Hematopoiesis has been considered hierarchical in nature, but recent data suggest that the system is not hierarchical and is, in fact, quite functionally plastic. Existing data indicate that engraftment and progenitor phenotypes vary inversely with cell cycle transit and that gene expression also varies widely. These observations suggest that there is no progenitor/stem cell hierarchy, but rather a reversible continuum. This may, in turn, be dependent on shifting chromatin and gene expression with cell cycle transit. If the phenotype of these primitive marrow cells changes from engraftable stem cell to progenitor and back to engraftable stem cell with cycle transit, then this suggests that the identity of the engraftable stem cell may be partially masked in nonsynchronized marrow cell populations. A general model indicates a marrow cell that can continually change its surface receptor expression and thus responds to external stimuli differently at different points in the cell cycle.
Introduction
We present here a different view of early hematopoiesis. This is not the standard dogma of many investigators in the field, but is built upon solid work by many laboratories over many years. This is a perspective, and thus naturally reflects the biases of the authors. These, in turn, have a basis in many published studies from our laboratory and other laboratories and also from extensive unpublished, but abstracted, data from our laboratory. It is appropriate to consider this contribution as speculative in nature.
The term chiaroscuro refers to the treatment of light and shade in painting. Our current view of the changing phenotype of the marrow stem cell suggests a chiaroscuro nature to this picture.
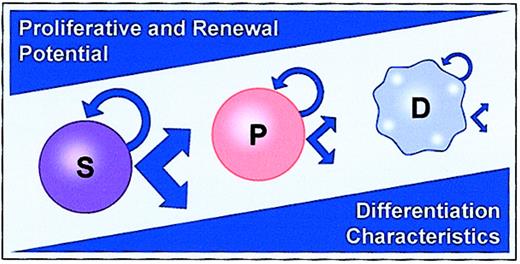
In general, models of stem cell regulation have been hierarchical.1,2 A primitive stem cell, with great potential, gives rise to a proliferating progenitor pool, which in turn gives rise to recognizable differentiated cells. During this process, proliferative potential is lost, while specific differentiated features are acquired. Presumptively, but without definite proof, there is also self-renewal at the most primitive stem cell level, and this is also lost with differentiation. Many data exist to support such a hierarchical model. Marrow cells have been separated with short- and long-term repopulation potential3,4 and progenitors have been characterized as exclusively committed to the production of restricted progeny.5,6 In addition, the clear expansion of different progenitor types in cytokine-stimulated in vitro culture with a loss of long-term engraftment capacity speaks to the existence of a progenitor hierarchy, at least at the more differentiated progenitor levels.7 8 A model encompassing these features is shown in Figure 1.
Classic hierarchical model of hematopoiesis.
S represents stem cell; P, progenitor; and D, differentiated cell.
Classic hierarchical model of hematopoiesis.
S represents stem cell; P, progenitor; and D, differentiated cell.
This model does not fit with all published data. For instance, the “daughter cell” or paired-progenitor experiments of Suda and colleagues9 indicate that a primitive progenitor cell can make totally different lineage choices during one cell cycle transit, such that, for example, one daughter cell forms an erythroid colony while the other daughter (or sister) cell forms a neutrophil-macrophage colony. Out of a total of 387 pairs evaluated, 68 pairs of colonies consisted of dissimilar combinations of cell lineages. Admittedly, these studies were carried out using spleen as a cell source with erythropoietin and an uncharacterized conditioned media as a source of stimulation. In addition, others have reported high degrees of congruence in the phenotype of individual colonies observed after replating single cells from colony starts.10 Despite these caveats, the elegant experiments of Suda and colleagues9would seem to weigh against an ordered hematopoietic stem cell hierarchy.
An intrinsic component of this hierarchical model is that the most primitive hematopoietic stem cell is a quiescent cell in G0 and a fount of potential without differentiated characteristics. It has generally been believed that primitive hematopoietic stem cells were dormant or quiescent and were thus protected from depletion or exhaustion.11,12 On the basis of a number of transplant studies, a clonal succession model has been proposed.13 This model proposes that the production of blood cells is maintained sequentially by one or just a few lymphohematopoietic stem cells, the residual stem cells remaining dormant. This model is consistent with reports evaluating the contributions of individually marked hematopoietic stem cells in transplant studies.14-16 This scenario formed the basis for the concept that hematopoietic stem cells are hierarchically ordered based on their relative quiescence.17,18 Other investigators have presented data indicating that hematopoietic stem cells are functioning concurrently, continuously cycling and contributing to blood cell production.19-21 This latter view suggests that hematopoietic stem cell quiescence is relative, the cells being relatively quiescent compared with more differentiated progenitor cells, but not dormant, often proceeding through cell cycle and undergoing cell division. Certainly, stem cells, as evaluated at any one point in time, are relatively quiescent. Our laboratory's standard stem cell separation is based on this relative quiescence, separating lineage-negative, rhodamine dull and Hoechst dull (Lin−RhodullHodull) cells,22-27 although other separations based on Sca-1 and c-kit epitopes include a small percentage of proliferating cells.28-30 In studies on Hoechst-stained side population marrow stem cells, Goodell et al31 determined that between 1% and 3% of these cells were in S/G2/M. Previous studies with cell cycle–specific killing drugs did not distinguish between cells that are truly dormant and cells that are either in prolonged cell cycle or intermittently entering and exiting cell cycle at a slow rate. In previous studies of hematopoietic cell cycling the cells analyzed were populations with functional characteristics of late progenitors.32-37 Bone marrow repopulating ability had been assessed with 7 days of bromodeoxyuridine (BrdU) labeling and a turnover time of more than 11.5 days was demonstrated.36 Labeling after 32 days of exposure to BrdU was assessed by one group.37 They showed a T½ of 21 days for colony-forming unit spleen (CFU-S) on day 14, but in this study long-term repopulating cells were not assessed.
Bradford and colleagues, studying long-term repopulating cells as represented by Lin−RhodullHodull–separated murine marrow cells and using in vivo exposure to BrdU, evaluated the proliferative history of these stem cells over longer periods of time in vivo.38 They studied mice continuously exposed to BrdU in their drinking water and then assessed the percentage of Lin−RhodullHodull marrow stem cells labeling over different time intervals of BrdU administration. BrdU is incorporated into DNA during DNA synthesis, and thus labeling with this agent is an accurate measure of whether a cell has transited S phase while progressing through the cell cycle. These researchers isolated Lin−RhodullHodull cells from these BrdU-exposed mice. They found that 60% (± 14%) of these primitive stem cells were labeled after 4 weeks and showed a time to 50% labeling (T½) of 19 days. Cheshier and colleagues, using a different mouse strain and a different stem cell separation, confirmed these data; they showed more rapid labeling of stem cells.30 We evaluated whether these data could be explained by DNA damage and repair, rather than proliferation, and after extensive studies came to the conclusion that the BrdU incorporation did, in fact, indicate proliferation of these primitive stem cells over time.39 We studied BALB/c mice and Lin−RhodullHodull cells in a fashion identical to that of Bradford et al38 and obtained virtually identical results. Thus, when the cycling status of stem cells is approached over a longer time frame, it appears that these relatively quiescent cells are either in a prolonged active cell cycle or, perhaps more likely, repeatedly entering and leaving the cell cycle. The latter possibility is supported by studies showing that transplanted stem cells rapidly enter cell cycle40,41 and that primitive marrow stem cells are easily induced into cell cycle on in vitro cytokine exposure.42 Abkowitz and colleagues,43 using stochastic modeling and computer simulation to study the replication, apoptosis, and differentiation of murine hematopoietic cells, estimated that murine hematopoietic stem cells replicated (on average) every 2.5 weeks. When this approach was used in cats, heterozygous for glucose-6-phosphate dehydrogenase, different estimates of hematopoietic stem cell replication rate (1 per 8.3 to 10 weeks) were derived. Thus this present work, along with the work of others, indicates that virtually all primitive marrow stem cells, while relatively quiescent, pass through the cell cycle over a varying period of time, depending on mouse strain and possibly the specifics of the stem cell separation.
Stem cell genes and functions
In a similar vein, while these cells do not express terminal differentiation functions, they robustly express certain stem cell functions. They are highly motile, showing very rapid directed movement and rapid membrane deformation on cytokine exposure.44These cells also express a large number of stem cell–specific genes. Phillips et al45 have reported on 2119 nonredundant gene products from fetal liver hematopoietic stem cells, using a subtracted cDNA library to generate a micro array chip. They have identified several genes specific to fetal liver hematopoietic cells. In addition, when comparing fetal hematopoietic cells with adult hematopoietic cells (Lin−Rhodull Hodullc-kit+Sca-1+), they found several genes that were coexpressed on fetal and adult stem cells as well as genes specific for either fetal or adult stem cells. Park et al46 have also reported on murine hematopoietic stem cells gene profiling. They used a 5000-cDNA array obtained by subtraction of cDNA from lineage-positive cell populations and studied both hematopoietic adult stem cells and multipotent progenitors (with minimal self-renewal capacity). Genes primarily expressed in stem cells were transcription factors, RNA binding proteins, chromatin modifiers, and protein kinases. In differential gene display studies using a gel-based method that uses the display of 3′ end fragments of cDNA generated by cutting with specific enzymes,47 we have identified a total of 637 differentially expressed genes in murine Lin−RhodullHodull cells compared with differentiated (Lin+) cells (S. Weissman et al, unpublished data, 2001). These genes include 411 unknowns and 19 different gene categories. These categories included transcription factors (22), protein synthesis regulators (11), surface proteins (11), mitochondrial sequences (10), RNA metabolism proteins (10), signaling pathway factors (9), and cytokines (8). In separate studies, we have also shown that Lin−RhodullHodull marrow cells express adhesion proteins48,49 and cytokine receptors50 on the cell surface. These data indicate a different picture of the primitive marrow stem cell than is usually envisioned—a functional cell in which a large number of stem cell–specific, as opposed to differentiated, functions are manifest (Figure 2).
Stem cell–specific functions.
This figure illustrates proteopodia membrane extensions and adherence to a mesenchymal stromal cell (M). Surface-based symbols illustrate cell surface–based receptors.
Stem cell–specific functions.
This figure illustrates proteopodia membrane extensions and adherence to a mesenchymal stromal cell (M). Surface-based symbols illustrate cell surface–based receptors.
In addition, work by others has indicated that marrow-derived stem cells may express lineage-specific genes prior to commitment.51-53 These studies indicate that primitive hematopoietic stem cells express a wide variety of genes, including a number of transcription factors. Presumptively, transcription factor access to DNA is a major determinant of such gene expression.
Cell cycle transit and chromatin modulation
Chromatin remodels during cell cycle progression. In previous studies chromatin modulation at the B-globin and lysozyme gene loci were evaluated54-57 and myeloid specificcis-regulatory elements showed a specific chromatin pattern at the lysozyme locus in myelomonocytic cells at different differentiation stages. This chromatin pattern was also found in multipotent hematopoietic progenitors, but it disappeared when cells differentiated into the erythroid lineage. Evaluation of the chromatin structure of multipotent hematopoietic cells indicated that the lineage-associated genes, globin, myeloperoxidase, immunoglobulin H (IgH), and CD3δ have accessible control regions before unilineage commitment.55-59 Other work indicates that chromatin remodeling factors may be recruited in one phase of the cell cycle for ultimate action in a later phase, which in turn results in changes in transcriptional programs. Consistent with the model of a fluctuating cell cycle–based stem cell phenotype and asymmetric division is the proposal of McConnell and Kaznowski60 that cell cycle passage could determine the fate of cells derived from stem cell division and renew stem cell multipotency. Studying laminar determination in the developing neocortex, these investigators transplanted embryonic neural progenitor cells into older host brains. The fate of the transplanted neurons correlated with the position of the progenitors in the cell cycle at the time of transplantation. Daughters of cells transplanted in S phase migrated to layer 2/3, while progenitors transplanted later in the cell cycle produced daughters that were committed to their normal deep-layer fate. These studies indicate that the cell fate of the immediate stem cell progeny is restricted by environmental signals shortly after S phase; passage through the next S phase then restores multipotency. These observations might be explained by alterations in chromatin structure during DNA replication. Such alterations could have marked effects on gene expression and therefore on cell fate.
This hypothesis is supported by studies in yeast61 62showing that chromatin-remodeling factors are recruited during M/G1 and chromatin is modeled in the next G1. Thus a reasonable sequence of events would be chromatin remodeling with cytokine-induced cell cycle transit leading to varying levels of DNA access to transcription factors, followed by alterations in patterns of gene expression.
In models of stem cell–cycle progression and division, there are several possibilities. The stem cell could have a symmetric division with the production of 2 identical stem cells, a symmetric division with the production of 2 differentiating cells, or an asymmetric division with the production of 1 stem cell and 1 differentiated cell. Most models assume that, on a population basis, stem cells would show asymmetric division; the other alternatives would lead to either hyperproliferation or stem cell exhaustion. These events might also be modulated by cellular apoptosis. The above-described stem cell phenotypic shifts with cell cycle transit and the concept of asymmetric divisions form the basis for the model shown in Figure3.
Fluctuating stem cell phenotype and genotype with cell cycle transit.
Chromatin modulation and resulting changes in access of transcription factors to different control regions. Shown is an asymmetric division in which a stem cell (S) produces a phenotypically similar stem cell and a cell (D) destined for terminal differentiation. Cylinders represent DNA chromatin coverage; boxes, active transcription factors.
Fluctuating stem cell phenotype and genotype with cell cycle transit.
Chromatin modulation and resulting changes in access of transcription factors to different control regions. Shown is an asymmetric division in which a stem cell (S) produces a phenotypically similar stem cell and a cell (D) destined for terminal differentiation. Cylinders represent DNA chromatin coverage; boxes, active transcription factors.
Changing engraftable multipotent stem cell phenotype with cell cycle transit
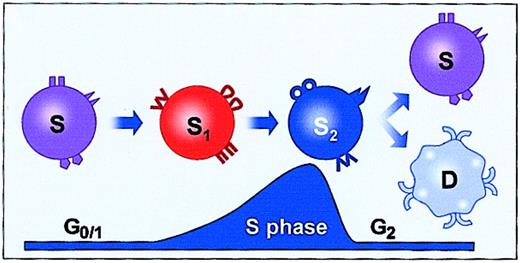
We have been investigating the phenotype of marrow stem cells as they progress through the cell cycle under cytokine stimulation. Initial studies showed that cytokine stimulation in vitro with interleukin-3 (IL-3), interleukin-11 (IL-11), interleukin-6 (IL-6), and steel factor for 48 hours resulted in a loss of engraftment capacity at 3 to 24 weeks and that longer-term engraftment was most strongly affected.63-65 Early engraftment, at 1 to 3 weeks, was either modestly augmented or unaffected. Subsequent studies evaluated the engraftment capacity of BALB/c or C57BL/6J marrow cells cultured in IL-3, IL-6, IL-11, and steel factor in nonadherent Teflon bottles every 2 to 4 hours from 24 to 80 hours of culture.66 Separate studies had determined the cell cycle status of Lin−RhodullHodull marrow cells under the same cytokine and culture conditions.42These hematopoietic stem cells isolated on the basis of quiescence progressed in a highly synchronized fashion through the cell cycle, entering S phase at about 18 hours and completing the first population doubling at about 36 to 38 hours. These studies showed dramatic and reversible fluctuations in 8-week and 6-month engraftment capacity at 2- to 4-hour intervals, with a nadir and recovery appearing in the first cell cycle transit. Cell cycle–related shifts in the functional and surface phenotype of both murine and human primitive hematopoietic stem cells have been observed by a number of investigators. Fleming and colleagues showed functional heterogeneity associated with the cell cycle status of murine stem cells.67 They found that purified lineage-negative Sca-1+ and thy-1lowcells showed decreased engraftment in S/G2/M as compared with G1. Orschell-Traycoff et al68 showed that when engrafting phenotypes of Sca-1+Lin− stem cells were Hoechst fractionated into G0 /G1 or S/G2/M, cells with long-term engraftment capacity were found in G0/G1. Gothot et al,69studying primitive human progenitor in bone marrow and peripheral blood, found multipotent progenitors in G0 or early G1, while lineage-restricted granulomonocytic progenitors were more abundant in late G1. These investigators used Hoechst 33342 and pyronin to isolate cells. The same group found that the repopulating capacity of human mobilized peripheral blood CD34+ cells in nonobese diabetic/severe combined immunodeficient mice (NOD/SCID ) was increased in G0 as opposed to G1.70 In a similar vein, Glimm and colleagues, studying human cord blood with lymphomyeloid repopulating activity in NOD/SCID mice, showed that transplantable stem cell activity was restricted to the G1fraction, even though colony forming cells (CFCs) and long-term culture initiating cells (LTC-ICs) were equally distributed between G0/G1 and S/G2/M.71G0 cells had (and were defined by) reduced K167 and cyclin D expression and low Hoechst expression. CD34+ cord blood cells stimulated with steel factor, thrombopoietin, and Flt-3 ligand showed functional differences between G0 and G1cell cycle phases, as reported by Summers et al.72 They showed a 1000-fold increase in granulocyte-macrophage colony forming cells (GM-CFCs) in G0 as compared with G1, a 250-fold increase in burst-forming unit-erythroid (BFU-e), and a 600-fold increase in CD34+ cells. Finally, in studies of murine hematopoietic stem cells, using 2 different stem cell separative approaches, long-term in vivo engraftment was shown to be better in G0 than in G1 and in G0/G1 than in S/G2/M.73 74
Studies of adhesion protein cell-surface expression on Lin−RhodullHodull and Lin−Sca-1+ hematopoietic stem cells with cell cycle transit showed fluctuations of α-4, α-5, α-6, β-1, L-selectin, and platelet-endothelial cell adhesion molecule-1 (PECAM-1) at different points in the cell cycle.48,49 A probable causal role for these fluctuations in the engraftment fluctuations was shown by homing studies in which it was found that CFDA-SE (5- (and -6)-carboxyfluorescein diacetate succinimidyl ester)–labeled Lin−Sca+ stem cells cultured in IL-3, IL-6, IL-11, and steel factor showed a marked depression in homing at a time when engraftment and α-4 were also markedly depressed (48 hours of culture).75 A schematic summarizing the results with marrow cells cultured in IL-3, IL-6, IL-11, and steel factor is shown in Figure 4. These results indicated that engraftment was reversibly lost at late S/early G2 and recovered in the next G1.
Fluctuating engraftment phenotype with cytokine-induced cell cycle transit.
Based on studies of murine marrow stem cells stimulated with IL-3, IL-6, IL-11, and steel factor.31 32 Engraftment is lost in late S early G2 and recovers in the next G1. ▾, ●, and ⋄ represent adhesion molecules.
In addition, Yamaguchi et al,76 studying CD34+ human progenitors, showed different adhesive characteristics and very-late antigen 4 (VLA-4) expression in the G0/G1 and S/G2/M phases of the cell cycle. A wide variety of other genes, including cytokine receptors, were also modulated with cycle progression.50Other gene families that were either down-regulated or up-regulated in purified murine hematopoietic stem cells after 48 hours' culture in IL-3, IL-6, IL-11, and steel factor included genes involved in energy metabolism, cell cycle regulation, signaling pathways, transcription fate, cytoskeleton, apoptosis regulation, membrane trafficking, RNA metabolism, and chromatin.77 In addition, a total of 246 unknown genes were modulated. These studies indicate that a large variety of genes are modulated with cell cycle transit, presumably underlying the shifting stem cell phenotype seen with cell cycle transit (Figure 5). All together, these studies showed a reversible fluctuating engraftment phenotype associated with cell cycle phase position, which is associated with a loss of homing and a modulation of the stem cell genotype.
Cell cycle–related fluctuations in gene expression.
The primitive marrow stem cells express a large variety of genes, and that expression changes with cell cycle transit. S represents engraftable stem cell; D, differentiated cell.
Cell cycle–related fluctuations in gene expression.
The primitive marrow stem cells express a large variety of genes, and that expression changes with cell cycle transit. S represents engraftable stem cell; D, differentiated cell.
Stem/progenitor cell inversions
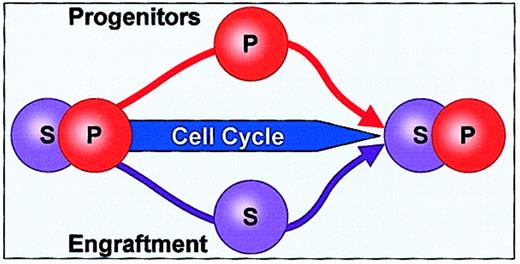
Differentiation was also assessed with cell cycle transit, this time with different cytokines and with 2 different culture systems. Unseparated BALB/c or C57BL/6J murine marrow cells were cultured in Teflon bottles or in microgravity rotating wall vessels in the presence of thrombopoietin, FLT3, and steel factor, and progenitor and engraftable stem cell levels were determined at various times in culture corresponding to different points in stem cell–cycle transit.78 These studies showed that there were marked and reversible increases in progenitor cell (high-proliferative-potential colony-forming cell [HPP-CFC] or colony-forming unit culture [CFU-C]) numbers during the first cell cycle transit. Remarkably, these increases in progenitor cells were tightly linked to decreases in engraftment capacity (8-week competitive engraftment into lethally irradiated mice), and generally, when the progenitor numbers returned to baseline there was a reciprocal change in engraftment capacity. A total of 20 marked increases in HPP-CFC or CFU-C numbers were observed, and in all but one case this increase was linked to a significant decrease in engraftable stem cells. These progenitor/stem cell shifts were significant at P < .0001. With longer times in cytokine culture, the progenitors expanded, indicating that the immediate kinetics were not those that might be seen in the setting of progenitor exhaustion. In a smaller number of observations, when the number of progenitors was decreased, the number of engraftable stem cells was elevated or at baseline. In general, in these experiments, when engraftable stem cell levels decreased and then returned to baseline, they did not show increases. This is consistent with an asymmetric-division stem cell model. We have termed these phenomena “stem cell inversions.” Others have reported similar findings. Knaan-Shanzer79 has proposed that there is “no hierarchy within the primitive hemopoietic stem cell compartment.” This investigator, studying umbilical cord blood cells, showed that primitive CD34+CD33−,38−,71−NOD/SCID repopulating cells were interconvertible with the less primitive CD34+CD33+,38+,71+cells in culture. These latter showed practically “no NOD/SCID repopulating activity.” The less primitive cells could give rise to the more primitive cells. Finally, Kirkland and Borokov80have developed a phase space model of hemopoiesis and stem cell proliferation and differentiation, which is used to describe the differentiative state of cells in 2 or more dimensions. In this model the stem cell population is viewed as a continuum, rather than being composed of discrete states.
Such a model includes the possibility that some daughter cells will have greater “stemness” than the parent cells in a renewing model. This is also consistent with our observations of stem/progenitor cell inversions. A schematic encompassing these observations is presented in Figure 6.
Stem cell/progenitor cell inversion.
As marrow stem cells progress through the cell cycle, there is an inverse correlation between progenitor and stem cells. When progenitors increase, engraftable stem cells decrease, and this is reversible.
Stem cell/progenitor cell inversion.
As marrow stem cells progress through the cell cycle, there is an inverse correlation between progenitor and stem cells. When progenitors increase, engraftable stem cells decrease, and this is reversible.
The stem cell continuum
These studies indicate that previous views of the stem cell hierarchy, at least at the more primitive stem/progenitor cell levels, may be untenable. Rather than a hierarchical transition from stem to progenitor cell, it appears that a fluctuating continuum exists, in which the phenotype of these primitive marrow cells shifts from one state to another and back. Presumptively, these phenotypic shifts are based on chromatin remodeling associated with cell cycle transit, which reversibly alters the surface phenotype of the stem cell, which then determines its response to environmental stimuli. This model would also be consistent with an asymmetric model of stem cell regulation in steady state hematopoiesis in which one daughter cell of a division returns to its base primitive (stem/progenitor) cell phenotype, while the other daughter cell commits to a differentiation pathway or an apoptotic death.
The masked stem cell hypothesis
If the phenotype of the stem cell reversibly modulates with cell cycle transit, with a predominantly progenitor phenotype at one point in the cycle and a predominantly engraftable multipotent stem cell phenotype at another point in the cycle, then estimates of long-term engraftable stem cell incidence in a cell population are probably underestimates. In essence, the identity of the stem cell would be masked at certain points in the cycle, and in a nonsynchronized population of cells, at any one point in time, a number of true stem cells would not be detectable. This could be termed the “masked” or “stealth” stem cell concept.
A unified stem cell theory
The present model builds upon a large base of previously accomplished work.81 It suggests that cell cycle transit, a fundamental characteristic of primitive hematopoietic stem cells, is associated with a continually changing stem cell phenotype and that, at least at the more primitive stem/progenitor cell levels, a fluctuating continuum, rather than a stem cell/progenitor hierarchy, may exist. Thus, outcomes would be determined by the changing cell cycle surface phenotype of the stem cell, that is, its receptor expression, and the delivered environmental stimuli, that is, fluid-phase or membrane-based cytokines, adhesion protein, or other ligands.
This provides, ultimately, a very flexible system for hematopoietic regulation, in which multiple different outcomes could occur sequentially, dependent on cell cycle phase and specific microenvironment. One can speculate that such flexibility might also hold for the recently described transdifferentiation of marrow stem cells to nonhematopoietic cells in different tissues, although at present there are no data addressing this point. This theory is presented in model form in Figure 7.
The chiaroscuro stem cell model.
As early marrow stem cells move through cell cycle transit, chromatin coverage changes, resulting in activation of different transcription regions and thus different gene expression. This change underlies the reversibly shifting phenotype of stem cells. Here stem cells are shown altering their phenotype from hematopoietic engraftable stem cell (S) to hematopoietic progenitor 1 (P1) to hematopoietic progenitor 2 (P2) with different phenotypes (eg, proteopodia) and back to hematopoietic engraftable stem cell. Cylinders represent DNA chromatin coverage; boxes, active transcription factors.
The chiaroscuro stem cell model.
As early marrow stem cells move through cell cycle transit, chromatin coverage changes, resulting in activation of different transcription regions and thus different gene expression. This change underlies the reversibly shifting phenotype of stem cells. Here stem cells are shown altering their phenotype from hematopoietic engraftable stem cell (S) to hematopoietic progenitor 1 (P1) to hematopoietic progenitor 2 (P2) with different phenotypes (eg, proteopodia) and back to hematopoietic engraftable stem cell. Cylinders represent DNA chromatin coverage; boxes, active transcription factors.
We wish to thank Dr Abby Maizel for his careful critique and helpful suggestions.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-04-1246.
Supported by grants PO1 DK50222-02, PO1 HL56920-04, and RO1 DK27424-19.
References
Author notes
Peter J. Quesenberry, Center for Stem Cell Biology, Roger Williams Medical Center, 825 Chalkstone Ave, Providence, RI 02908-4735; e-mail: pquesenberry@rwmc.org.