), CD45RA (2H4, IgG1), phycoerythrin (PE)–conjugated IgG1 and IgG2b control, CD3 (SΚ7, IgG1), CD34 (My10, IgG1), CD33 (P67.6, IgG1), CD14 (My4, IgG2b), HLA-DR (L243, IgG2a), allo-phycocyanin–conjugated moAb CD11c (S-HCL-3, IgG2b) (BD Biosciences, Sydney, Australia); PE-conjugated moAbs CD83 (HB15, IgG2b), CD40 (MAB89, IgG1) (Coulter-Immunotech, Sydney, Australia); PE-conjugated moAbs CD86 (IT2.2, IgG2b), CD123 (7G3, IgG2a) (PharMingen, Sydney, Australia); PE-conjugated moAbs against cytokines interferon gamma (IFN-γ) and IL-4 and control moAb IgG1 (PharMingen, San Diego, CA). The moAb CD209 (AZN-D1, IgG1) was available as part of the 7th Leukocyte Differentiation Antigen Workshop. FITC sheep antimouse (SAM) imunoglobulin F(ab')2fragments were purchased from Silenus (Melbourne, Australia).
Generation of blood CD11c+ DCs and Mo-DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll/Hypaque gradient (Pharmacia Uppsala, Sweden) (density = 1.077 g/mL) centrifugation and maintained in RPMI-1640 medium (Invitrogen, Melbourne, Australia) supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine and heat-inactivated human serum (HS; pooled AB serum [ABS] or autologous serum [AS]).
Blood CD11c+ DCs were prepared from PBMCs cultured overnight at a concentration of 1 × 107 cells/mL in RPMI/5% HS at 37°C and 5% CO2 in Falcon polystyrene 100 mm standard dish (BD Biosciences). Cultured PBMCs were labeled with a mix of CD3, CD19, CD14, CD16, and CD11b moAbs. After incubation with magnetic beads (QIAGEN, Valencia, CA) labeled cells were removed by magnetic immunodepletion using MACS Separation Columns (Miltenyi Biotec, Sydney, Australia). Cells were recovered from negative fraction (Lin− cells) labeled with FITC-SAM and CD11c moAb and Lin−CD11c+ DCs further purified by fluorescence-activated cell-sorter scanner (FACS) sorting (FACS Vantage; BD Biosciences).
Mo-DCs were prepared from T-cell–depleted PBMCs obtained by rosetting with neuramidase-treated sheep erythrocytes (ERs). The ER-negative cells containing 1 × 106 CD14+ cells were cultured in Falcon 6-well plates (BD Biosciences) (3 mL/well) in RPMI/5% ABS or AS supplemented with recombinant human GM-CSF (200 U/mL) (Schering-Plough, Sydney, Australia) and IL-4 (50 U/mL) (Sigma, St Louis, MO). In some experiments, ER-negative cells were cultured in RPMI/1% AS supplements with 1000 U/mL GM-CSF and 1000 U/mL IL-4. The resulting Mo-DCs used after 7 days of culture, with or without the addition of 1 μg/mL lipopolysaccharide (LPS) (Sigma), 30% allogeneic monocyte conditioned medium (MCM) at day 5 of culture to differentiate/activate the Mo-DCs. The latter are hereafter referred to as “ActMo-DCs.” The media and cytokines were not changed during the culture period. The viabilities of each preparation were carefully monitored (see “Results”).
Preparation of responder T lymphocytes
CD3+ lymphocytes were purified from PBMCs after labeling with CD19, CD16, CD14, CD11b, HLA-DR moAbs and depletion using immunomagnetic beads and FACS sorting as described above for preparation of blood DCs. CD45RA+CD4+lymphocytes were purified by including CD8 and CD45RO moAbs in the mixture used for isolation of CD3+ lymphocytes and magnetic beads separation and FACS sorting. Purities were generally more than 95%. Freshly isolated T lymphocytes were used for TT and keyhole limpet hemocyanin (KLH) antigen presentation assays and frozen/thawed T lymphocytes were used to enable the identical responder to be used in comparative mixed leukocyte responses (MLRs).
TT-specific T-cell clones were generated from PBMCs cultured in RPMI/5% ABS in the presence of 0.1 μg/mL TT. IL-2 (1 U/mL) was added at day 7 of culture, cells were expanded for a further 5 days and then frozen in liquid N2. Expanded cells were more than 90% CD3+ lymphocytes. These TT-responsive cell clones were thawed and expanded for 3 more days in medium with 1 U/mL IL-2 before use in TT antigen presentation assays.
Morphologic examination
To assess DC morphology, cytospins were prepared by cytocentrifuging 3 × 104 to 5 × 104 DCs onto a glass slide at 1000 rpm for 5 minutes using a cytospin-3 centrifuge (Shandon, Trace Scientific, Melbourne, Australia). Cytospins were air-dried and stained using May-Grünwald-Giemsa stain, then examined by light microscopy. For transmission, electron microscopy DCs were fixed in 3% glutaraldehyde, 4% paraformaldehyde plus 0.8% calcium chloride, 0.1 sodium cacodylate buffer (pH 7.4), and postfixed in 1% aqueous OsO4, stained in block with 5% aqueous uranyl acetate, dehydrated in graded ethanol solutions and propylene oxide then embedded in Epon/Araldite epoxy resin. Ultrathin sections were cut using a Leica UCT ultramicrotome, stained in Reynold lead citrate and observed in a JEOL 1200EXII TEM (Department of Pathology, Electron Microscope Unit, University of Queensland, Brisbane, Australia).
Cell labeling
Cells were incubated with saturating concentrations of moAbs, washed, and for indirect labeling further labeled with FITC-SAM. For double labeling, 10% mouse serum was used to block before incubating with PE- or allo-phycocyanin–conjugated moAbs. Data were acquired using a FACS Calibular Flow Cytometer (BD Biosciences) and at least 5000 events were acquired from each specimen.
Intracellular cytokine production was assessed in T lymphocytes expanded with PMA (10-20 ng/mL) plus ionomycin (1 ng/mL) (Sigma) for 6 hours. GolgiPlug (PharMingen) was added during the last 2 of 6 hours to accumulate most of the cytokine protein in the Golgi complex. Cells were labeled with CD3 moAb, fixed/permeabilized according to the manufacturer's instructions (Caltag Laboratories, Burlingame, CA) and then labeled with moAbs against cytokine IFN-γ or IL-4.
Functional assays
Medium RPMI/5% ABS supplemented with 10 mM HEPES, 1 mM nonessential amino acid, and 2 mM L-glutamine was used for all functional assays. Allogeneic MLRs were established by adding a range of blood CD11c+ DCs, Mo-DCs, or ActMo-DCs to 1 × 105 allogeneic CD3+ lymphocyte (DC/T ratio; 1:10-1000) in 96-well round-bottom microtiter plates. Proliferation was measured by adding 1 μCi (0.037 MBq) [3H] thymidine for the last 16 hours of a 6-day culture period. Responses were reported as a mean of triplicate or quadruplicate, counts per minute (cpm) ± SD. Control wells containing only T lymphocytes or DCs alone always incorporated less than 500 cpm [3H] thymidine.
TT (CSL, Melbourne, Australia) and KLH (Sigma) were used to test secondary and primary antigen-specific T-lymphocyte responses. The DCs were loaded with 0.01 μg/mL to 10.0 μg/mL TT or with 25 μg/mL to 100 μg/mL KLH at 37°C for at least 30 minutes (maximum 3 hours). TT-loaded DCs were cultured with 1 × 105 autologous CD3+ lymphocyte or with 5 × 104 TT-specific T-cell clones (DC/T ratio; 1:5-40) in 96-well round-bottom microtiter plates for 6 or 3 days, respectively. KLH-loaded DCs were cultured with 1 × 105 autologous CD45RA+CD4+ T lymphocyte (DC/T ratio; 1:20) in 96-well round-bottom microtiter plates for 6 days. During the last 16 hours of each culture period, cells were pulsed with 1 μCi (0.037 MBq) [3H] thymidine. Responses were reported as a mean of triplicate cpm ± SD or as stimulation index (SI; mean cpm from optimal antigen concentration/mean cpm from no antigen control).
Statistical analysis
Differences between groups were compared by Student 2-tailed paired t test, assuming equal variances. Values ofP < .05 were considered significant.
Results
Characteristics of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs
PBMCs from healthy donors were cultured overnight in RPMI/HS (ABS or AS) prior to the preparation of blood CD11c+ DCs. The Lin− cells obtained following immunomagnetic bead negative selection were divided into 3 subsets based on their level of CD11c expression; these are: CD11c+, CD11cdim, and CD11c−. The blood CD11c+ DC subset (Figure1A, Region 1 [R1]) expressed high levels of the HLA-DR, CD86, CD40, and CD83 antigens. These were further identified as CD33+ myeloid blood DCs (data not shown) which were CD123+ and lacked CD209 antigen.
Comparison of phenotypic profiles of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
(A) Blood CD11c+ DCs were obtained by lineage negative (Lin−) selection using magnetic beads and further sorting for CD11c+ cells (R1, a gate used for sorting). Histograms show expression of the cell-surface antigens on blood CD11c+ DCs in region R1 (dotted line, isotype control moAb; solid line, specific moAb). (B) Mo-DCs generated as described in “Patients, materials, and methods” had low levels of CD1a expression in most cases. Histograms show expression of the antigens on Mo-DCs and ActMo-DCs (generated in RPMI/5% AS, 200 U/mL GM-CSF, and 50 U/mL IL-4 and activated by LPS) in region R2 (dotted line, isotype control moAb; thin solid line, specific moAb on Mo-DCs; thick solid line, specific moAb on ActMo-DCs).
Comparison of phenotypic profiles of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
(A) Blood CD11c+ DCs were obtained by lineage negative (Lin−) selection using magnetic beads and further sorting for CD11c+ cells (R1, a gate used for sorting). Histograms show expression of the cell-surface antigens on blood CD11c+ DCs in region R1 (dotted line, isotype control moAb; solid line, specific moAb). (B) Mo-DCs generated as described in “Patients, materials, and methods” had low levels of CD1a expression in most cases. Histograms show expression of the antigens on Mo-DCs and ActMo-DCs (generated in RPMI/5% AS, 200 U/mL GM-CSF, and 50 U/mL IL-4 and activated by LPS) in region R2 (dotted line, isotype control moAb; thin solid line, specific moAb on Mo-DCs; thick solid line, specific moAb on ActMo-DCs).
The Mo-DCs derived after 7 days in vitro culture with 5% AS and supplemented with GM-CSF 200 U/mL and IL-4 50 U/mL lacked CD1a antigen as reported26 in 21 out of 24 experiments (Figure 1B, Region 2 [R2]) but expressed HLA-DR, CD86, and CD40. They lacked CD123 but expressed low levels of CD209. When differentiated/activated by LPS during the last 2 days of culture, the resulting ActMo-DCs increased the expression of HLA-DR, CD86, CD40, CD123, and CD209 antigens and acquired surface expression of the CD83 antigen (Figure1B). When the level of expression (Δ mean fluorescence intensity [Δ MFI]) of these markers was compared between blood CD11c+ DCs, Mo-DCs, or ActMo-DCs, the similar level of HLA-DR (Δ MFL; 5426 vs 1365) CD40 (1344 vs 2259), CD86 (2485 vs 1857), and CD83 (83 vs 60) was found (Figure 1A-B, Table1). The use of ABS or AS made no differences to either blood CD11c+ DCs or Mo-DCs phenotype.
We also assessed phenotype of Mo-DCs generated in RPMI/ 1% AS, GM-CSF 1000 U/mL, and IL-4 1000 U/mL with or without LPS or MCM. There were no differences in phenotype between Mo-DCs generated under different culture conditions. ActMo-DCs activated with LPS or MCM showed similar phenotype (Table 1).
The absolute yield of blood CD11c+ DCs obtained appeared to vary among individuals and with the sorting conditions. Starting with approximately 400 mL whole blood producing 230 × 106 to 480 × 106 (mean ± SD; 342 ± 79 × 106) PBMCs, we obtained 0.8 × 105 to 22.8 × 105 (mean ± SD; 7.0 ± 5.6 × 105; n = 20) blood CD11c+DCs (> 95% purity). These yields are consistent with our assessment of the theoretically achievable number of blood CD11c+ DCs available from 400 mL blood.51 The yields of Mo-DCs were also variable. Using 1.6 × 106 to 9.0 × 106 (mean ± SD; 5.5 ± 3.7 × 106) CD14+ monocytes obtained from approximately 40 mL whole blood yielded 5.8 × 105to 43 × 105 (mean ± SD; 24.5 ± 12.6 × 105; n = 24) Mo-DCs and 4.5 × 105 to 44 × 105 (mean ± SD; 29.5 ± 19.9 × 105; n = 24) ActMo-DCs in culture with 5% AS, GM-CSF 200 U/mL and IL-4 50 U/mL. The use of ABS or AS, different concentration of serum or cytokines made no difference to either blood CD11c+ DCs or the Mo-DCs yield (data not shown).
Morphology of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs
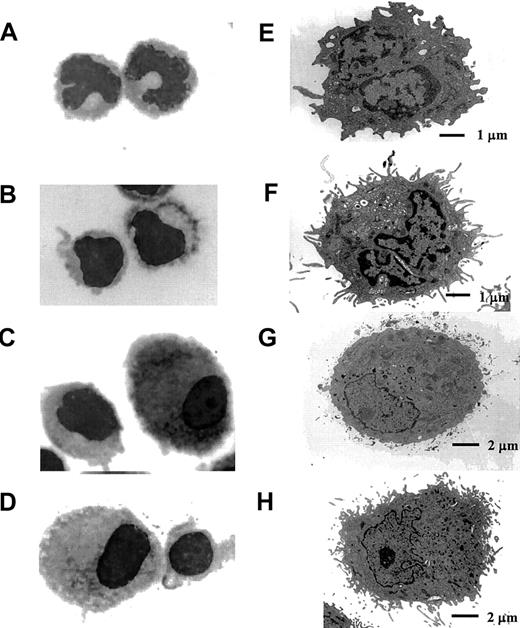
Blood CD11c+ DCs isolated both from fresh PBMCs and from PBMCs after overnight culture were purified by FACS sorting. Mo-DCs and ActMo-DCs were collected after 7 days culture (with or without LPS). Each DC preparation was examined by light microscopy (May-Grünwald-Giemsa staining) (Figure2A-D, ×1000) and electron microscopy (EM) (Figure 2E-H). The freshly isolated blood CD11c+ DCs had varied morphologically, with many cells having an irregular surface and a lobulated nucleus (Figure 2A) whereas blood CD11c+DCs isolated after overnight culture had more cytoplasmic projections with a minimally indented nucleus (Figure 2B). At an ultrastructural level, freshly isolated blood CD11c+ DCs lacked prominent endoplasmic reticulum and had fewer dendrites (Figure 2E) compared to their cultured counterparts (Figure 2F). In contrast, Mo-DCs were larger cells with irregular cytoplasm but retained a large rounded and eccentrically located nucleus (Figure 2C-D). ActMo-DCs generated in the presence of LPS (Figure 2H) developed much more prominent dendrites than did the blood CD11c+ DCs or Mo-DCs (Figure 2E,G).
The morphology of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
(A-D) May-Grünwald-Giemsa–stained DCs (× 1000) and (E-H) electron microscopic analysis of DCs. (A,E) Directly isolated blood CD11c+ DCs and (B,F) blood CD11c+ DCs after overnight culture. (C,G) Mo-DCs and (D,H) ActMo-DCs differentiated/activated with LPS.
The morphology of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
(A-D) May-Grünwald-Giemsa–stained DCs (× 1000) and (E-H) electron microscopic analysis of DCs. (A,E) Directly isolated blood CD11c+ DCs and (B,F) blood CD11c+ DCs after overnight culture. (C,G) Mo-DCs and (D,H) ActMo-DCs differentiated/activated with LPS.
Allostimulatory activity of DCs
Varying numbers of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs derived from the same donor were tested for their ability to induce proliferation of the same responding CD3+lymphocytes. In an initial set of 10 experiments we compared proliferative responses induced by blood CD11c+ DCs, by Mo-DCs generated in RPMI/ 5% ABS or AS, GM-CSF 200 U/mL, and IL-4 50 U/mL, and by LPS ActMo-DCs. Of 10 cases tested, one did not induce any reponses. This case was excluded from the results as it may have been compatible by chance. The remaining 9 indicated that there was some heterogeneity in the relative effectiveness of the different DC preparations as allostimulatory cells. Using up to 300 DCs/well (DC/T ratio; 1:300), there was no significant difference among proliferative responses induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs, in most cases. At 3000 DCs/well (DC/T ratio; 1:30), blood CD11c+ DCs induced lower T-cell proliferative responses than Mo-DCs in 5 cases (Figure 3B-F), similar responses in 2 cases (Figure 3A,G), and higher responses in 2 cases (Figure 3H-I). When compared with ActMo-DCs, blood CD11c+ DCs induced lower proliferation in 6 cases (Figure3A,C-G), similar responses in one case (Figure 3B), and higher responses in 2 cases (Figure 3H-I). At 10 000 DCs/well (DC/T ratio; 1:10), blood CD11c+ DCs induced lower proliferative responses than Mo-DCs in 4 cases (Figure 3A-D), similar proliferative responses in 3 cases (Figure 3E-G), and higher proliferative responses in 2 cases (Figure 3H-I). When compared with ActMo-DCs, blood CD11c+ DCs induced lower proliferative responses in 7 cases (Figure 3A-G), similar proliferative responses in one case (Figure 3I), and higher in one case (Figure 3H).
Allogeneic MLR induced by blood CD11c+ DCs, Mo-DCs and ActMo-DCs.
(A-I) Increasing numbers of blood CD11c+ DCs (▴), Mo-DCs (generated in RPMI/5% ABS or AS, GM-CSF 200 U/mL, IL-4 50 U/mL) (●), or ActMo-DCs (activated with LPS) (○) were prepared from the same donor and cultured with 1 × 105 allogeneic CD3+ lymphocytes (DC/T ratio; 1:10-1000). (J) Increasing numbers of blood CD11c+ DCs (▴), Mo-DCs generated in RPMI/5% AS GM-CSF 200 U/mL, IL-4 50 U/mL (●), and ActMo-DCs activated with LPS (○) or with MCM (■) were prepared from the same donor and cultured with 1 × 105 allogeneic CD3+ lymphocytes (DC/T ratio; 1:10-1000). (K) Increasing numbers of blood CD11c+ DCs (▴), Mo-DCs generated in RPMI/1% AS, GM-CSF 1000 U/mL, IL-4 1000 U/mL (●), and ActMo-DCs activated with LPS (○) or with MCM (■) were prepared from the same donor and cultured with 1 × 105 allogeneic CD3+ lymphocytes (DC/T ratio; 1:10-1000). Results shown are presented as the mean ± SD cpm of triplicate wells.
Allogeneic MLR induced by blood CD11c+ DCs, Mo-DCs and ActMo-DCs.
(A-I) Increasing numbers of blood CD11c+ DCs (▴), Mo-DCs (generated in RPMI/5% ABS or AS, GM-CSF 200 U/mL, IL-4 50 U/mL) (●), or ActMo-DCs (activated with LPS) (○) were prepared from the same donor and cultured with 1 × 105 allogeneic CD3+ lymphocytes (DC/T ratio; 1:10-1000). (J) Increasing numbers of blood CD11c+ DCs (▴), Mo-DCs generated in RPMI/5% AS GM-CSF 200 U/mL, IL-4 50 U/mL (●), and ActMo-DCs activated with LPS (○) or with MCM (■) were prepared from the same donor and cultured with 1 × 105 allogeneic CD3+ lymphocytes (DC/T ratio; 1:10-1000). (K) Increasing numbers of blood CD11c+ DCs (▴), Mo-DCs generated in RPMI/1% AS, GM-CSF 1000 U/mL, IL-4 1000 U/mL (●), and ActMo-DCs activated with LPS (○) or with MCM (■) were prepared from the same donor and cultured with 1 × 105 allogeneic CD3+ lymphocytes (DC/T ratio; 1:10-1000). Results shown are presented as the mean ± SD cpm of triplicate wells.
We also made Mo-DCs using RPMI/1% AS and the higher concentration of cytokines (GM-CSF 1000 U/mL and IL-4 1000 U/mL) and activated them with LPS and MCM. We compared these preparations with blood CD11c+ DCs and with Mo-DCs generated in RPMI/5% AS, GM-CSF 200 U/mL, and IL-4 50 U/mL, and activated with LPS and MCM. Comparative studies revealed that Mo-DCs generated in RPMI/1% AS, GM-CSF 1000 U/mL, and IL-4 1000 U/mL induced similar T-cell proliferative responses as Mo-DCs generated in RPMI/5% AS, GM-CSF 200 U/mL, and IL-4 50 U/mL (Figure 3J-K). ActMo-DCs compared with Mo-DCs induced higher T proliferative responses, but this result was less consistent than expected. In addition, LPS was confirmed to be a good maturation stimulus and ActMo-DCs activated by LPS induced higher proliferative responses than ActMo-DCs activated by MCM (Figure 3J-K).
No influence on the stimulatory effect of either blood CD11c+ DCs, Mo-DCs, or ActMo-DCs was noted according to whether ABS or AS was used for cellular preparations (data not shown).
Initial optimization of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs for antigen loading
It is likely that different DC preparations may require different antigen loading conditions for optimal antigen presentation.20 27 To test this, we analyzed the T-lymphocyte proliferative responses induced by DCs loaded with TT (10 μg/mL) or KLH (100 μg/mL) for periods varying from 0 to 3 hours. Although there were some variations among donors, these experiments established that blood CD11c+ DCs loaded with TT for 1 to 2 hours induced maximal TT-specific T-lymphocyte proliferation (data not shown). KLH-specific T-lymphocyte proliferation was maximal when blood CD11c+ DCs were loaded with KLH for 2 to 3 hours (data not shown). Mo-DCs and ActMo-DCs required a similar incubation time in order to generate maximal proliferative responses. Therefore, we exposed all types of DCs to TT for 2 hours and to KLH for 3 hours.
Comparison of the ability of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs to present the recall TT antigen
To test the ability of DCs to induce recall antigen-specific T-lymphocyte responses, we cultured CD3+ lymphocytes (1 × 105) from known responders with different numbers of each DC (DCs; 2500, 5000, 10 000/well), which had been loaded with TT (0.01, 0.1, 1, 10 μg/mL) for 2 hours. As shown in Figure4, a dose-dependent (antigen-specific) T-lymphocyte proliferation was induced using antigen-loaded blood CD11c+ DCs (Figure 4A) but the optimal TT concentration differed between individuals. The specific T-cell response induced by Mo-DCs or ActMo-DCs also varied between individuals (Figure 4B-D). Increasing the numbers of DC stimulators increased the T-lymphocyte proliferative responses but TT nonspecific autologous reactive responses also occurred at higher DC/T ratios (Figure 4A,C-D). In general, minimal TT nonspecific autologous CD3+lymphocyte proliferation resulted at 5000 DCs/well.
TT-specific proliferative responses induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
TT-specific T-lymphocyte responses induced by a graded number (2500/well-10 000/well) of blood CD11c+ DCs (▴), Mo-DCs (produced in RPMI/5% AS, GM-CSF 200 U/mL, and IL-4 50 U/mL) (●) or ActMo-DCs (produced in RPMI/5% AS, GM-CSF 200 U/mL, and IL-4 50 U/mL and activated with LPS) (○) loaded with 0 μg/mL to 10 μg/mL TT for 2 hours. CD3+ lymphocytes (1 × 105/well) from TT known responders were used in A through D (DC/T ratio; 1:10-40), while TT-specific T-cell clones (5 × 104/well) were used in E and F (DC/T ratio; 1:5-40). Graphs A and B related to case 2, C and D to case 5, and E and F to case 12. Results are presented as the mean ± SD cpm of triplicates.
TT-specific proliferative responses induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
TT-specific T-lymphocyte responses induced by a graded number (2500/well-10 000/well) of blood CD11c+ DCs (▴), Mo-DCs (produced in RPMI/5% AS, GM-CSF 200 U/mL, and IL-4 50 U/mL) (●) or ActMo-DCs (produced in RPMI/5% AS, GM-CSF 200 U/mL, and IL-4 50 U/mL and activated with LPS) (○) loaded with 0 μg/mL to 10 μg/mL TT for 2 hours. CD3+ lymphocytes (1 × 105/well) from TT known responders were used in A through D (DC/T ratio; 1:10-40), while TT-specific T-cell clones (5 × 104/well) were used in E and F (DC/T ratio; 1:5-40). Graphs A and B related to case 2, C and D to case 5, and E and F to case 12. Results are presented as the mean ± SD cpm of triplicates.
Blood CD11c+ DCs loaded with 0.1 μg/mL TT for 2 hours induced in some instances more than 100-fold higher specific T-lymphocyte proliferation compared with Mo-DCs or ActMo-DCs (DC/T ratio; 1:20), (mean ± SD cpm: blood CD11c+ DCs; 33 471 ± 22 982, Mo-DCs; 6829 ± 3739, ActMo-DCs; 8120 ± 8878, n = 7; P = .0217 for blood CD11c+ DCs vs Mo-DCs; P = .0269 for blood CD11c+ DCs vs ActMo-DCs). However, when loaded with 1 μg/mL TT there were no significant differences among the proliferative responses induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs (mean ± SD cpm: blood CD11c+DCs; 42 367 ± 25 744, Mo-DCs; 18 787 ± 23 069, ActMo-DCs; 17 157 ± 18 463, n = 8; P > .05 for blood CD11c+ DCs vs Mo-DCs and for blood CD11c+ DCs vs ActMo-DCs).
When TT-specific T-cell clones (5 × 104) were stimulated by TT-loaded blood CD11c+ DCs, Mo-DCs, or ActMo-DCs, similar proliferative responses were noted independent of the dose of TT used to load the DCs (Figure 4E-F). In addition, proliferative responses of TT-specific T-lymphocyte lines were always lower (Figure 4E-F) than proliferative responses of CD3+ lymphocytes (Figure 4A-D).
The data were further evaluated using the SI in order to avoid the influence of the variable TT nonspecific responses generated by the different preparations and individuals. The blood CD11c+DCs appeared to generate more consistent specific increases in the SI. When loaded with 0.1 μg/mL of TT blood CD11c+ DCs (DC/T ratio; 1:20) produced a higher SI than Mo-DCs in 5 out of 6 cases and ActMo-DCs in 6 out of 6 cases (Table2). When loaded with 1 μg/mL TT blood CD11c+ DCs produced a higher SI than Mo-DCs in 5 out of 7 cases and ActMo-DCs in 6 out of 7 cases (Table 2).
Comparison of the ability of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs to present the primary KLH antigen
We compared the ability of blood CD11c+ DCs, Mo-DCs generated in culture with 5% AS, GM-CSF 200 U/mL, and IL-4 50 U/mL and ActMo-DCs activated with LPS to generate primary T-lymphocyte responses. Blood CD11c+ DCs, Mo-DCs, and ActMo-DCs were loaded with different concentrations of KLH (25 μg/mL to 100 μg/mL) for 3 hours and then incubated with autologous CD45RA+CD4+ lymphocytes (1 × 105/well) (DC/T ratio; 1:20). Similar T-lymphocyte proliferative responses were observed using blood CD11c+DCs, Mo-DCs, or ActMo-DCs loaded with 100 μg/mL KLH (Figure5A-B). Variable T-lymphocyte responses were observed using DC preparations loaded with 25 μg/mL KLH and interpreting these was difficult because of KLH nonspecific autologous proliferative responses.
KLH-specific proliferative responses induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
(A,B) KLH-specific T-lymphocyte responses induced by 5 × 103 blood CD11c+ DCs, Mo-DCs, and ActMo-DCs (generated in RPMI/5%AS, GM-CSF 200 U/mL, IL-4 50 U/mL and activated with LPS) loaded with 0 μg/mL to 100 μg/mL KLH for 3 hours (DC/T ratio; 1:20). Shown are 2 representative experiments with mean ± SD. (C,D) Paired KLH-specific T-lymphocyte responses induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs loaded with 100 μg/mL KLH or (E,F) with 25 μg/mL KLH are shown as SI from 4 experiments.
KLH-specific proliferative responses induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
(A,B) KLH-specific T-lymphocyte responses induced by 5 × 103 blood CD11c+ DCs, Mo-DCs, and ActMo-DCs (generated in RPMI/5%AS, GM-CSF 200 U/mL, IL-4 50 U/mL and activated with LPS) loaded with 0 μg/mL to 100 μg/mL KLH for 3 hours (DC/T ratio; 1:20). Shown are 2 representative experiments with mean ± SD. (C,D) Paired KLH-specific T-lymphocyte responses induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs loaded with 100 μg/mL KLH or (E,F) with 25 μg/mL KLH are shown as SI from 4 experiments.
KLH-specific T-cell proliferation was also evaluated by SI. In all 4 experiments the SI induced by blood CD11c+ DCs loaded with 100 μg/mL KLH was greater than that achieved with either Mo-DCs or ActMo-DCs loaded with the same concentration of KLH (Figure 5C-D). This also occurred in 3 out of 4 experiments, in which the DCs were loaded with 25 μg/mL KLH (Figure 5E-F).
Comparison of the ability of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs to generate antigen-specific IFN-γ– and IL-4–producing T-lymphocyte responses
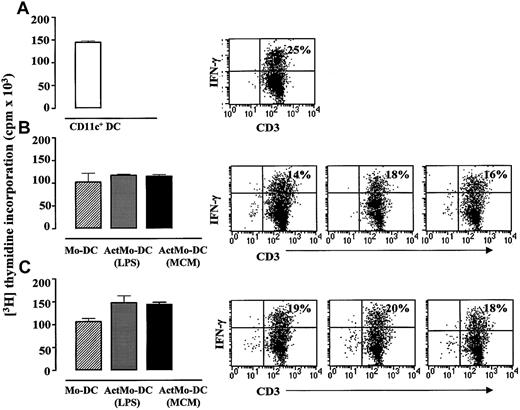
Blood CD11c+ DCs, Mo-DCs, or ActMo-DCs were cultured with allogeneic CD45RA+CD4+ lymphocytes for 5 days (DC/T ratio; 1:20) and the responding T lymphocytes analyzed for IFN-γ (Th1) and IL-4 (Th-2) producers. When cultured with CD45RA+CD4+ lymphocytes, blood CD11c+ DCs induced more IFN-γ–producing CD3+lymphocytes than did Mo-DCs or ActMo-DCs (mean ± SD: 14% ± 9%; 7% ± 4% and 6% ± 5%, n = 3;P > .05, respectively) (Figure6A-C). Mo-DCs cultured in RPMI/5% AS, GM-CSF 200 U/mL, IL-4 50 U/mL induced similar number of IFN-γ producing CD3+ lymphocytes as Mo-DCs cultured in RPMI/1% AS, GM-CSF 1000 U/mL, IL-4 1000 U/mL (Figure 6B-C). Low number or no IL-4 producers (0%-4%) were detected in all of these experiments (data not shown). It was noteworthy that the number of IFN-γ–producing CD3+ lymphocytes did not parallel the proliferative responses of allogeneic CD45RA+CD4+ lymphocytes induced either by blood CD11c+ DCs, Mo-DCs, or ActMo-DCs (Figure 6A-C).
IFN-γ production in T lymphocytes in the allogeneic MLR induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
Allogeneic CD45RA+CD4+ T-lymphocyte proliferation and intracellular IFN-γ production induced by 1 × 104 (A) blood CD11c+ DCs, (B) Mo-DCs and ActMo-DCs (produced in RPMI/5% AS, GM-CSF 200 U/mL, IL-4 50 U/mL and activated with LPS or MCM), (C) Mo-DCs and ActMo-DCs (produced in RPMI/1% AS, GM-CSF 1000 U/mL, IL-4 1000 U/mL and activated with LPS or MCM) (DC/T ratio; 1:10). The percentages of T lymphocytes producing IFN-γ are indicated on the dot plots. One representative experiment from the 3 experiments performed is shown.
IFN-γ production in T lymphocytes in the allogeneic MLR induced by blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
Allogeneic CD45RA+CD4+ T-lymphocyte proliferation and intracellular IFN-γ production induced by 1 × 104 (A) blood CD11c+ DCs, (B) Mo-DCs and ActMo-DCs (produced in RPMI/5% AS, GM-CSF 200 U/mL, IL-4 50 U/mL and activated with LPS or MCM), (C) Mo-DCs and ActMo-DCs (produced in RPMI/1% AS, GM-CSF 1000 U/mL, IL-4 1000 U/mL and activated with LPS or MCM) (DC/T ratio; 1:10). The percentages of T lymphocytes producing IFN-γ are indicated on the dot plots. One representative experiment from the 3 experiments performed is shown.
The TT-loaded DCs did not induce detectable cytokine-producing T lymphocytes during 6 days of culture with autologous CD3+or CD45RA+CD4+ lymphocytes. Even in the presence of TT throughout the 3-day culture period only up to 2% of IFN-γ–producing T lymphocytes were detected. This occurred despite a strong proliferative response (data not shown) again dissociating the 2 readouts of T-lymphocyte response.
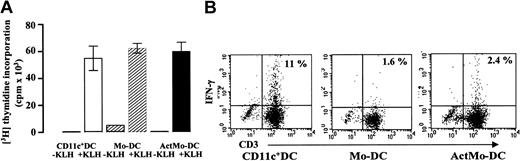
Blood CD11c+ DCs, Mo-DCs, or ActMo-DCs, pulsed with 100 μg/mL KLH for 3 hours were cultured with 1 × 105autologous naive CD45RA+CD4+ lymphocytes for 6 days (DC/T ratio; 1:20) and the responding T lymphocytes analyzed for IFN-γ producers. Despite inducing similar T-lymphocyte proliferative responses (Figure 7A), the KLH-pulsed blood CD11c+ DCs generated 5- to 8-fold more INF-γ–producing CD3+ lymphocytes than did the Mo-DCs or ActMo-DCs (Figure 7B). Similar results were obtained in 3 separate experiments (mean ± SD; blood CD11c+ DCs; 11.7% ± 4%, Mo-DCs; 1.9% ± 0.6%, ActMo-DCs; 3.7% ± 1.8%, blood CD11c+ DCs vs Mo-DCs; P = .0454, blood CD11c+ DCs vs ActMo-DCs; P = .0158).
IFN-γ production in T lymphocytes induced by KLH-loaded blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
(A) Autologous CD45RA+CD4+ lymphocyte proliferation and (B) intracellular IFN-γ production induced by 5 × 103 blood CD11c+ DCs, Mo-DCs, and ActMo-DCs (produced in RPMI/5% AS, GM-CSF 200 U/mL, IL-4 50 U/mL and activated with LPS) loaded with 100 μg/mL KLH for 3 hours (DC/T ratio; 1:20). The percentages of T lymphocytes producing IFN-γ are indicated on the dot plots. One representative experiment from the 3 experiments performed is shown.
IFN-γ production in T lymphocytes induced by KLH-loaded blood CD11c+ DCs, Mo-DCs, and ActMo-DCs.
(A) Autologous CD45RA+CD4+ lymphocyte proliferation and (B) intracellular IFN-γ production induced by 5 × 103 blood CD11c+ DCs, Mo-DCs, and ActMo-DCs (produced in RPMI/5% AS, GM-CSF 200 U/mL, IL-4 50 U/mL and activated with LPS) loaded with 100 μg/mL KLH for 3 hours (DC/T ratio; 1:20). The percentages of T lymphocytes producing IFN-γ are indicated on the dot plots. One representative experiment from the 3 experiments performed is shown.
Discussion
These experiments are, to our knowledge, the first reported attempt to compare the preformed, in vivo–generated, circulating myeloid blood CD11c+ DCs with their in vitro–generated Mo-DC counterpart. We deliberately chose to use the purified myeloid blood CD11c+ DCs to compare with Mo-DCs because the blood “lymphoid” CD123hi DC population is thought to have a different origin and function.1 2 In undertaking these comparisons, we also endeavored to prepare the different DC populations in conditions as close as possible to physiologic conditions. In particular, we prepared the blood CD11c+ DCs and Mo-DCs from the same donor in medium supplemented with HS and used fresh, not frozen, DC preparations in all experiments. Furthermore, we optimized antigen concentrations and loading time to provide optimal antigen-specific T-lymphocyte proliferative responses. The results indicate clear morphologic, phenotypic, and functional differences between blood CD11c+ DCs and Mo-DCs prepared in the one laboratory (by one person). The most striking finding is that blood CD11c+ DCs are more efficient than Mo-DCs in polarizing the proliferating T lymphocytes into Th1 effectors.
The preparation of blood DCs may involve various techniques including density gradient separation,28,29 and a variety of immunoselection procedures.1 The resulting blood DC populations are heterogeneous and for these experiments we chose to use a pure myeloid CD11c+ DC population. Our27 and other data30 suggested that a period of in vitro blood DC differentiation/activation was essential for optimal antigen responses. Therefore, we chose to isolate the blood CD11c+ DCs from PBMCs cultured in medium supplemented with HS. Previous data indicated that both ABS and AS human serum sources were able to induce differentiated/activated blood CMRF-44+ DCs with potent antigen-presenting activity27 and we wished to avoid fetal calf serum (FCS) in any potential clinical preparation and as antigen in our in vitro assays. Likewise, ABS or AS was used to prepare Mo-DCs replacing FCS in a protocol13 that has been shown to be effective in producing cell preparations with similar properties to those generated in medium with FCS. However, LPS was used to differentiate/activate Mo-DCs in order to provide a stable mature phenotype.31 Also, LPS was compared with MCM used in a clinical setting.
As previously published there was minimal expression of CD1a on Mo-DCs derived in culture with HS.26 Cao et al20have described the influence of different serum additives on Mo-DC generation and have evaluated the resulting yield, phenotype, and function. We also noted little difference between ABS and AS in our experiments, as has been reported previously.27 In addition, we confirmed that neither serum source was superior for the induction of antigen-specific responses.
Our phenotypic analysis of blood CD11c+ DCs isolated from PBMCs cultured overnight confirmed the expected phenotype. However, the direct comparison with Mo-DCs prepared from the same individual revealed no differences, which may be relevant for interpreting the functional data. First, the surface density of HLA-DR and costimulatory CD40 and CD86 molecules on the blood CD11c+ DCs was similar to that on Mo-DCs or even to the ActMo-DCs. Surface CD83 was readily detected on blood CD11c+ DCs but was not found on Mo-DCs and is only modestly expressed on ActMo-DCs. It is relevant that blood CD11c+ DCs after culture express CD123 but Mo-DCs lack CD123. This molecule is known to be inducible on myeloid blood CD11c+ DCs but the higher density on “lymphoid” blood DCs (CD11c−cells) still allowed discrimination between these cell types. CD209 (DC-SIGN) was recently described on Mo-DCs but this study again confirmed that this C-type lectin is not expressed on blood DCs.32,33 Other very relevant differences between blood CD11c+ DCs and Mo-DCs is their expression of the related multilectins, notably the CD206 (macrophage mannose receptor) and CD205 (DEC-205) as described by Kato et al.34 Some data are also emerging to suggest they have different expression of the toll receptors35 and chemokine receptors.36
The allogeneic MLR has been used to assess alloantigen- presenting capability of DC preparations for decades.37 We attempted to compare the alloantigen-presenting capability of blood CD11c+ DCs, Mo-DCs, and ActMo-DCs using the same T-lymphocyte responders. The data produced in 9 separate experiments reflect the wide variability in the MLR proliferative responses and indicate how even opposing conclusions might be drawn from the smaller data sets produced in different laboratories. Our data suggest that blood CD11c+ DCs stimulate lesser allogeneic proliferative responses than Mo-DCs. Undoubtedly, the MLR is heavily influenced by minor technical differences38 and this caused difficulties in interpreting different publications.
On the other hand, T lymphocytes cultured with blood CD11c+DCs generated more IFN-γ–producing effectors than those cultured with Mo-DCs or ActMo-DCs. It has been reported that multiple factors including the type of DCs,39 the factors used to differentiate/activate DCs,18,40 the duration of DC activation,41,42 and the DC:T ratio43influence the number and type of effector cells generated in the allogeneic MLR. It was reported recently that blood CD11c+DCs and Mo-DCs, after 48 hours of culture with GM-CSF/IL-4, generated a mix of Th1/Th2 effectors, but after further stimulation with CD40L predominantly Th1 effectors were generated.44 Our data indicate that both blood CD11c+ DCs differentiated/activated within PBMCs for a short period in vitro and Mo-DCs generated with GM-CSF/IL-4 and differentiated/activated with LPS or MCM generate predominantly Th1 effectors.
Testing the induction of human primary antigen-specific T-lymphocyte responses in vitro is difficult. Primary and restimulation assays are probably limited by the T-lymphocyte precursor frequency as we have suggested previously using blood DCs.27 For this reason we used the KLH antigen, which generates measurable primary T-lymphocyte responses. Our data suggest that blood CD11c+ DCs loaded with KLH in vitro in optimal conditions (concentration of antigen, loading time) induced a higher SI of T-lymphocyte proliferation than Mo-DCs or ActMo-DCs. Furthermore, blood CD11c+ DCs had the ability to polarize proliferative cells to Th1 effectors, whereas Mo-DCs and ActMo-DCs failed to polarize proliferating cells. It has been shown that chemokine(s) produced by activated/differentiated DCs attract T lymphocytes and enhance encounters between DCs and T lymphocytes.45,46 Prolonged antigen stimulation, multiple rounds of stimulation, the presence of polarized cytokine (IL-12 or IL-4), and costimulatory signals, all contribute to the generation of Th1 or Th2 effectors.47
We did not anticipate that the presentation of the memory or recall TT antigen would reveal a difference between blood CD11c+ DCs and Mo-DCs. Upon antigen recognition, memory T lymphocytes respond much more rapidly even in absence of costimulation than naive T cells.47 Proliferative responses were detected in both subsets of memory T cells (effector memory and central memory) even 10 years after vaccination.48 Surprisingly, we detected low T-lymphocyte proliferative responses using TT-specific clones in the culture with antigen-loaded DCs. However, we did note the apparently superior antigen-loading ability of blood CD11c+ DCs with a low concentration of TT. We showed that blood CD11c+ DCs loaded with small amounts of TT could induce significant proliferative responses, whereas Mo-DCs and ActMo-DCs need to be challenged with higher amounts of antigen (Figure 4). Cao et al20 also reported poor TT-specific T-lymphocyte proliferative responses using Mo-DCs.
The conditions that favor the expansion of memory T cells are not completely understood, but if blood CD11c+ DCs take up and process TT more effectively than Mo-DCs, they may present more antigen peptide complex to responding T lymphocytes and generate greater proliferative responses. Indeed, it has been shown that T lymphocytes form better synapses with DCs that offer higher amounts of antigen.49 It will be important to understand whether blood CD11c+ DCs can offer higher amounts of antigen than Mo-DCs and ActMo-DCs, since they lack putative antigen receptors such as CD209 (Figure 2),33 CD206, and CD205.34
These studies were performed to provide some guidance about the DC preparation we might take into clinical studies. We have established blood DC counting methods50 and documented the influence of surgical stress16 and cyclophosphamide/G-CSF on blood DC counts in multiple myeloma and non-Hodgkin lymphoma patients (S.V., M. Kim, D. Khalil, et al, manuscript submitted, July 2002). These latter results and new data suggest that reasonable yields of blood DCs can be obtained by pheresis and furthermore by moAb-based purification with effective in vitro function.52 It might be anticipated, that as blood DCs are destined to traffic to the tissues, antigen loading in vitro will be entirely compatible with their preprogrammed function, including migration to draining lymph nodes. Our own and others experience confirms that Mo-DCs are also readily obtained, providing care is paid to their extended in vitro culture. However, optimizing their antigen uptake, costimulatory potential and migration capacity may be a difficult process. Clearly, Mo-DCs administered to patients have generated immune responses including clinical responses with tumor regression.3
Remarkable advances in our understanding of the role of blood DCs and Mo-DCs in generating and maintaining the different subsets of memory/effector T lymphocytes have shed new light on the regulation of the immune responses. Once we understand what the ideal T-lymphocyte responders require, this new knowledge will help develop better therapies for controlling the immune response. At present it is sensible for investigators to refine both types of DC preparations. Ultimately the clinical data and practical issues will decide which DC preparation clinicians will select.
The authors wish to thank Dr Chris Schmidt for providing MCM, Mr Len Brown for assistance with flow cytometry, Mrs Dalia Khalil for assistance in performing experiments, Dr Alejandró López for helpful discussions, Mrs Debra Croft and Mr Noel Williams for organizing blood volunteers, and Mrs Tanya Hansen for help in preparing the manuscript. The authors also wish to thank the volunteers who provided blood specimens.
Supported by a Mater Medical Research Institute grant, the J.P. Kelly Research Foundation, and the Kanae Foundation for Life and Socio-Medical Science.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Derek N. J. Hart, Mater Medical Research Institute, Aubigny Pl, Raymond Terr, South Brisbane, Queensland 4101 Australia; e-mail: dhart@mmri.mater.org.au.