Abstract
Hematologists are often needed to assist with the management of microangiopathic emergencies in pregnancy. A firm understanding of the diagnosis and management of preeclampsia with severe features, hemolysis elevated liver enzyme and low platelet syndrome, and disseminated intravascular coagulation, which are the most common causes of microangiopathic emergencies, is critical. However, being able to consider when other microangiopathic emergencies (acute fatty liver of pregnancy, congenital and acquired thrombotic thrombocytopenic purpura, complement mediated microangiopathy, antiphospholipid syndrome) should be considered is imperative. The hematologist and obstetric team should work together to optimize the care of common as well as rare hematologic emergencies.
Learning Objectives
Identify the epidemiology and common presentations of labor and delivery microangiopathic emergencies including DIC, HELLP, and preeclampsia
Outline the diagnosis of preeclampsia, distinguish it from HELLP syndrome, and learn the management similarities and differences between the two
Review the etiology and typical management of DIC in pregnancy
Identify when other microangiopathic emergencies should be considered
CLINICAL CASE
A 30-year-old patient, gravida 2 para 1, presents at 29 weeks with shortness of breath, epigastric pain, and headache. She has a history of immune thrombocytopenia in the setting of systemic lupus erythematosus for which she has received multiple immunosuppressive medications. She also has a history of multiple venous thromboembolisms (VTEs), for which she is currently on long-term anticoagulation with enoxaparin 1 mg/kg twice daily. She received a course of high dose prednisone early in pregnancy (developed significant psychiatric side effects) and has received intravenous immunoglobin every 3 weeks for the past 8 to 10 weeks, maintaining a platelet count of around 80 000/µL.
Introduction
Common reasons for hematology consultation in hospitals are anemia and thrombocytopenia beyond what is expected for gestational age1 and especially when identified in an acutely ill pregnant patient. The differential diagnoses include the usual causes of cytopenias that hematologists are familiar working through as well as pregnancy-specific etiologies with which consultants are less comfortable. The acuity of presentation and speed at which a working diagnosis and treatment plan need to be formulated pose a unique challenge to the clinical team. Prioritization of interventions, which include emergent delivery, requires prompt identification features that support one diagnosis over another, acknowledging that microangiopathic emergencies overlap in their pathophysiology and share many common features.
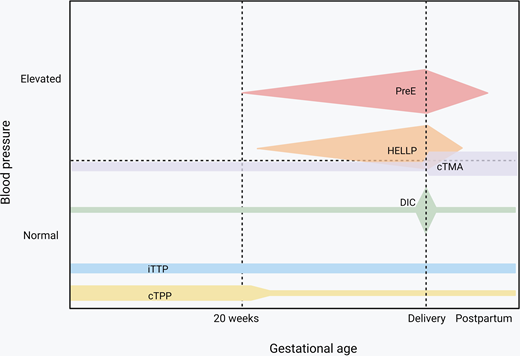
Endothelial activation and consumptive coagulopathy are the main mechanisms that drive microangiopathies resulting from pregnancy-specific conditions as well as those that occur in pregnancy but are not unique to pregnancy.2 Hypertensive disorders of pregnancy with hematologic features include preeclampsia and preeclampsia with severe features, which, by definition, occur after week 20 of gestation and can present post partum. Hemolysis elevated liver enzyme and low platelet (HELLP) syndrome involves liver endothelial dysfunction that results in consumptive coagulopathy and has some shared features in clinical presentation with acute fatty liver of pregnancy (AFLP). Disseminated intravascular coagulation (DIC) can result from systemic medical illness or trauma in the context of pregnancy but is most frequently observed secondary to placental abruption and postpartum hemorrhage. Congenital thrombotic thrombocytopenia purpura (cTTP), though rare, is most likely to be seen in pregnant patients, especially those that develop severe thrombocytopenia before 20 weeks; in contrast, acquired/immune TTP (iTTP) is the subtype of thrombotic thrombocytopenia purpura (TTP) that most hematologists are more familiar diagnosing and managing.3 Complement-mediated disorders, in particular those that predominantly affect the kidneys, are seen with increased frequency during pregnancy and especially in the postpartum period but are not exclusive to pregnancy.
Preeclampsia
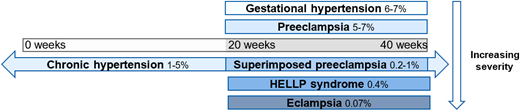
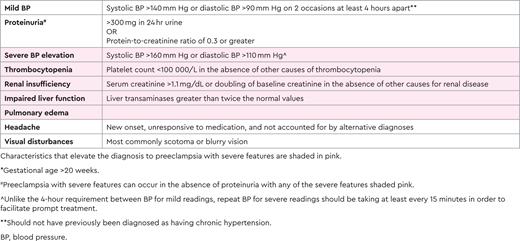
Preeclampsia impacts 2%-8% of all pregnancies4 and is part of the spectrum that includes hypertensive disorders in pregnancy, ranging from gestational hypertension to HELLP syndrome (Figure 1), and is the most common microangiopathic emergency in pregnancy.5 It is important to note that not all cases of preeclampsia include microangiopathy. Most preeclampsia cases present during the last few weeks of pregnancy, with only 10% of patients developing the condition prior to 34 weeks and 5% developing it after delivery.6,7 The diagnostic criteria of these hypertensive disorders of pregnancy, yet the nuances are important. All of these disorders occur after the first 20 weeks of pregnancy.8,9 Prior to 20 weeks of pregnancy, other causes should be pursued more seriously, as it is extremely rare that these disorders present earlier.10,11 Gestational hypertension is elevated blood pressure (>140 mm Hg systolic or >90 mm Hg diastolic) without any other symptoms, evidence of end organ damage, or laboratory criteria.8,9 It is crucial to be able to distinguish preeclampsia from preeclampsia with severe features. Preeclampsia is diagnosed with elevated blood pressure (>140 mm Hg systolic or >90 mm Hg diastolic) plus symptoms, end organ dysfunction, or laboratory derangement (Table 1).8,9 Proteinuria with elevated blood pressure represents most of the cases of preeclampsia without severe features; most of the other diagnostic criteria represent a severe feature, thus elevating the severity of the disease.8
Spectrum of hypertensive disorders during pregnancy and their prevalence. Gestational hypertension is defined by new-onset elevations in blood pressure (<140/90 mmHg) after 20 weeks of gestation, whereas preeclampsia is also accompanied by proteinuria and/or end-organ dysfunction. Chronic hypertension is present prior to 20 weeks of gestation or continues >12 weeks into the postnatal period and can occur in concert with preeclampsia. Hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome is classified as a subset of preeclampsia, and eclampsia is a complication of preeclampsia characterized by the addition of seizures.
Spectrum of hypertensive disorders during pregnancy and their prevalence. Gestational hypertension is defined by new-onset elevations in blood pressure (<140/90 mmHg) after 20 weeks of gestation, whereas preeclampsia is also accompanied by proteinuria and/or end-organ dysfunction. Chronic hypertension is present prior to 20 weeks of gestation or continues >12 weeks into the postnatal period and can occur in concert with preeclampsia. Hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome is classified as a subset of preeclampsia, and eclampsia is a complication of preeclampsia characterized by the addition of seizures.
CLINICAL CASE (continued)
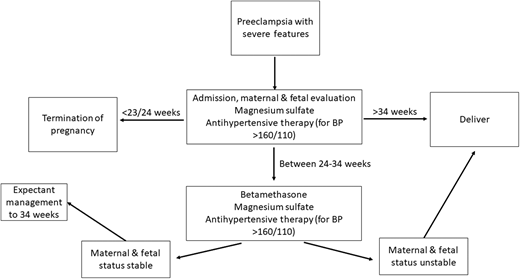
Vital signs in OB triage were blood pressure, 172/88; pulse, 61 beats per minute; saturation of peripheral oxygen (SpO2), 83%; and temperature, 98.8 degrees Fahrenheit. Labs were significant for platelets of 32 000/µL, aspartate transaminase (AST)of 1560 U/L, alanine transaminase (ALT) of 1222 U/L, and creatinine of 0.83 mg/dL. Lactate dehydrogenase was markedly elevated at 3452 U/L, and haptoglobin was normal at 39 mg/dL. There were 0-2 schistocytes per high power field on peripheral smear. Fetal heart tracing remained reassuring. Given the blood pressure on presentation and laboratory abnormalities, preeclampsia with severe features was the top diagnosis, with a question of whether she met criteria for HELLP syndrome. With her underlying hematologic disorders, the hematology team was consulted, magnesium sulfate was started, betamethasone was administered (for fetal lung maturation), and antihypertensive medications were administered.
Preeclampsia with severe features and preeclampsia without severe features are managed differently and in a gestational-age-dependent fashion. For all preeclampsia, the only resolution of the disease is birth, as the disease is thought to arise from the placenta.8,9 Therefore, at premature gestational ages, care is taken to provide supportive care and symptomatic management for the mother in order to gain greater gestational age for the fetus. This tradeoff of risks and benefits is done differently based on gestational age and disease severity.8 At term (37 weeks or greater), all patients diagnosed with preeclampsia should be promptly delivered.8,9,12 A diagnosis of preeclampsia, however, is not an indication for a cesarean delivery, which should be reserved for obstetric indications. In pregnancies with preeclampsia with severe features, patients should also be given magnesium sulfate for seizure prevention as well as antihypertensive treatment (labetalol, hydralazine, or nifedipine) to maintain blood pressure less than 160/110 mm Hg.8,9
At a preterm delivery (<37 weeks), severity of disease strongly impacts management decisions. For example, in preeclampsia with severe features and a gestational age >34 weeks, delivery is standard of care, while those with preeclampsia without severe features are monitored closely with planned delivery at 37 weeks' gestation.8,9 In those with preeclampsia with severe features, the stability of the pregnant person dictates whether or not the patient should be managed expectantly or delivered more promptly.8,9 Additionally, confirming that the diagnosis is preeclampsia with severe features and not another condition is crucial.3 As there is overlap in the clinical criteria of these syndromes and distinguishing one from another can be challenging (Table 2), maternal fetal medicine and hematology should work together to diagnose the patient.3,13 Patients with preeclampsia with severe features are particularly at risk for poor outcomes, including seizures, strokes, placental abruption, stillbirth, and death. In patients who are preterm with preeclampsia with severe features, delivery versus expectant management depends on patient stability.8,9,14 The unstable patient will be delivered immediately, the somewhat stable patient (when the patient's life or that of their fetus is not imminently at risk) will be given a 48-hour course of betamethasone (for fetal lung maturity), and the stable patient will be managed as an inpatient until 34 weeks (Figure 2).8,9 While delivery is the ultimate cure for preeclampsia, immediate resolution does not always occur. In fact, platelet nadir typically occurs at ~24 hours post partum, and returns toward normal approximately 3 days later.15 Resolution of elevated creatinine and liver function follow the same trends.15 Therefore, in patients who continue to have severe, persistent, or worsening thrombocytopenia and/or rising creatinine and have liver transaminases beyond the first 24-48 hours postpartum, alternative diagnoses should be strongly considered.3,15
Comparison of clinical features and specific management of preeclampsia with severe features (PES)/HELLP syndrome, DIC, TTP, and C-TMA
| Clinical feature . | PES/HELLP . | TTP . | DIC . | C-TMA . |
|---|---|---|---|---|
| Incidence (per 105 pregnancies) | 1000 | 1 | 130§ | Unknown. May be similar to TTP |
| Time of occurrence during pregnancy/post partum | By definition, occurs after 20 weeks; more common near term and within 3 days post partum | May occur throughout pregnancy, but most common near term and several weeks post partum | Typically at the time of delivery (independent of gestational age) but can occur in the setting of acute illness | May occur throughout pregnancy, but most common post partum |
| Blood pressure | Typically, >160/110 mm Hg, but could be >140/90 mm Hg | Normal | Normal or hypotensive | High, related to acute kidney injury |
| Neurologic abnormalities | Minor (headache, vision changes). Less common: eclamptic seizures, PRES, stroke | Severe in 30% (transient focal defects, seizure, stroke); minor in 30% | None | Inconsistent, but up to 50% of patients |
| MAHA, thrombocytopenia | Moderate | Severe | Variable | Moderate |
| Kidney injury | Mild | Mild | Mild | Severe |
| Liver function tests: ALT, AST | Markedly increased ALT, AST | Normal or slightly increased | Normal (as long as liver dysfunction is not the driver) | Normal |
| Typical course following delivery | Improvement within 24-36 hours | No improvement within 36 hours | Improvement if driven by obstetric complication | Increasing serum creatinine |
| Specific management | Delivery of infant is curative | Plasma infusion or plasma exchange, immunosuppression if acquired autoimmune TTP suspected | Transfusion support, correction of the underlying cause | Anticomplement agent |
| Clinical feature . | PES/HELLP . | TTP . | DIC . | C-TMA . |
|---|---|---|---|---|
| Incidence (per 105 pregnancies) | 1000 | 1 | 130§ | Unknown. May be similar to TTP |
| Time of occurrence during pregnancy/post partum | By definition, occurs after 20 weeks; more common near term and within 3 days post partum | May occur throughout pregnancy, but most common near term and several weeks post partum | Typically at the time of delivery (independent of gestational age) but can occur in the setting of acute illness | May occur throughout pregnancy, but most common post partum |
| Blood pressure | Typically, >160/110 mm Hg, but could be >140/90 mm Hg | Normal | Normal or hypotensive | High, related to acute kidney injury |
| Neurologic abnormalities | Minor (headache, vision changes). Less common: eclamptic seizures, PRES, stroke | Severe in 30% (transient focal defects, seizure, stroke); minor in 30% | None | Inconsistent, but up to 50% of patients |
| MAHA, thrombocytopenia | Moderate | Severe | Variable | Moderate |
| Kidney injury | Mild | Mild | Mild | Severe |
| Liver function tests: ALT, AST | Markedly increased ALT, AST | Normal or slightly increased | Normal (as long as liver dysfunction is not the driver) | Normal |
| Typical course following delivery | Improvement within 24-36 hours | No improvement within 36 hours | Improvement if driven by obstetric complication | Increasing serum creatinine |
| Specific management | Delivery of infant is curative | Plasma infusion or plasma exchange, immunosuppression if acquired autoimmune TTP suspected | Transfusion support, correction of the underlying cause | Anticomplement agent |
The incidence of preeclampsia with severe features is 1 case/100 pregnancies.3 The incidence of TTP associated with pregnancy is estimated from Oklahoma TTP Registry data. Five patients have had TTP associated with pregnancy during 19 years, 1996-2014. Centers for Disease Control in the US state that in 2013, there were 12 births/1000 population36; the Oklahoma TTP Registry region has a population of approximately 2 × 106. Therefore, the Oklahoma TTP Registry region would have approximately 24 000 births/year, 456 000 births/19 years. Five patients with pregnancy-associated TTP/456,000 births is approximately 1 patient/105 pregnancies.
Overall prevalence is low, but risk is highest in patients with placental abruption and amniotic fluid embolism.
MAHA, microangiopathic hemolytic anemia; PES, preeclampsia with severe feature; PRES, posterior reversible encephalopathy syndrome.
CLINICAL CASE (continued)
After being diagnosed with HELLP syndrome and considering her systemic compromise and overall clinical decline, the patient urgently underwent a cesarean delivery. On the first postpartum day, her lab values were as follows: platelet count, 19 000/µL; AST, 2536 U/L; ALT, 1446 U/L; and creatinine, 1.79 mg/dL. By the second day post partum, platelets and creatinine continued to worsen and were 20 000/µL, and 4.77 mg/dL, respectively, while her liver enzymes began improving with AST of 451 U/L and ALT of 531 U/L. Maternal fetal medicine and hematology consulted the nephrology service to evaluate her for complement mediated thrombotic microangiopathy (C-TMA). A heparin drip was used instead of enoxaparin because of her compromised renal function, while dialysis was considered when her creatinine peaked at 7.17 mg/dL on postpartum day 4. Ultimately, her hemolysis parameters normalized, and her renal function began recovering. By postpartum day 9, her creatinine had dropped to <2.0 mg/dL and was thought to be part of preeclampsia/HELLP and acute kidney injury.
HELLP syndrome
HELLP syndrome occurs in 0.5%-0.9% of pregnancies but in 10%-20% of those with preeclampsia with severe features.13,16 Most often, HELLP presents prior to delivery, but in up to 30% of cases, it can present post partum, typically within the first 48 hours of delivery.16,17 The hallmark of HELLP syndrome is microangiopathic hemolytic anemia, so laboratory values are most useful in confirming this diagnosis.3,15 HELLP is considered to occur on the spectrum of hypertensive disorders of pregnancy, but can also be considered a variant of preeclampsia, as up to 15% present without hypertension or proteinuria.18 It is critical to determine whether the patient has an obstetric disease, HELLP, or an alternative diagnosis, such as TTP or C-TMA (Table 2). Because C-TMA is rare, collaboration between the hematology and maternal fetal medicine departments is paramount.13 Determining the etiology is especially important in pregnant patients, as HELLP syndrome requires delivery, whereas nonobstetric etiologies may benefit from treatment of the underlying problem rather than delivering. In general, HELLP syndrome is managed similarly to preeclampsia with severe features (Figure 2) but often requires further supportive care, transfusion of blood products, and intensive-care-unit admission, as these patients can be critically ill.8,18 In the postpartum period, HELLP should typically improve within 24-48 hours and rapidly resolve.3,15 In cases where the patient fails to improve, alternative diagnoses must be considered. Work-up should include TTP or C-TMAs.
DIC
Disseminated intravascular coagulation in pregnancy is uncommon, occurring in less than 1% of pregnancies, yet represents a leading cause of maternal morbidity and mortality worldwide.19,20 The cause of DIC can be obstetric or nonobstetric; however, obstetric causes of DIC are the primary focus of this discussion. Obstetric causes of DIC include massive obstetric hemorrhage (typically from atony or laceration), HELLP, preeclampsia, spontaneous or missed abortion, intrauterine fetal demise, placental abruption, sepsis, AFLP, and amniotic fluid embolism.19,20 It is important to note that coagulation parameters change throughout pregnancy, and normal laboratory values during pregnancy are not the same as those outside of pregnancy, or even comparable across all trimesters. Therefore, when evaluating coagulation parameters in a pregnant patient, it is important to reference trimester-specific values (Table 3).20 Disseminated intravascular coagulation occurs because of a disrupted balance in normal hemostasis and regulatory mechanisms. These pathways are tightly regulated, and a tip toward or away from normal can result uncontrolled thrombin and fibrinogen-fibrin split product generation, and, consequently, endothelial activation and coagulopathy resulting in DIC.19,20 Scoring systems for DIC that take into account the expected changes in laboratory parameters, such as the pregnancy-modified International Society of Thrombosis and Hemostasis DIC score, have been developed.19 Although these scoring systems have not been validated in obstetrics, they can be used to guide management.19 The diagnosis is typically made based on a combination of clinical and laboratory parameters, with platelet count, prothrombin time and/or partial thromboplastin time, fibrinogen, and fibrin-split products being most commonly used.20
Coagulation parameters in the nonpregnant and pregnant states stratified by first, second, and third trimesters
| Coagulation parameters . | Nonpregnant adult . | 1st Trimester . | 2nd Trimester . | 3rd Trimester . |
|---|---|---|---|---|
| D-dimer (micrograms/mL) factor (%) | 0.22-0.74 | 0.05-0.95 | 0.32-1.29 | 0.13-1.7 |
| Factor V | 50-150 | 75-85 | 72-96 | 60-88 |
| Factor VII | 50-150 | 100-146 | 95-153 | 149-211 |
| Factor VIII | 50-150 | 90-210 | 97-312 | 143-353 |
| Factor IX | 50-150 | 103-172 | 154-217 | 164-235 |
| Factor XI | 50-150 | 80-127 | 82-144 | 65-123 |
| Factor XII | 50-150 | 78-124 | 90-151 | 129-194 |
| Fibrinogen (mg/dL) | 233-496 | 244-510 | 291-538 | 373-619 |
| INR | 0.9-1.04 | 0.89-1.05 | 0.85-0.97 | 0.80-0.94 |
| PTT, activated (s) | 26.3-39.4 | 24.3-38.9 | 24.2-38.1 | 24.7-35.0 |
| Protein C, functional (%) | 70-130 | 78-121 | 83-133 | 67-135 |
| Protein S, functional activity (%) | 65-140 | 57-95 | 42-68 | 16-42 |
| tPA (ng/mL) | 1.6-13 | 1.8-6.0 | 2.4-6.6 | 3.3-9.2 |
| tPA inhibitor-1 (ng/mL) | 4-43 | 16-33 | 36-55 | 67-92 |
| Coagulation parameters . | Nonpregnant adult . | 1st Trimester . | 2nd Trimester . | 3rd Trimester . |
|---|---|---|---|---|
| D-dimer (micrograms/mL) factor (%) | 0.22-0.74 | 0.05-0.95 | 0.32-1.29 | 0.13-1.7 |
| Factor V | 50-150 | 75-85 | 72-96 | 60-88 |
| Factor VII | 50-150 | 100-146 | 95-153 | 149-211 |
| Factor VIII | 50-150 | 90-210 | 97-312 | 143-353 |
| Factor IX | 50-150 | 103-172 | 154-217 | 164-235 |
| Factor XI | 50-150 | 80-127 | 82-144 | 65-123 |
| Factor XII | 50-150 | 78-124 | 90-151 | 129-194 |
| Fibrinogen (mg/dL) | 233-496 | 244-510 | 291-538 | 373-619 |
| INR | 0.9-1.04 | 0.89-1.05 | 0.85-0.97 | 0.80-0.94 |
| PTT, activated (s) | 26.3-39.4 | 24.3-38.9 | 24.2-38.1 | 24.7-35.0 |
| Protein C, functional (%) | 70-130 | 78-121 | 83-133 | 67-135 |
| Protein S, functional activity (%) | 65-140 | 57-95 | 42-68 | 16-42 |
| tPA (ng/mL) | 1.6-13 | 1.8-6.0 | 2.4-6.6 | 3.3-9.2 |
| tPA inhibitor-1 (ng/mL) | 4-43 | 16-33 | 36-55 | 67-92 |
Reproduced with permission from Cunningham and Nelson, Disseminated intravascular coagulation syndromes in obstetrics, Obstet Gynecol. 2015;126(5):999-1011.20 Copyright © 2015.
INR, international normalized ratio; PTT, partial thromboplastin time; tPA, tissue plasminogen activator.
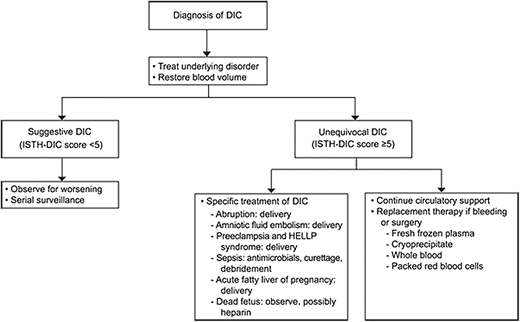
Successful management of DIC is grounded on identification and treatment of the underlying cause concurrent with product replacement and circulatory support (Figure 3).20 Importantly, delivery and pregnancy termination/abortion must be carefully considered in these situations, as evacuating the uterus often saves the pregnant patient's life. Early initiation of the massive transfusion protocol and use of tranexamic acid in cases of obstetric hemorrhage have significantly improved maternal morbidity and mortality and are considered standard of care in the U.S.19,20 The on-call hematologist may be called to help identify or confirm the underlying coagulopathy and may be asked for assistance in balancing ongoing risks of bleeding and thrombosis.
Treatment algorithm for clinical management of DIC in obstetric syndromes. ISTH, International Society of Thrombosis and Hemostasis. Reproduced with permission from Cunningham and Nelson, Disseminated intravascular coagulation syndromes in obstetrics, Obstet Gynecol. 2015;126(5):999-1011.20 Copyright © 2015.
Treatment algorithm for clinical management of DIC in obstetric syndromes. ISTH, International Society of Thrombosis and Hemostasis. Reproduced with permission from Cunningham and Nelson, Disseminated intravascular coagulation syndromes in obstetrics, Obstet Gynecol. 2015;126(5):999-1011.20 Copyright © 2015.
Other microangiopathic emergencies
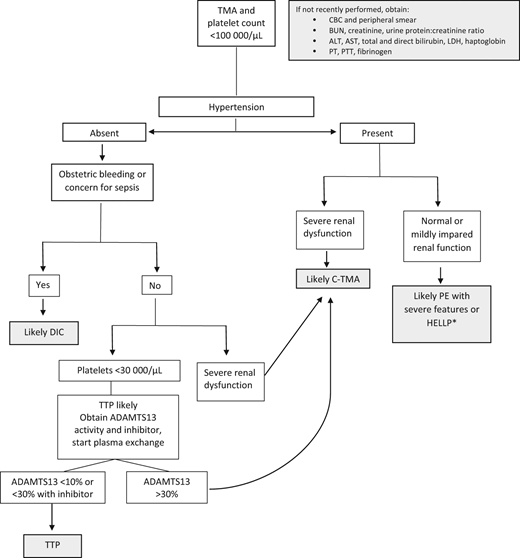
Though less common, the following conditions are nonetheless important because they are strongly associated with morbidity and mortality: AFLP, TTP (both inherited and acquired), and C-TMAs, including atypical hemolytic uremic syndrome and catastrophic antiphospholipid antibody syndrome. Figure 4 includes an algorithm for the management of microangiopathic emergencies in pregnancy.
Algorithm for clinical evaluation of microangiopathy in pregnancy. ADAMTS13; BUN, blood urea nitrogen; CBC, complete blood count; C-TMA, complement-mediated thrombotic microangiopathy; LDH, lactate dehydrogenase; PE, preeclampsia; PT, prothrombin time; PTT, activated partial thromboplastin time; TMA, thrombotic microangiopathy. *Occasionally severe renal failure occurs with HELLP, but the recovery is typically more rapid than in C-TMA.
Algorithm for clinical evaluation of microangiopathy in pregnancy. ADAMTS13; BUN, blood urea nitrogen; CBC, complete blood count; C-TMA, complement-mediated thrombotic microangiopathy; LDH, lactate dehydrogenase; PE, preeclampsia; PT, prothrombin time; PTT, activated partial thromboplastin time; TMA, thrombotic microangiopathy. *Occasionally severe renal failure occurs with HELLP, but the recovery is typically more rapid than in C-TMA.
From the hematology perspective, identifying TTP is a priority because treatment must be initiated early to prevent fatality. A markedly decreased ADAMTS13 (under 10% or under 30% with presence of an inhibitor) is specific and diagnostic of TTP; however, this laboratory assay is not widely available and is mostly performed by large academic and reference laboratories.21 The absence of hypertension and presence of severe thrombocytopenia prior to 20 weeks is helpful in distinguishing TTP from preeclampsia and HELLP.3 Treatment is typically initiated and directed by the hematology and transfusion medicine teams, with plasma infusion initiated for cTTP or plasma exchange for iTTP.22 Delivery of the infant is not expected to lead to improvement, and hence TPP should be considered in microangiopathies that persist in the postpartum period.
Identification of catastrophic antiphospholipid antibody syndrome is important in patients with known antiphospholipid antibody syndrome, those with underlying connective tissue disorders, and patients whose presentation includes VTE; unlike arterial events, VTE is not part of the classical presentation of other microangiopathies. Immunosuppression and plasmapheresis23 in addition to delivery should be considered.
Conclusion
While obstetric microangiopathic emergencies may not be at the forefront of a hematologist's mind, the close collaboration of hematology with the obstetric team in these cases is crucial for optimal patient care. Management of these serious conditions are tremendously complicated, with both a maternal and fetal life often at risk. Starting with a broad differential diagnosis, and using clues provided by clinical features (gestational age, blood pressure, clinical presentation) and laboratory studies (severity of platelet count drop, peripheral smear findings, screening coagulation studies, creatine, liver enzymes), will help clinicians formulate a treatment plan. Decision-making will be a dynamic process, with frequent reevaluation of clinical progress and changes in laboratory parameters. By working together to provide optimal care, hematology and obstetric teams will improve patient outcomes.
Acknowledgment
Jennifer Jury McIntosh receives funding through the National Heart, Lung, and Blood Institute (HL150340-01).
Conflict-of-interest disclosure
Juliana Perez Botero: no competing financial interests to declare.
Jennifer Jury McIntosh: no competing financial interests to declare.
Off-label drug use
Juliana Perez Botero: nothing to disclose.
Jennifer Jury McIntosh: nothing to disclose.