Abstract
A 3-year-old child with chronic granulomatous disease was brought to the transplant clinic by his parents. The patient has a history of Aspergillus fumigatus pneumonia, which required mechanical ventilation, and sepsis, resulting in several intensive care stays. He has failure to thrive and developmental delay. His parents are seeking guidance whether allogeneic hematopoietic cell transplantation (HCT) is a reasonable treatment option given concerns about his upfront major health limitations. Based on the original HCT-Comorbidity Index (CI), this child's risk for nonrelapse mortality (NRM) would be negligible with a score of 0. With use of the validated youth-nonmalignant HCT-CI, the score increases to 5, due to prior mechanical ventilation (+3), history of fungal infection (+1), and being underweight (+1), with at least 2-fold increase in risk of NRM. The role of developmental delay is unclear and not currently validated to prognosticate survival. While HCT was ultimately recommended in this case, the family was counseled to have a more realistic sense of NRM risk.
Learning Objectives
Comorbidity assessment via the youth-HCT-CI is key in understanding risks of mortality before HCT
Other factors, such as neuropsychiatric, socioeconomic, and metabolic risks, should be taken into account when counseling youth HCT candidates
CLINICAL CASE
A 3-year-old child with chronic granulomatous disease was brought to the transplant clinic by his parents. The patient has a history of Aspergillus fumigatus pneumonia, which required mechanical ventilation, and sepsis, resulting in several intensive care stays. He has failure to thrive and developmental delay. His parents are seeking guidance whether allogeneic hematopoietic cell transplantation (HCT) is a reasonable treatment option given concerns about his upfront major health limitations.
Introduction
Despite improvements in hct procedures and supportive care, nonrelapse mortality (NRM) remains a major contributor to death after allogeneic HCT, accounting for >60% of early deaths in those ≤18 years of age.1 To assist in determining an individual patient's risk for NRM, the Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI) was created to capture incremental organ impairments and was validated to predict overall survival (OS) in both malignant2 and nonmalignant3 diseases. While its discriminative appeal has been proclaimed for its value in helping transplant physicians counsel recipients of all ages on transplant risks, the HCT-CI score has been rarely used by pediatric transplant physicians due to concern of its applicability to this age group.4 Children have been found to have fewer comorbidities per the HCT-CI compared to adults, resulting in relatively lower scores (0-1),5 despite the fact that they continue to experience substantial NRM.1 This suggests that either the definitions used to assess HCT-CI do not comprehensively apply to children or that there are additional risk factors that are missing for this age group.6 This perspective also applies to adolescents and young adults (AYA)7 ; although they are older and therefore more likely to have testable organ dysfunction compared to infants and children, AYA also tend to have other nondisease related concerns, such as environmental and social dysfunction, that can affect OS after HCT. “Sentiment” tends to be more emotional. Finally, nonmalignant diseases represent a large percentage of indications for allogeneic HCT in children and AYA, but many of these diseases are individually unique and are predisposed to developing distinct organ toxicities that may not be fully reflected in the HCT-CI. It is also possible that these disease-defining comorbidities may be treated or cured by HCT, therefore becoming less problematic for predicting OS. Here, we discuss 1) updated applications of the HCT-CI in children and AYA populations (collectively named youth) and 2) other ways to improve pretransplant risk assessment in youth.
Updating the HCT-CI to be more inclusive of youth risk factors
Strategy 1: Expanding the HCT-CI definitions
One method for improving applicability of HCT-CI for youth is to expand definitions and include new items that are prevalent in this age group.8,9 For example, lack of pulmonary function testing in young children limited proper evaluation of their pulmonary comorbidities. To overcome this limitation, the definition was expanded to include history of mechanical ventilation. History of previously treated fungal infection was counted toward expanding infection comorbidity. Definitions for renal comorbidities were expanded to include the estimated glomerular filtration rate (eGFR) using either the Bedside Schwartz equation for children or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for young adults. Finally, failure to thrive or being underweight was added to the index to capture nutritional deficiencies more comprehensively. These additions were first tested in training sets and then validated in independent validation sets with discriminatory capacity confirmed by C-statistic (Table 1).
Comorbidity list with corresponding weight distributions compared between HCT-CI and youth HCT-CI
| Comorbidity . | Definition . | HCT-CI . | Expanded youth HCT-CI . | Simplified youth HCT-CI . | |||
|---|---|---|---|---|---|---|---|
| Malignant . | Nonmalignant . | Malignant . | Nonmalignant . | ||||
| Arrhythmia | Atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias | 1 | 1 | 1 | 0 | 0 | |
| Cardiac disease | Coronary artery disease, congestive heart failure, myocardial infarction, or EF ≤50% on most recent test | 1 | 1 | 1 | 1 | 1 | |
| Heart valve disease | Except asymptomatic mitral valve prolapse | 3 | 3 | 3 | 3 | 3 | |
| Inflammatory bowel disease | Crohn disease or ulcerative colitis | 1 | 1 | 1 | 0 | 1 | |
| Diabetes | Requiring treatment with insulin or oral hypoglycemics, but not diet alone | 1 | 1 | 1 | 1 | 1 | |
| Psychiatric disease | Requiring psychiatric consult or treatment in the last 4 weeks | 1 | 1 | 1 | 0 | 0 | |
| Cerebrovascular | Any history of transient ischemic attack, subarachnoid hemorrhage, or cerebrovascular accident | 1 | 1 | 1 | 0 | 1 | |
| Infection* | Requiring antimicrobial treatment for serious infection that continues through conditioning | 1 | 1 | 1 | 1 | 1 | |
| Or history of invasive fungal disease (proven, suspected, and/or documented) | N/A | ||||||
| Obesity* | >35 kg/m2 | 1 | 1 | 1 | 1 | 0 | |
| BMI >95th percentile by CDC guidelines for (≤18 years old) | N/A | ||||||
| Underweight* | Underweight: BMI <5th percentile by CDC guidelines for (≤18 years old) or <18 kg/m2 (>18 years old) | N/A | 0 | 1 | 0 | 1 | |
| Mild hepatic disease | Chronic hepatitis, bilirubin >upper limit of normal to 1.5 × upper limit of normal, or AST/ALT upper limit of normal to 2.5 × upper limit of normal | 1 | 1 | 1 | 0 | 0 | |
| Moderate/severe hepatic disease | Liver cirrhosis, bilirubin >1.5 × upper limit of normal, or AST/ALT >2.5 × upper limit of normal | 3 | 3 | 3 | 3 | 3 | |
| Mild renal disease* | Creatinine >2 mg/dL or prior renal transplant | or on dialysis | 2a | N/A | N/A | N/A | N/A |
| or eGFR 60-89 mL/min/1.73 m2 (by Bedside Schwartz calculation for <18 years old, CKD-EPI calculation for ≥18 years old) | N/A | 2 | N/A | 2 | N/A | ||
| eGFR 60-89 mL/min/1.73 m2 (by Bedside Schwartz calculation for <18 years old, CKD-EPI calculation for ≥18 years old) | N/A | N/A | 1 | N/A | 1 | ||
| Moderate/severe renal disease* | Creatinine >2 mg/dL, or prior renal transplant, or on dialysis | 2a | N/A | N/A | N/A | N/A | |
| Creatinine >2 mg/dL, on dialysis, or prior renal transplant | or eGFR <60 mL/min/1.73 m2 (by Bedside Schwartz calculation for <18 years old, CKD-EPI calculation for ≥18 years old) | N/A | N/A | 2 | N/A | 2 | |
| On dialysis | N/A | 3 | N/A | 3 | N/A | ||
| Moderate pulmonary disease | Corrected diffusion capacity of carbon monoxide and/or FEV1 66%-80%, or dyspnea on slight activity | 2 | 2 | 2 | 0 | 0 | |
| Severe pulmonary disease* | Corrected diffusion capacity of carbon monoxide and/or FEV1 ≤65%, dyspnea at rest, or requiring oxygen | 3 | 3 | 3 | 3 | 3 | |
| Or prior history of mechanical ventilation | N/A | ||||||
| Peptic ulcer disease | Confirmed by endoscopy and requiring treatment | 2 | 2 | 2 | 0 | 2 | |
| Rheumatologic disease | Systemic lupus, rheumatoid arthritis, polymyositis, mixed connective tissue disease, or polymyalgia rheumatica requiring treatment | 2 | 2 | 2 | 0 | 2 | |
| Prior solid tumor | At any point in patient's history, excluding nonmelanoma skin cancer, leukemia, lymphoma, or multiple myeloma; does not count if patient is being transplanted for indication of solid tumor | 3 | 3 | 3 | 3 | 3 | |
| Comorbidity . | Definition . | HCT-CI . | Expanded youth HCT-CI . | Simplified youth HCT-CI . | |||
|---|---|---|---|---|---|---|---|
| Malignant . | Nonmalignant . | Malignant . | Nonmalignant . | ||||
| Arrhythmia | Atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias | 1 | 1 | 1 | 0 | 0 | |
| Cardiac disease | Coronary artery disease, congestive heart failure, myocardial infarction, or EF ≤50% on most recent test | 1 | 1 | 1 | 1 | 1 | |
| Heart valve disease | Except asymptomatic mitral valve prolapse | 3 | 3 | 3 | 3 | 3 | |
| Inflammatory bowel disease | Crohn disease or ulcerative colitis | 1 | 1 | 1 | 0 | 1 | |
| Diabetes | Requiring treatment with insulin or oral hypoglycemics, but not diet alone | 1 | 1 | 1 | 1 | 1 | |
| Psychiatric disease | Requiring psychiatric consult or treatment in the last 4 weeks | 1 | 1 | 1 | 0 | 0 | |
| Cerebrovascular | Any history of transient ischemic attack, subarachnoid hemorrhage, or cerebrovascular accident | 1 | 1 | 1 | 0 | 1 | |
| Infection* | Requiring antimicrobial treatment for serious infection that continues through conditioning | 1 | 1 | 1 | 1 | 1 | |
| Or history of invasive fungal disease (proven, suspected, and/or documented) | N/A | ||||||
| Obesity* | >35 kg/m2 | 1 | 1 | 1 | 1 | 0 | |
| BMI >95th percentile by CDC guidelines for (≤18 years old) | N/A | ||||||
| Underweight* | Underweight: BMI <5th percentile by CDC guidelines for (≤18 years old) or <18 kg/m2 (>18 years old) | N/A | 0 | 1 | 0 | 1 | |
| Mild hepatic disease | Chronic hepatitis, bilirubin >upper limit of normal to 1.5 × upper limit of normal, or AST/ALT upper limit of normal to 2.5 × upper limit of normal | 1 | 1 | 1 | 0 | 0 | |
| Moderate/severe hepatic disease | Liver cirrhosis, bilirubin >1.5 × upper limit of normal, or AST/ALT >2.5 × upper limit of normal | 3 | 3 | 3 | 3 | 3 | |
| Mild renal disease* | Creatinine >2 mg/dL or prior renal transplant | or on dialysis | 2a | N/A | N/A | N/A | N/A |
| or eGFR 60-89 mL/min/1.73 m2 (by Bedside Schwartz calculation for <18 years old, CKD-EPI calculation for ≥18 years old) | N/A | 2 | N/A | 2 | N/A | ||
| eGFR 60-89 mL/min/1.73 m2 (by Bedside Schwartz calculation for <18 years old, CKD-EPI calculation for ≥18 years old) | N/A | N/A | 1 | N/A | 1 | ||
| Moderate/severe renal disease* | Creatinine >2 mg/dL, or prior renal transplant, or on dialysis | 2a | N/A | N/A | N/A | N/A | |
| Creatinine >2 mg/dL, on dialysis, or prior renal transplant | or eGFR <60 mL/min/1.73 m2 (by Bedside Schwartz calculation for <18 years old, CKD-EPI calculation for ≥18 years old) | N/A | N/A | 2 | N/A | 2 | |
| On dialysis | N/A | 3 | N/A | 3 | N/A | ||
| Moderate pulmonary disease | Corrected diffusion capacity of carbon monoxide and/or FEV1 66%-80%, or dyspnea on slight activity | 2 | 2 | 2 | 0 | 0 | |
| Severe pulmonary disease* | Corrected diffusion capacity of carbon monoxide and/or FEV1 ≤65%, dyspnea at rest, or requiring oxygen | 3 | 3 | 3 | 3 | 3 | |
| Or prior history of mechanical ventilation | N/A | ||||||
| Peptic ulcer disease | Confirmed by endoscopy and requiring treatment | 2 | 2 | 2 | 0 | 2 | |
| Rheumatologic disease | Systemic lupus, rheumatoid arthritis, polymyositis, mixed connective tissue disease, or polymyalgia rheumatica requiring treatment | 2 | 2 | 2 | 0 | 2 | |
| Prior solid tumor | At any point in patient's history, excluding nonmelanoma skin cancer, leukemia, lymphoma, or multiple myeloma; does not count if patient is being transplanted for indication of solid tumor | 3 | 3 | 3 | 3 | 3 | |
This is a summary table combining the three different HCT-CI scores that have been validated in assessing pre-HCT comorbidities. Definitions of comorbidities and weighted scores are listed. Refer to original citations for accompanying hazard ratios and confidence intervals that justify weights assigned. New definitions that apply to the pediatric/AYA population (or youth scores) are indicated with an *. Please note that youth classification is further divided into 2 groups based on malignant or nonmalignant disease status. In validation models, having an underlying malignant or nonmalignant disease made a difference in weighted scores when using the simplified youth HCT-CI to assess inflammatory bowel disease, cerebrovascular disease, obesity, being underweight, renal disease, peptic ulcer disease, and rheumatologic disease. N/A is listed for those new definitions that are part of the youth HCT-CI measures that are not part of the original HCT-CI. For renal disease, the definitions are different for malignant compared to non-malignant conditions. Adapted from Sorror et al., Blood 2005; Friend et al., TCT 2023; and Broglie et al., TCT 2023.2,8,9
In the original HCT-CI, there was only one category listed for renal disease. With the pediatric definitions, the renal category is now split into mild or moderate/severe. The renal disease HCT-CI score is currently listed in both the mild and moderate/severe categories to reflect this.
Items modified for youth population (pediatric/AYA) include revised definitions or new definitions.
ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; CDC, Centers for Disease Control and Prevention; N/A, not applicable.
Strategy 2: Simplifying the HCT-CI to remove comorbidities that are not prevalent in pediatrics
Despite being developed and validated in both adult and pediatric populations, adult patients and their risk factors were overly represented in the development of the HCT-CI, therefore creating the impression that it is a more adult-focused scale.2 To address this issue, comorbidities with hazard ratios <1.2 were eliminated, as these would indicate comorbidities that had minimal contribution to the predictive capacity of the model in the youth population.8,9 Comorbidities that were eliminated in both the malignant and nonmalignant groups were arrhythmia, psychiatric disease, mild hepatic disease, moderate pulmonary disease, and peptic ulcer disease. Additional items removed for malignant diseases included inflammatory bowel disease, cardiovascular disease, being underweight, peptic ulcer disease, and rheumatologic disease, while obesity was the only additional factor removed for nonmalignant diseases (Table 1).
The new HCT-CI for youth
Applying these two strategies, two studies were performed. Youth with nonmalignant diseases (n = 2815) who received their first allogeneic HCTs between 2008 and 2017 were included in the first study to develop the youth-nonmalignant-HCT-CI (ynHCT-CI).9 These modifications resulted in 39% of patients having an increase in their ynHCT-CI scores with an increased hazard of mortality compared to those whose score remained the same (hazard ratio, 1.41; 95% CI, 1.01-1.98). Performance of the new model was slightly better than that of the original HCT-CI (C-statistic estimates of 65.8 versus 64.3, respectively), but the main advantage was that it represented a more youth- focused scale. Likewise, 5790 youths with malignant diseases contributed to development of the youth-malignant-HCT-CI.8 The youth-maliginant-HCT-CI led to an increase in comorbidity scores for 23% of youth patients and was associated with a significant risk of NRM (HR, 1.34; 95% CI, 1.02-1.74). The 3-year survival rates were 62.9%, 53.3%, and 50.1%, respectively for scores 0, 1-2 and ≥3.
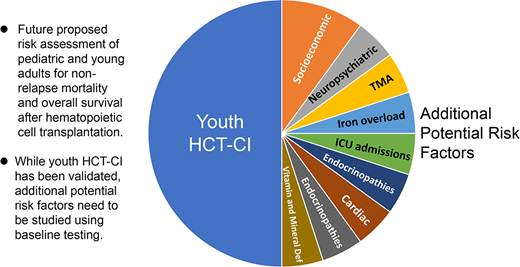
Future directions and other considerations
Further fine-tuning of the youth HCT-CI will require thoughtful attention to data collected in future prospective studies. Psychiatric comorbidities could be expanded to include certain behaviors (eg, aggression) that have that been connected to untreated depression or anxiety in children.10 Another example is better defining comorbidities linked to specific primary nonmalignant diseases. For instance, redefining iron overload by using T2* magnetic resonance imaging or including the number of intensive care hospitalizations could better capture the burden of hemoglobinopathy-associated risk factors. It is also unknown whether elevated pre-HCT baseline biomarkers, such as urine protein to creatinine ratio or soluble C5b-9 levels, could capture individuals at higher risk for life-threatening post-HCT complications such as transplant-associated microangiopathy (Table 2).11
Additional variables that could be validated as pre-HCT risk factors in pediatric patients
| Cardiac |
| Prolonged QTc |
| Endocrinopathies |
| Metabolic syndrome |
| Thyroid dysfunction |
| Low cortisol production |
| Growth delays |
| Hypertriglyceridemia |
| Low activity levels/sedentary lifestyle |
| Gastrointestinal |
| Pneumatosis |
| Pancreatitis |
| Poorly-diversified microbiome |
| Hypoalbuminemia/hypoproteinemia |
| Genetic variants (HLA and non-HLA genes) |
| Intensive care |
| Number of hospitalizations |
| Extracorporeal membrane oxygenation |
| Iron overload |
| Number of red blood cell transfusions received |
| T2*MRI |
| Neuropsychiatric concerns |
| Developmental delay |
| Isolation |
| Poor resiliency |
| Behavior concerns |
| Attention deficit hyperactivity disorders |
| Poor sleep patterns |
| Low scores on validated, age-appropriate patient-reported outcomes |
| Pain requiring scheduled opioid medications |
| Predisposition to transplant-associated microangiopathy |
| High inflammatory markers (e.g., C reactive protein) preceding conditioning |
| High baseline terminal complement system pathway factors (eg, soluble C5b-9) |
| High urine protein to creatinine ratio |
| Socioeconomic factors/access to care |
| Noncompliance with treatments |
| Homelessness |
| Poor family support system |
| Social isolation |
| Food insecurity |
| Financial toxicity/insurance concerns |
| Amount of school/work missed |
| Marginalized populations/disparities in care |
| Vitamin and mineral deficiencies |
| Vitamin D |
| Iron |
| Zinc |
| Cardiac |
| Prolonged QTc |
| Endocrinopathies |
| Metabolic syndrome |
| Thyroid dysfunction |
| Low cortisol production |
| Growth delays |
| Hypertriglyceridemia |
| Low activity levels/sedentary lifestyle |
| Gastrointestinal |
| Pneumatosis |
| Pancreatitis |
| Poorly-diversified microbiome |
| Hypoalbuminemia/hypoproteinemia |
| Genetic variants (HLA and non-HLA genes) |
| Intensive care |
| Number of hospitalizations |
| Extracorporeal membrane oxygenation |
| Iron overload |
| Number of red blood cell transfusions received |
| T2*MRI |
| Neuropsychiatric concerns |
| Developmental delay |
| Isolation |
| Poor resiliency |
| Behavior concerns |
| Attention deficit hyperactivity disorders |
| Poor sleep patterns |
| Low scores on validated, age-appropriate patient-reported outcomes |
| Pain requiring scheduled opioid medications |
| Predisposition to transplant-associated microangiopathy |
| High inflammatory markers (e.g., C reactive protein) preceding conditioning |
| High baseline terminal complement system pathway factors (eg, soluble C5b-9) |
| High urine protein to creatinine ratio |
| Socioeconomic factors/access to care |
| Noncompliance with treatments |
| Homelessness |
| Poor family support system |
| Social isolation |
| Food insecurity |
| Financial toxicity/insurance concerns |
| Amount of school/work missed |
| Marginalized populations/disparities in care |
| Vitamin and mineral deficiencies |
| Vitamin D |
| Iron |
| Zinc |
HLA, human leukocyte antigen; MRI, magnetic resonance imaging. While not exhaustive, many of these entities have been evaluated as possible contributors to poor health and/or medical complications in transplant and/or nontransplant patients and may contribute to acute and long-term complications in areas of human health that could impact NRM and OS after HCT in youths.
Aside from comorbidities, other risk factors could further enhance our understanding of HCT risks in youths. These include categories such as neuropsychiatric conditions and socioeconomic factors.12 Finally, genetic variants found in recipients and their donors could adversely impact outcomes and are an emerging area of research.13,14 A list of these potential factors is detailed in Table 2. Future prospective studies are needed to enhance risk-assessment potential for youth recipients of allogeneic HCT.
CLINICAL CASE (revisited)
Based on the original HCT-CI, this child's risk for NRM would be negligible with a score of 0. With use of the validated ynHCT-CI, the score increases to 5, due to prior mechanical ventilation (+3), history of fungal infection (+1), and being underweight (+1), with at least a 2-fold increase in risk of NRM. The role of developmental delay is unclear and not currently validated to prognosticate survival. While HCT was ultimately recommended in this case, the family was counseled to have a more realistic sense of NRM risk.
Conclusions
The HCT-CI was introduced almost two decades ago and has been reliable in providing data-driven survival predictions for transplant recipients. The newly validated youth scores can further assist transplant physicians in counseling families with children. When counseling patients about transplants, additional risk factors (Table 2) need to be considered, especially those that may be discovered in future studies and added to the current models.
Recommendations15
Based on our review of the literature, we offer the following recommendations:
The HCT-CI assessment should be performed in all pediatric patients undergoing HCT to assess risk of NRM. (Grade 1A).
Either the expanded or simplified youth HCT-CI, which provide improved definitions for certain pediatric comorbidities, should be assessed in all youth to determine risk of NRM, although real-life experience using this new scale is limited. (Grade 1B).
At this time, we suggest consideration of other pretransplant risk factors reported in the literature that could contribute to mortality after HCT. While they have not been validated to date in the transplant setting, future studies could broaden their applicability. (Grade 1C).
Acknowledgments
We would like to acknowledge the contributions of Drs Larisa Broglie and Brian Friend to this body of work. We thank Ms Helen Crawford for her assistance in manuscript preparation. M.L.S. was funded by research grant award R01 CA227092 from the National Cancer Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict-of-interest disclosure
Monica S. Thakar: on the scientific advisory boards for Proteios Technology and ImmunoVec.
Mohamed L. Sorror: reports consultancy for and receiving an honorarium from Jazz Pharmaceuticals and receiving a research grant from Massachusetts General Hospital and BlueNote.
Off-label drug use
Monica S. Thakar: Nothing to disclose.
Mohamed L. Sorror: Nothing to disclose.