Abstract
Hydrocortisone reduces the number of inflammatory leukocytes within tissues, but thus far the site of action on the multistep adhesion cascade leading to leukocyte extravasation has not been identified. We have recently developed a noninvasive in vivo reflected-light confocal microscopy technique to study this at sites of inflammation in human patients. In the present study, we evaluated the effect of preoperative intravenous hydrocortisone treatment on leukocyte trafficking after conjunctival inflammation induced by cataract surgery in human subjects in vivo. The surgery generated leukocyte rolling along the endothelial lining of conjunctival vessels. While preoperative hydrocortisone did not reduce the number of rolling cells, it significantly raised the velocity of individual rolling leukocytes and concomitantly reduced leukocyte emigration into conjunctival tissue. Immunohistology of conjunctival biopsies excised from the individuals studied provided circumstantial evidence that endothelial P-selectin might play a role in the surgery-induced up-regulation of the leukocyte rolling. Furthermore, hydrocortisone reduced surgery-induced P-selectin induction, suggesting a role for this selectin in the regulation of local leukocyte traffic into sites of inflammation in human conjunctiva. Taken together, these results suggest that control of the rolling velocity might be an effective way to adjust leukocyte traffic in vivo in human subjects.
Introduction
Hydrocortisone has been used as an anti-inflammatory drug for several decades,1,2 and we asked whether its efficacy may be due to its modifying the multistep leukocyte adhesion cascade, resulting in the generation of local inflammatory lesions. If hydrocortisome is targeted toward leukocyte trafficking, its anti-inflammatory effects can, at least theoretically, result from changes in the number of rolling leukocytes or in their rolling behavior or in the actual extravasation phase through the interendothelial junctions.3-6
We have recently introduced a novel noninvasive application of confocal reflected-light microscopy enabling direct and repeatable analysis and quantification of conjunctival inflammations in human patients.7 The human conjunctiva can be used in these analyses, as it is semitransparent, and the tissue is normally devoid of leukocytes, which greatly facilitates the identification of newly emigrated leukocytes during the inflammatory reaction. Standard phacoemulsification cataract surgery was used to induce a rapid and very reproducible model of local conjunctival inflammatory reaction. The hallmarks of this surgery-induced inflammation are (1) the rapid increase in number of rolling leukocytes, which are almost exclusively granulocytes, and (2) the velocity of these rolling leukocytes, which is very slow, which is a prerequisite for (3) the extravasation of leukocytes from the blood circulation into the conjunctival tissue.7
In the present study, we evaluated the effect of preoperative intravenous hydrocortisone treatment on leukocyte trafficking after conjunctival inflammation induced by cataract surgery in human subjects in vivo. The surgery generated leukocyte rolling along the endothelial lining of conjunctival vessels. Whereas preoperative hydrocortisone did not reduce the number of rolling cells, it significantly elevated the velocity of individual rolling leukocytes and concomitantly reduced leukocyte emigration into conjunctival tissue. Immunohistology of conjunctival biopsies, excised from the individuals studied, suggested that P- and L-selectin, but not E-selectin, might play a role in the surgery-induced up-regulation of the leukocyte rolling. Furthermore, hydrocortisone diminished surgery-induced P-selectin induction, suggesting a role for this selectin on the regulation of local leukocyte traffic into sites of inflammation in human conjunctiva.
Patients, materials, and methods
Patients
Eight otherwise healthy patients with senile cataracts were enrolled for the study. Informed consent was obtained from all patients, and the study protocol was reviewed and accepted by the Committee on Ethics of the Helsinki University Eye and Ear Hospital and followed the tenets of the Declaration of Helsinki. Exclusion criteria were the use of anticoagulative, immunosuppressive, or anti-inflammatory therapies or any combination of these. All patients were examined by the novel confocal reflected-light microscopy (described below) 1 to 7 days before the surgical cataract operation and 24 hours afterward, during the most prominent inflammation. Blood leukocyte counts and their subclasses were analyzed at both time points.
The cataract operation was performed according to the standard phacoemulsification technique under subtenon anesthesia. Half the patients (4 of 8) received a single intravenous hydrocortisone injection (250 mg) 15 minutes before the operation. The surgery began with a temporal conjunctival incision and cauterization of bleeding episcleral vessels. The wounds were self-sealing without stitches, and the conjunctiva was apposed to the wound by cautery. All patients received 3 mg/mL topical ofloxacin (Exocin; Allergan, Irvine, CA) as 1 to 2 drops × 4 for 3 preoperative days, and 1 mg/mL dexametasone and 2 mg/mL chloramphenicol (Oftan Dexachlora; Santen, Tampere, Finland) as 1 to 2 drops × 4 for 3 postoperative weeks.
In vivo confocal microscopy
A tandem scanning confocal microscope was used (TSCM, Model 165A; Tandem Scanning, Reston, VA). The objective lens of the microscope (cone tip, 24 ×; gel contact; numerical aperture, 0.6; working distance, 0-1.5 mm, including a floating tip retraction mechanism) was adjusted to give an en faceview of the bulbar conjunctival vessels about 3 to 6 mm lateral to the limbus. The setup and operation of the confocal microscope have been described.7-9 Briefly, a 100-W mercury lamp supplied the illumination, and real-time images were captured by means of a low-light video camera (VE-1000 Sit System; Dage-MTI, Michigan City, IN). The black level, gain, and kilovoltage were automatically controlled. With this objective and camera, the field of view was 450 × 360 μm, and the optical slice thickness (z-axis resolution) was 9 μm. From the camera, the video stream was directly captured by a video card (Matrox Marvel G-400; Matrox Electronic Systems, Dorval, QC, Canada) in a second computer by Matrox PC-VCR software and saved online to the hard disk in Windows AVI (Microsoft, Seattle, WA) format. The final video analysis was performed with Adobe Premiere 5.1 (Adobe Systems, San Jose, CA), and image analysis and processing were performed with Adobe Photoshop 5.0. The in vivo confocal microscopy for analysis of leukocyte trafficking in conjunctival venules was performed twice for each patient; before surgery and 24 hours after the operation.
Leukocyte rolling and histological image parameters
Vessel diameters and the number of rolling cells were measured from all vessels. Mean centerline flow velocity was counted for 3 to 5 freely moving bright cells by measuring average movement in 4 subsequent frames. Vessels with flow above 500 μm/s were included in the analysis. The number of rolling cells was counted for each vessel with continuous flow and a sharp image from the cells passing an imaginary horizontal line in the vessel, which was fixed in one of the local landmarks in the vessel area to eliminate the effect of small movements. The number of extravasated leukocytes in the focal plane of the conjunctival stroma was counted from an area adjacent to the vessels where rolling had been analyzed.
Immunohistochemistry
The glycan epitopes on L-selectin ligands were identified by the monoclonal antibodies (mAbs) on formalin-fixed and paraffin-embedded conjunctival specimens. The mAb 2F3 (5 μg/mL)10 (Pharmingen, San Diego, CA) is anti–sialyl Lewis x (anti-sLex) mAb, and it requires presence of α2,3 sialylation and α1,3 fucosylation of the lactosamine. The mAb MECA-79 (1:100 culture supernatant, from S. Jalkanen) requires 6-sulfation of the core 1 O-glycan decoration of L-selectin ligands.11 We also used 10 μg/mL anti–vascular cellular adhesion molecule-1 (anti–VCAM-1) mAb (1.4C3; Novocastra Laboratories, Newcastle, United Kingdom). A polyclonal Ab was used against P-selectin (10 μg/mL) (Pharmingen); E-selectin (5 μg/mL) (HP9017; HyCult, Uden, The Netherlands); and intercellular adhesion molecule-1 (ICAM-1) (5 μg/mL) (HP9018; HyCult). Isotype-matched mouse and rat immunoglobulin G (IgG), IgM, and rabbit polyclonal Ab served as negative control reagents with the same concentration on the same slide (parallel section) as the specific Ab. Immunohistochemistry was performed according to the relevant ABC Elite Kit protocols (Vector Laboratories, Burlingame CA), with the use of pretreatment in citrate buffer, pH 5, 2 × 5 minutes (mAbs 2F3 and MECA-79) or at pH 3 (P- and E-selectin and ICAM-1), and incubation of primary Ab overnight at 4°C (except E-selectin and ICAM-1, which were incubated for 1 hour at room temperature [RT]). A separate protocol for the anti–VCAM-1 mAb was used according to the manufacturer's instructions (incubation of primary mAb overnight at 4°C).
The reactivity of mAbs was evaluated by J.K., who had no knowledge of the pathological diagnosis of the specimens. The number of all vessels (CD34+ mAb) from a biopsy was calculated with the use of × 400 magnification to get the total number of vessels per section. The staining was scored either positive or negative for each vessel. To eliminate inherent variations between individuals in the number of vessels analyzed, the number of specific Ab-reactive vessels was divided by the number of CD34+ vessels from the same specimen (× 100%), yielding the percentage of specific Ab-reactive vessels in one patient. The mean ± SD was calculated from these normalized percentage values. E-selectin was not expressed in specimens taken either before or during inflammation, although we were able to induce a strong and specific staining in an inflamed skin biopsy specimen treated with 100 ng/mL lipopolysaccharide (LPS) and incubated in Dulbecco modified Eagle medium (DMEM) for 4 hours in 37°C in 5% CO2 atmosphere.
Statistics
The Mann-Whitney U test was used to compare the different groups (hydrocortisone treated versus untreated) against each parameter. The Wilcoxon signed rank test was used to compare the same patient group, for example, cortisone treated, at different time points. P < .05 was considered significant.
Results
The cataract surgery in the 8 otherwise healthy individuals induced a strong local inflammatory response in every patient, as reported previously.7 To study the effects of hydrocortisone administration on leukocyte trafficking, 4 of 8 patients received a single preoperative intravenous hydrocortisone injection. The analysis was performed so that the number of vessels, the vessel diameter, analysis time, and hemodynamics were similar in these 2 groups (Table1), and thus the differences listed below were interpreted as resulting from the surgical trauma–induced inflammatory reaction, hydrocortisone treatment, or both, rather than from sampling errors.
Hydrocortisone reduced surgery-induced slow rolling of leukocytes
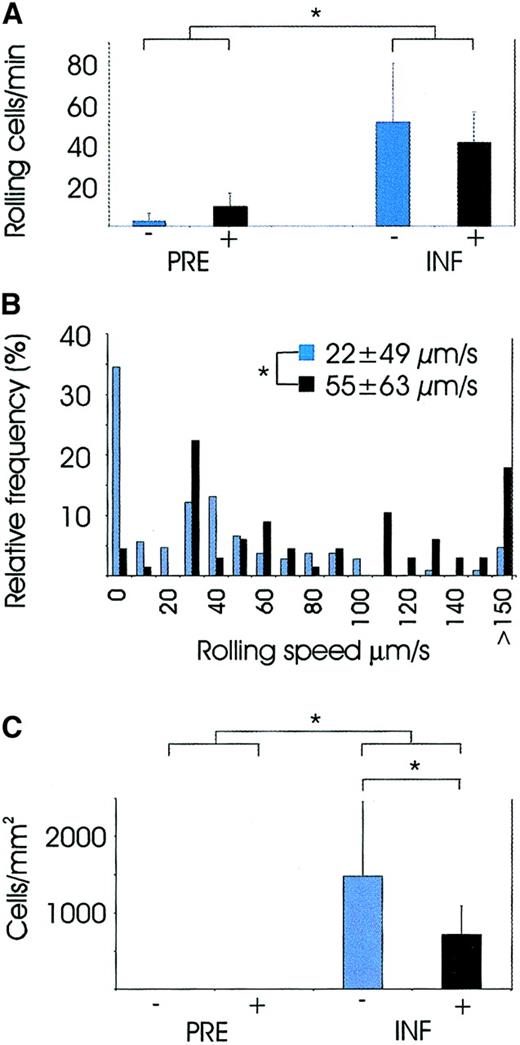
Cataract surgery induced a strong inflammatory reaction, characterized by an 8-fold increase in the number of rolling leukocytes, that is, 6.1 ± 3.4 rolling leukocytes per minute (mean ± SD) were observed in the conjunctival venules in the specimens taken before the inflammation, whereas up to 46 ± 9.9 rolling leukocytes per minute were observed in them during inflammation (P = .012, Wilcoxon signed rank test, pooled specimens with and without hydrocortisone treatment; Figures1A and 2A-B). Essentially all slowly rolling cells that could be identified in still video images by their nuclear morphology were granulocytes.
Video analysis of leukocytes at the site of inflammation.
PRE indicates analysis performed before surgery, and INF indicates analysis 24 hours after surgery when the inflammatory reaction was most vivid. Patients without hydrocortisone treatment are marked with blue bars and patients with hydrocortisone treatment with black. Pvalues between the groups were calculated with Mann-WhitneyU test. *P <.05. (A) Number of rolling cells in conjunctival venules was significantly higher in specimens taken during inflammation than in specimens taken before inflammation (groups pooled in the Figure), and hydrocortisone had no effect on this variable. (B) Relative frequencies of leukocyte rolling velocities in specimens taken during the inflammation, with or without preoperative hydrocortisone treatment. Leukocyte rolling in the nontreated patients was significantly slower than in the hydrocortisone-treated patients. (C) Number of extravasated leukocytes per square millimeter in conjunctival tissue. Specimens taken before the inflammation contained essentially no leukocytes, but the number was significantly elevated in the specimens taken during the inflammation versus those taken before the inflammation, and hydrocortisone treatment significantly reduced their numbers.
Video analysis of leukocytes at the site of inflammation.
PRE indicates analysis performed before surgery, and INF indicates analysis 24 hours after surgery when the inflammatory reaction was most vivid. Patients without hydrocortisone treatment are marked with blue bars and patients with hydrocortisone treatment with black. Pvalues between the groups were calculated with Mann-WhitneyU test. *P <.05. (A) Number of rolling cells in conjunctival venules was significantly higher in specimens taken during inflammation than in specimens taken before inflammation (groups pooled in the Figure), and hydrocortisone had no effect on this variable. (B) Relative frequencies of leukocyte rolling velocities in specimens taken during the inflammation, with or without preoperative hydrocortisone treatment. Leukocyte rolling in the nontreated patients was significantly slower than in the hydrocortisone-treated patients. (C) Number of extravasated leukocytes per square millimeter in conjunctival tissue. Specimens taken before the inflammation contained essentially no leukocytes, but the number was significantly elevated in the specimens taken during the inflammation versus those taken before the inflammation, and hydrocortisone treatment significantly reduced their numbers.
Further analysis of the distribution of velocities of individual rolling leukocytes conducted in the specimens taken during the inflammation showed that the velocity of rolling leukocytes in the conjunctival venules of the nontreated patients was 22 ± 49 μm/s (mean ± SD) (Figure 1B); in hydrocortisone-treated individuals, velocity was significantly elevated, that is, 55 ± 63 μm/s (P = .001 with Mann-Whitney U test). In the specimens taken during the inflammation of hydrocortisone-treated versus nontreated patients, the proportion of adherent or very slowly rolling cells, in particular (range, 0 to 20 μm/s), was significantly reduced (P = .007, with Mann-WhitneyU test).
Hydrocortisone treatment reduced leukocyte emigration into the tissue
The number of extravasated leukocytes was analyzed from adjacent conjunctival tissues by means of electronic video images as well as conventional histological conjunctival biopsies taken after the video microscopy from the same area where the microscopy was performed. Essentially no leukocytes were present in the conjunctival tissue in specimens taken before the inflammation with or without hydrocortisone treatment. In fact, surgery induced a significant elevation in the number of tissue-emigrated cells in nontreated patients (0 ± 0 versus 1480 ± 973; P = .005 with Wilcoxon signed rank test; Figures 1C and 2C). Intravenous preoperative hydrocortisone treatment significantly reduced the number of tissue-infiltrating leukocytes from 1480 ± 973 cells per square millimeter to 714 ± 374 cells per square millimeter (P = .013, Mann-Whitney U test; Figure 1C). All cells within conjunctival tissue that could be identified by their nuclear morphology in still video images were granulocytes. Just as in thein silico histology taken with the confocal video microscope, classical histology provided similar signs of conjunctival inflammation characterized by a large number of tissue-infiltrating leukocytes (Figure 2D).
Comparison of digital noninvasive video “biopsies” with classical invasive histological biopsy.
(A) Video image of conjunctival capillary from a specimen taken before the inflammation showing no rolling leukocytes near the inner surface of the vessel lined by endothelium and no leukocytes within the conjunctival stroma. (B) Strong tissue inflammation induced by local cataract surgery is visible just above the capillary diving into the surface pictured. Another hot spot of tissue-invading cells is visible at the upper right (white arrows). (C) Specimen taken during the inflammation with hydrocortisone pretreatment: capillary with several rolling leukocytes (white arrows) tethering to vascular endothelium. Several cells are visible within the tissue near the vessels. (D) A classical histological biopsy from the same patient as in panel B. Several tissue-infiltrating leukocytes stained with anti sLex antibodies (brown) are visible within the tissue; 3 intravascular leukocytes and red blood cells (blue) are depicted within a vessel cross-section marked with white dotted lines. A 1-minute video (Cataract version 2 video; size, 12 Mb; format, quick time movie) demonstrating the leukocyte rolling during inflammation in capillaries, extravasated cells within the tissue, as well as the effects of hydrocortisone on these parameters is available via the University of Helsinki Web site (http://www.helsinki.fi/science/rdd/renkonen/publications.html). Original magnification A-C, × 200; D, × 400.
Comparison of digital noninvasive video “biopsies” with classical invasive histological biopsy.
(A) Video image of conjunctival capillary from a specimen taken before the inflammation showing no rolling leukocytes near the inner surface of the vessel lined by endothelium and no leukocytes within the conjunctival stroma. (B) Strong tissue inflammation induced by local cataract surgery is visible just above the capillary diving into the surface pictured. Another hot spot of tissue-invading cells is visible at the upper right (white arrows). (C) Specimen taken during the inflammation with hydrocortisone pretreatment: capillary with several rolling leukocytes (white arrows) tethering to vascular endothelium. Several cells are visible within the tissue near the vessels. (D) A classical histological biopsy from the same patient as in panel B. Several tissue-infiltrating leukocytes stained with anti sLex antibodies (brown) are visible within the tissue; 3 intravascular leukocytes and red blood cells (blue) are depicted within a vessel cross-section marked with white dotted lines. A 1-minute video (Cataract version 2 video; size, 12 Mb; format, quick time movie) demonstrating the leukocyte rolling during inflammation in capillaries, extravasated cells within the tissue, as well as the effects of hydrocortisone on these parameters is available via the University of Helsinki Web site (http://www.helsinki.fi/science/rdd/renkonen/publications.html). Original magnification A-C, × 200; D, × 400.
Induced endothelial expression of P-selectin and ligands for L-selectin on vessels at sites of inflammation
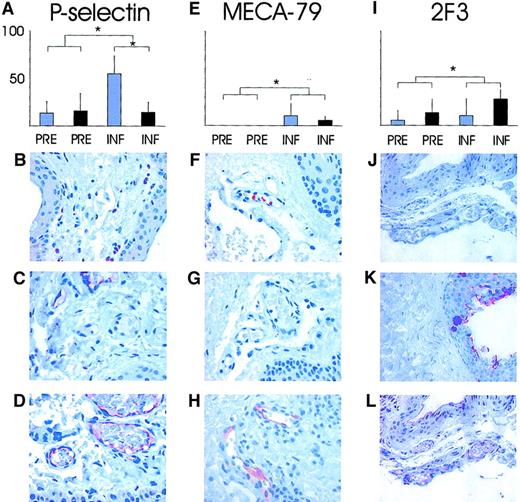
To help elucidate the molecular mechanisms of these observations, we took conjunctival biopsies for immunohistological analysis. The presence of endothelial P- and E-selectin, sulfo-sLex glycans (detected with mAbs MECA-79 and 2F3) known to decorate L-selectin ligands, as well as ICAM-1 and VCAM-1 were analyzed immunohistochemically. Of the small conjunctival venules, 15% were P-selectin–positive in biopsies taken before the inflammation with or without hydrocortisone treatment. The proportion of P-selectin–reactive venules was significantly elevated after surgery in the nontreated patients' specimens taken during compared with specimens taken before the inflammation (55% ± 19%;P < .05, Wilcoxon signed rank test; Figure3A-D). Concomitantly, in the specimens taken during the inflammation, significantly greater reduction occurred in the percentage of venules reactive with endothelial P-selectin in hydrocortisone-treated than in nontreated patients (55% ± 19% versus 15% ± 10%; P = .034, Mann-Whitney Utest; Figure 3A).
Analyses of endothelial expression of P-selectin and sulfated sLex glycans on L-selectin ligands in conjunctiva.
The percentage of conjunctival venules expressing P-selectin (A); MECA-79, reactive with sulfated extended core 1 mucin–type O-glycans (E); 2F3 detecting sLex epitopes in the specimens taken before and during the inflammation without (blue bars) and with (black bars) hydrocortisone pretreatment (I). P values between the groups were calculated with Mann-Whitney U test. *P < .05. (B) (F) (J) Isotype-matched background controls visible with essentially no reactivity. (C) (G) (K) Staining of specimens taken before the inflammation where a small proportion of vessels react with anti–P-selectin antibodies (brown in panel C), but no MECA-79 reactivity is recorded. The 2F3 stains epithelial sLex, but the endothelium lining the vessels is negative. (D) (H) (L) Staining of specimens taken during the inflammation for endothelial P-selectin, MECA-79, and 2F3 with reactive endothelium within vessels (red-brown) is visible in all panels. Original magnification, × 200.
Analyses of endothelial expression of P-selectin and sulfated sLex glycans on L-selectin ligands in conjunctiva.
The percentage of conjunctival venules expressing P-selectin (A); MECA-79, reactive with sulfated extended core 1 mucin–type O-glycans (E); 2F3 detecting sLex epitopes in the specimens taken before and during the inflammation without (blue bars) and with (black bars) hydrocortisone pretreatment (I). P values between the groups were calculated with Mann-Whitney U test. *P < .05. (B) (F) (J) Isotype-matched background controls visible with essentially no reactivity. (C) (G) (K) Staining of specimens taken before the inflammation where a small proportion of vessels react with anti–P-selectin antibodies (brown in panel C), but no MECA-79 reactivity is recorded. The 2F3 stains epithelial sLex, but the endothelium lining the vessels is negative. (D) (H) (L) Staining of specimens taken during the inflammation for endothelial P-selectin, MECA-79, and 2F3 with reactive endothelium within vessels (red-brown) is visible in all panels. Original magnification, × 200.
Another key endothelial adhesion molecule participating in the leukocyte rolling and extravasation, E-selectin, was expressed neither in specimens taken before nor during inflammation. We could induce a strong and specific E-selectin staining in a skin biopsy used as a positive control treated with LPS for 4 hours in vitro, indicating that the negative results in conjunctival biopsies were reliable (data not shown).
The sulfated glycans (extended core 1 mucin–type O-glycan)12 detected by MECA-79 were essentially absent from the endothelium of normal conjunctival venules, but were significantly and strongly elevated with inflammation in a small proportion of vessels (8%; P < .05 compared with specimens taken before the inflammation with Wilcoxon signed rank test). Similarly, the sLex-type glycans decorating L-selectin ligands on the endothelium were weakly expressed in a small proportion of normal conjunctival venules, but they were significantly induced with inflammation as detected by mAb 2F3 (9% in specimens taken before the inflammation versus 21% specimens taken during the inflammation;P = .042, Wilcoxon signed rank test). Hydrocortisone treatment, however, had no significant impact on the expression of either MECA-79 (Figure 3E-H) or sLex epitopes (Figure 3I-L). These results suggest that L-selectin ligands were induced in a subset of vessels, but that hydrocortisone pretreatment had no significant effect on them.
Analysis of the expression of ICAM-1 and VCAM-1 showed neither ICAM-1 nor VCAM-1 to be present in the vessels in conjunctival biopsies, indicating that leukocyte trafficking in a sterile surgical inflammation model might be dependent on interactions between P- and L-selectin and their ligands but is not dependent on E-selectin, ICAM-1, or VCAM-1.
Discussion
We show in this study that surgery induced a strong and rapid granulocyte-dominated inflammation, characterized by strong induction of leukocyte rolling on conjunctival venules, which we monitored in real-time noninvasive confocal microscopy. Intravenous hydrocortisone may down-regulate the number of tissue-infiltrating cells, most likely by preventing the adherence of slowly rolling cells to the inner walls of the conjunctival venules. Furthermore, immunohistological biopsies excised from the same conjunctival sites suggest that at least endothelial P-selectin and ligands for L-selectin, but not E-selectin, may participate in the rapid up-regulation of the surgery-induced leukocyte rolling and that the hydrocortisone effect of preventing the slow rolling of leukocytes could be partially mediated by down-regulation of endothelial P-selectin expression in vivo.
Corticosteroids inhibit arteriolar leukocyte rolling and their transmigration through the vascular wall in the postcapillary venules,13,14 and in rodent models E-selectin is crucial in controlling the rolling velocities of the leukocytes.15The surgery-induced inflammation in one rodent model was dependent on P- and L-selectin, but not on E-selectin expression.16Contrary to this, in a recent human study, incision of the skin under aseptic conditions did not change the levels of endothelial P-selectin, ICAM-1, or VCAM-1, but induced E-selectin expression. These findings may indicate either that different organs respond in an independent manner, that there is variation between species with regard to these responses, or that both are the case.
During the past decade, these selectin-mediated functional interactions have been studied extensively in animal models. Genetic knockout mutations of several combinations of the selectins (P-, E-, and L-selectin)17 or 1 or 2 α1,3-fucosyltransferase enzymes responsible for the biosynthesis of the fucosylated selectin ligands18,19 cause gross alterations in leukocyte traffic from blood circulation to the tissue sites. Although selectins are evolutionally conserved, there are major differences between mouse and human in the repertoire of genes responsible for enzymes that in turn participate in the generation of ligands for selectins.20We thus believe that the only way to sort out the mechanisms of glycan-dependent leukocyte trafficking into sites of inflammation in human patients is to perform studies in human subjects. Along this line, we have already initiated a characterization of the selectin-recognizing glycoforms of endothelial CD34 in human patients21 and developed an in vivo technique to quantify leukocyte traffic into inflammatory lesions in human patients.7 The present surgical inflammation differs from that previous one, in which no biopsies were taken from the wound area; thus, a more vigorous inflammation was induced in the present study. These differences may explain the higher number of adherent or very slowly rolling cells in the control group of this study than in the previous one.7
Our data obtained from immunohistological analysis of the conjunctival biopsies suggest that induction of endothelial P-selectin and ligands for L-selectin, but not E-selectin, ICAM-1, or VCAM-1, might be involved in the surgery-induced inflammation, which caused a rapid increase in rolling leukocytes, and especially in the large proportion of adherent and very slowly rolling leukocytes at sites of inflammation in our surgical patients without corticosteroids. Furthermore, the hydrocortisone treatment prevented the slow rolling and was shown to take place concomitantly with the down-regulation of endothelial P-selectin expression. Taken together, these results suggests that current anti-inflammatory drugs may also have a potential influence on leukocyte trafficking in vivo. Finally, we conclude that direct noninvasive monitoring of inflammatory reactions in human patients can be carried out in medically relevant inflammatory models.
We thank Eeva Linnolahti, Esko Järvinen, Veli-Pekka Suomalainen, and Heikki Saaren-Seppälä for providing histological biopsies and for smooth collaboration, and Minna Vesaluoma for aid with statistics.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-04-1017.
Supported by grants from the Academy of Finland (R.R.), Technology Development Center of Finland (R.R.), and the Sigrid Juselius Foundation (R.R. and T.M.T.T.); the Rector's grant, Helsinki University (T.M.T.T.); research funds from the Helsinki University Central Hospital (T.M.T.T., R.R., and T.P.); and the Eye Foundation (M.H. and J.A.O.M.).
M.H. and J.A.O.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Risto Renkonen, Rational Drug Design Program, Biomedicum and Haartman Institute, PO Box 63, FIN-00014 University of Helsinki, Finland; e-mail:risto.renkonen@helsinki.fi.