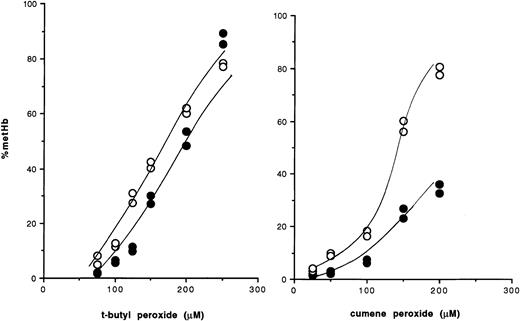
Red cells from mice with a disrupted glutathione peroxidase-1 (GSHPx-1) gene have no GSHPx activity, since GSHPx-1 is the only isoform of GSHPx found in the erythrocyte. In a recent article inBlood,1 we reported that these enzyme-deficient red cells are not oxidized by exogenous hydrogen peroxide any faster than wild-type cells. This strongly supports the view that catalase is the preeminent enzyme protecting red cells from attack by exogenous hydrogen peroxide. However, this conclusion also raises a question about the role of GSHPx in the red cell. In this regard, we noted that while catalase is completely specific for H2O2, GSHPx is able to reduce organic peroxides as well, suggesting that the distinctive role of GSHPx might be to detoxify organic peroxides. To test this, wild-type and GSHPx-deficient red cells2 were exposed to a range of compounds known to hemolyze red cells (cumene peroxide, methylene blue, chloramphenicol, naphthalene, phenylhydrazine, t-butyl peroxide, primaquine, paraquat). Oxidation of hemoglobin (Hb) was used as an endpoint for oxidative damage. Preliminary studies also assayed K efflux, which is increased by organic peroxides.3 4 However, the alteration in K efflux was found to follow temporally the oxidation of Hb, indicating that Hb oxidation was an earlier indicator of oxidative damage. Of these compounds, the GSHPx-deficient red cells showed differential sensitivity only to organic peroxides. Figure1 shows a distinct and reproducible difference between wild-type and GSHPx-deficient cells in their sensitivity to organic peroxides.
Oxidation of hemoglobin in intact erythrocytes by organic peroxides.
● indicate wild-type red cells; ○, GSHPx-deficient red cells.
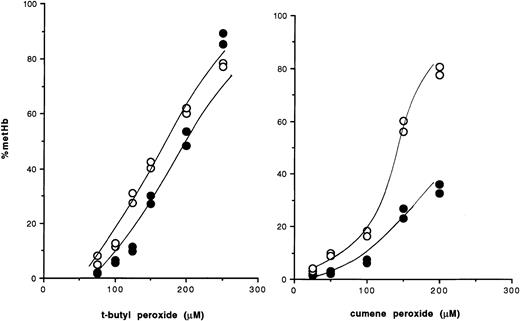
Oxidation of hemoglobin in intact erythrocytes by organic peroxides.
● indicate wild-type red cells; ○, GSHPx-deficient red cells.
What might be the evolutionary benefits of an erythrocyte mechanism for detoxifying organic peroxides? Are there circumstances when organic peroxides might arise in animal issues? Circumstantial evidence is found in the observation that all microorganisms have enzymatic activities, AhpC/F, that reduce organic peroxides.5-10Although these enzymes are peroxiredoxins and have no structural relationship to eucaryotic GSHPx, they exhibit similar catalytic capacities and are able to reduce cumene peroxide and t-butyl peroxide. These enzymes protect the bacterium against damage by organic peroxides, strengthening their functional similarity to GSHPx. Interestingly, deletion of genes for these organic peroxide reductases sometimes,9,10 but not always,8 attenuates the virulence of pathogenic strains, suggesting that organic peroxides may be part of the macrophage bactericidal response. Reactions between the H2O2 of the respiratory burst and unsaturated compounds in the cellular environment would be expected to generate toxic organic peroxides, providing a rationale for the reduced virulence phenotype of strains deficient in organic peroxidase reductase. It would be important for the host organism that its cells are also able to detoxify such organic peroxides. Thus, we suggest that protection against organic peroxides produced during phagocyte killing is a physiological role for GSHPx in red cells.
This work was supported by National Institutes of Health grant HL56421 (Y-S.H.) and the Ginopolis Fund of Children's Hospital of Michigan.