The article by Kunzmann and colleagues1brings attention to a potential complication of rituximab therapy, known as rituximab-induced tumor cell agglutination (RITCA), in patients with a high number of circulating lymphoma/leukemia cells. Although it is not clear whether the figures included in the report represent leukostasis secondary to high leukocyte count or true tumor cell agglutination (TCA), it is, nevertheless, an important observation and should alert clinicians who are undertaking such treatment of this potential untoward effect of rituximab therapy.
Severe infusion-related toxicities during treatment with rituximab have been reported in patients with a high number of circulating tumor cells and this complication has hitherto been attributed to cytokine release. It is conceivable that, at least in some cases as pointed out by Kunzmann and colleagues, the above phenomenon (ie, TCA) may either induce or contribute to the pathogenesis of various infusion-related toxicities. One of the important sequelae of this syndrome is peripheral circulatory failure. The management of such a hemodynamic disturbance routinely includes administration of intravenous fluids, either crystalloids (eg, normal saline) or various colloidal preparations such as gelofusin. Although these preparations are effective in restoring peripheral circulation and blood pressure, their effect on the coexisting pathologic process (ie, TCA) is not known.
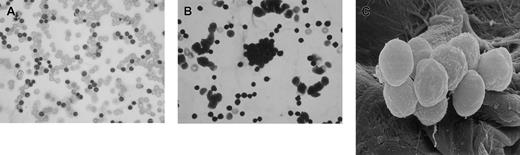
I would like to present some observations made during rituximab therapy in 2 patients with high circulating tumor (leukemic) cell counts (120 × 109/L and 256 × 109/L). Both patients were given prerituximab hydration therapy with normal saline followed by the drug at standard dose (375 mg/m2). Both patients experienced mild to moderate hypotension following the first dose of rituximab, but blood pressure was promptly restored by further administration of intravenous fluids (normal saline). Blood samples from these 2 patients were collected 6 hours after the first dose of rituximab infusion into RPMI medium and divided into 6 aliquots and incubated at 37°C for 2 hours with 1 mL of one of the following: normal saline, Hartman solution, hydroxy ethyl starch, dextran-40 (6%), dextran-70 (6%), or gelofusin. Aliquots of these suspensions were collected for light microscopy and scanning electron microscopy (SEM). Smears were prepared from the samples, stained with May-Grünwald-Giemsa (MGG) stain, and examined by light microscopy. The results show that none of the samples incubated in normal saline or Hartman solution exhibited TCA (Figure 1A) while varying degrees of TCA were noted with all colloid solutions. The most profound TCAs were observed with dextran-70 and gelofusin (Figure 1B,C).
Ex vivo agglutination of in vivo antibody-coated chronic lymphocytic leukemia cells (CLLs).
Cells incubated in normal saline (control) are shown in panel A, cells incubated in gelofusin showing TCA are shown in panel B, and SEM appearance of agglutinated CLL cells is shown in panel C. Original magnification: × 500.
Ex vivo agglutination of in vivo antibody-coated chronic lymphocytic leukemia cells (CLLs).
Cells incubated in normal saline (control) are shown in panel A, cells incubated in gelofusin showing TCA are shown in panel B, and SEM appearance of agglutinated CLL cells is shown in panel C. Original magnification: × 500.
Although this observation was made ex vivo, it nevertheless raises an important question whether similar changes (ie, TCA) may be induced in vivo by the above colloid preparations. It may, therefore, be reasonable to make a suggestion that colloids such as gelofusin, a commonly used intravenous preparation to restore blood pressure in patients with cardiovascular collapse, may induce or aggravate TCA in patients with a high number of circulating tumor cells and, therefore, should be used with caution during or soon after rituximab infusion. Further in vivo studies are needed to confirm the above ex vivo observations.
Reinvestigation of colloid administration's role in rituximab-induced tumor cell agglutination recommended
In a recent report of the fatal course of a rituximab-treated patient, we have demonstrated that tumor cell agglutination (TCA) might be a key mechanism of rituximab-induced adverse reactions. The pattern of intravascular leukostasis shown by histopathologic examinations was clearly different from findings in other patients with high leukocyte counts.1-1
Dr Muttuswamy Sivakumaran reports an interesting ex vivo finding regarding rituximab-induced tumor cell agglutination (RITCA). He suggests that various colloid preparations may induce or aggravate TCA in patients with a high number of circulating tumor cells, based on ex vivo observations in blood samples collected from 2 patients 6 hours after their first rituximab infusion.
In the course of his severe infusion-related syndrome, our reported patient also experienced hypotension, which was treated with hydroxy ethyl starch (250 mL 10% HES), after the administration of intravenous crystalloids (normal saline) did not increase blood pressure. Since HES was among the colloid preparations which induced TCA in Dr Sivakumaran's ex vivo experiments, we cannot exclude the possibility that the administration of this colloid preparation aggravated infusion-related toxicities in our patient and finally contributed to the fatal course.
The induction of blood cell agglutination after administration of HES has not been described before, even in patients with abnormally high blood cell numbers. However, it has been shown that HES can preserve rather than induce platelet agglutination during and after cardiopulmonary bypass surgery.1-2 The in vivo effect of colloid preparations after infusion of therapeutic monoclonal antibodies such as rituximab are not known. Therefore, we recommend a reinvestigation concerning the administration of colloids in all rituximab-related fatalities that have been reported to date to further evaluate their role in RITCA.
The ex vivo observations by Dr Sivakumaran confirm our results suggesting that TCA may play a role in the pathogenesis of rituximab-related toxicities, especially in patients with a high number of circulating tumor cells. Until the exact function of colloids in this syndrome has been elucidated, we support Dr Sivakumaran's suggestion that colloids should be avoided in the management of peripheral circulatory failure after rituximab infusion.