Abstract
Curative therapy with an allogeneic hematopoietic cell transplant (HCT) can now be offered to a wider patient population due to improvements in donor selection, transplant conditioning regimens, and supportive care measures. However, risk of transplant-related morbidity and mortality remains, and thus appropriate transplant candidate workup pre-HCT for risk stratification and a management plan after HCT is crucial for success of the procedure. These include understanding and identifying risk of underlying malignant disease relapse, graft-versus-host disease, and infectious complications a patient may be predisposed toward, irrespective of allogeneic donor type. Progress in these domains with new therapeutic paradigms allows for development of a treatment plan prior to HCT to mitigate these potential risks tailored to the patient's case. Herein, we present case studies to focus on factors that influence decision-making in HCT and the approaches and strategies used to optimize post-HCT outcomes based on the individual HCT recipient's clinical scenario to improve on these high-risk scenarios.
Learning Objectives
Identify the most common causes of allogeneic hematopoietic cell transplant treatment failure
Illustrate treatment options available to mitigate risk based on individual hematopoietic cell transplant recipient's risk
Define novel strategies used to improve outcomes of high morbidity and mortality in hematopoietic cell transplant
Introduction
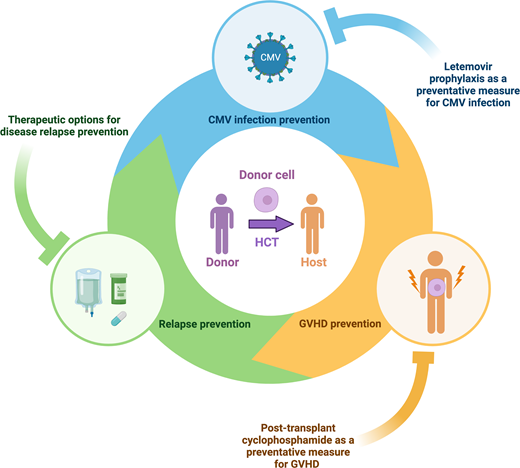
Transplantation of healthy hematopoietic stem cells from an allogeneic donor (HCT) is a treatment option to improve outcomes for otherwise incurable hematologic diseases. With the development of safer transplant conditioning regimens, supportive care measures, and enhanced donor selection methodologies, HCT can now be offered to a wider population. This is reflected by the rising number of annual HCT procedures (over 9000 performed domestically).1,2 As HCT poses risks (relapse, organ failure, treatment toxicity, graft-versus-host disease [GVHD]) (Figure 1),2 assessment entails a detailed patient evaluation to determine the most appropriate HCT treatment algorithm.3
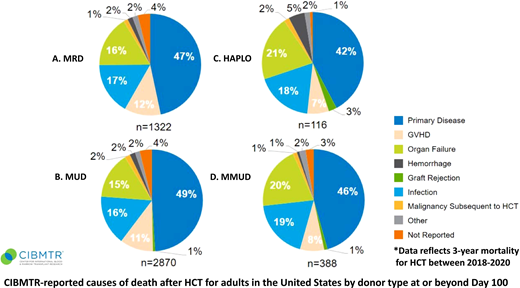
Center for International Blood and Marrow Transplant Research–reported causes of death after HCT for adults in the United States by donor type at or beyond day 100. HAPLO, haploidentical; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
Center for International Blood and Marrow Transplant Research–reported causes of death after HCT for adults in the United States by donor type at or beyond day 100. HAPLO, haploidentical; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
CLINICAL CASE 1
A 32-year-old woman with FMS-like tyrosine kinase 3–internal tandem duplication (FLT3-ITD) mutant acute myeloid leukemia (AML) is being considered for HCT. She underwent induction chemotherapy along with FLT3-ITD inhibitor followed by consolidative chemotherapy. She continues to have measurable residual disease via FLT3-ITD polymerase chain reaction–next-generation sequencing with a sensitivity of ~1 × 10−6. Her sister was identified as a fully matched sibling donor, and she proceeds to HCT using myeloablative conditioning (MAC). Day +30 bone marrow biopsy specimen confirms she is in morphologic remission and FLT3 polymerase chain reaction–next-generation sequencing is negative. She begins maintenance with gilteritinib, continues this for 2 years, and remains in remission post-HCT.
Drug-based maintenance therapies
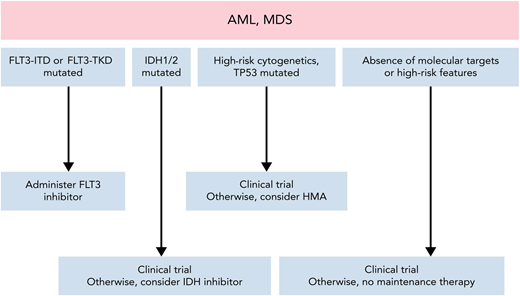
Disease relapse remains the leading cause of HCT failure,4,5 irrespective of donor type (Figure 1). Maintenance therapy may decrease the risk of relapse post-HCT, but broad application to all patients risks overtreatment.6 Thus, questions remain regarding identification of ideal patient subsets, minimal residual disease testing, duration of treatment, and drug intervention. Other considerations include (1) the underlying disease risk itself, (2) conditioning intensity, (3) remission status, (4) patient post-HCT recovery, and (5) toxicity risk of the maintenance therapy.6 Promising advances have been achieved with commercially available pharmacologic agents, with the majority in targeted maintenance therapy for AML and myelodysplastic syndrome (MDS) (Figure 2).
Approach to maintenance therapy for AML and MDS after HCT. Adapted from DeFilipp and Chen.6
Approach to maintenance therapy for AML and MDS after HCT. Adapted from DeFilipp and Chen.6
Maintenance post-HCT for AML/MDS
FLT3-ITD is common (~25% of AML), and incorporation of FLT3 inhibitors to upfront induction chemotherapy and routine use of HCT as consolidative therapy have improved outcomes.7 Despite these advances, risk of relapse post-HCT ranges from ~30% to 40%. Several prospective randomized trials evaluated efficacy of tyrosine kinase inhibitor maintenance with varying degrees of benefit in different patient populations. The SORMAIN (SORafenib Maintenance) trial enrolled 83 patients and closed early due to poor accrual after 5 years but showed a 2-year relapse-free survival (RFS) benefit with maintenance therapy given up to 2 years after HCT vs placebo (85.0% vs 53.3%, P = .002).8 In a larger phase 3 randomized placebo controlled trial (n = 202), Xuan et al9 demonstrated a similar benefit favoring a 6-month sorafenib maintenance regimen initiated 30 to 60 days after HCT with a 2-year leukemia-free survival to be 78.9% vs 56.6% over placebo (P < .0001). The RADIUS study, evaluating midostaurin, did not show an RFS benefit in the maintenance setting, although the study was underpowered to determine this.10 More recently, the highly anticipated results of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1506/MORPHO trial (NCT02997202) were presented. This study was a phase 3, randomized, double-blind, placebo- controlled multicenter, multinational, trial, randomizing 356 patients with FLT3-ITD AML to receive either gilteritinib 120 mg or placebo for a total of 2 years. While the study did not achieve its primary end point of RFS (hazard ratio [HR], 0.679; 95% CI, 0.46-1.005; 2-sided P = .052), the study did demonstrate the benefit of gilteritinib maintenance in patients with FLT3-ITD minimal residual disease pre- and post-HCT (HR, 0.2; 95% CI, 0.32-0.8; P = .0065) compared to placebo. Thus, maintenance therapy may be most beneficial for those with minimal residual disease.11
Data for other targetable mutations for myeloid neoplasms are in earlier stages of development. This includes mutations of IDH1 and IDH2. Several early phase studies have evaluated orally available small-molecule inhibitors, ivosidenib and enasidenib, IDH1 and IDH2 inhibitors, respectively, post-HCT. Fathi and colleagues12 reported a promising tolerance profile with maintenance enasidenib in a phase 1 setting with 63% of patients (n = 12) completing all 12 planned cycles of treatment with a 2-year relapse rate of 16% (95% CI, 3.7%-36%) for the cohort at a median follow-up for surviving patients of 25 months. Similarly, post-HCT maintenance ivosidenib was well tolerated when evaluated in the phase 1 setting (n = 18), with only 1 patient discontinuing maintenance due to an adverse event. Additionally, the study had a promising 2-year incidence of relapse, nonrelapse mortality (NRM), and overall survival (OS) of 19%, 0%, and 88%, respectively.13 Ongoing clinical trials will further delineate efficacy of maintenance with these agents.
As many high-risk mutations may not be targetable by current pharmacologic agents,14 other more broadly acting agents are of interest. Hypomethylating agents may be an attractive option given their tolerance and potential for increasing the graft- leukemia effect through increased tumor antigen expression and expanding immunomodulatory T regulatory cells.15 Existing data are conflicting and represent a mixture of patient populations and dosing schedules. Early-phase trials demonstrated promise with both 5-azacitidine (AZA) and decitabine.16-18 Oran and colleagues19 conducted a phase 3 randomized study of AZA maintenance (32 mg/m2/d intravenously × 5 days every 28 days for 12 cycles; median number delivered, 4) vs observation in 187 patients after HCT in high-risk AML and MDS but found that RFS and OS were not different in the 2 groups. Challenges in delivering this intravenous therapy post-HCT may be addressed by the oral formulation AZA, CC-486, which is currently being studied in a phase 3, randomized, placebo-controlled trial (Table 1; NCT04173533). Last, to potentiate the efficacy of hypomethylating agents, combination therapy with the BCL-2 inhibitor and BH3 mimetic, venetoclax (VEN), is being explored in high-risk myeloid diseases. Wei and colleagues evaluated the combination of decitabine/VEN prospectively in patients with high-risk AML (n = 17) and MDS (n = 3) based on prespecified prognostic criteria in phase 1 followed by a phase 2 study showing 2-year OS and event-free survival of 85% for both in this high-risk population.20 Additionally, phase 4 studies with AZA/VEN are also under way (NCT04161885).
Active trials for drug maintenance therapy after HCT for AML or MDS
| ClinicalTrials.gov identifier . | Phase . | Status . | Drug/schedule . | N . | Age . | Primary outcome . |
|---|---|---|---|---|---|---|
| Post-HCT FLT3-ITD maintenance | ||||||
| NCT02997202, CTN 1506 | 3 | Active, not recruiting | Gilteritinib 120 mg daily vs placebo for 2 years | 356 | >18 | 96-month RFS |
| NCT03690115, PONALLO trial | 2 | Active, not recruiting | Ponatinib 30 mg daily for 1 year | 77 | 18-70 | 24-month RFS |
| Post-HCT IDH 1/2 maintenance | ||||||
| NCT03728335 | 1 | Active, not recruiting | Enasidenib (dose unspecified) daily Q28 days for 24 cycles | 15 | >18 | Incidence of AEs |
| NCT03564821 | 1 | Active, not recruiting | Ivosidenib 500 mg daily dose escalation study | 18 | 18-75 | MTD |
| NCT04522895 | 2 | Active, not recruiting | Enasidenib 100 mg daily Q28 days for 12 cycles | 50 | >18 | Incidence of AEs |
| Post-HCT HMA-based maintenance | ||||||
| NCT01995578 | 2 | Active, not recruiting | Azacitadine 32 mg/m2 Q5 days every 28 days starting days +60-120 post-HCT up to 1 year post-HCT for ~8-10 cycles | 32 | 1-75 | 24-month relapse rate |
| NCT04173533 | 3 | Recruiting | Oral azacitidine (CC-486) 14 days for each 28-day cycle for 12 cycles | 324 | ≥16 | 12-month RFS |
| NCT03613532 | 1 | Recruiting | Azacitidine 5 doses with venetoclax 14 doses for 8-12 cycles or oral decitabine/cedazuridine 3 doses with venetoclax 14 doses for 8 cycles | 68 | ≥18 | MTD |
| NCT04128501 | 2 | Recruiting | Azacitidine days 1-5 + venetoclax days 1-7 Q4-8 weeks for up to 12 cycles. Dosing unspecified | 125 | 18-75 | RFS |
| NCT04161885, VIALE-T | 3 | Recruiting | Azacitidine days 1-5 + venetoclax days 1-28 Q28 for 6 cycles. Dosing unspecified | 424 | >18 | MTD and RFS |
| NCT03843528 | 1 | Recruiting | Azacitidine days 1-5 Q28 days. Vorinostat concurrently on days 1-7 and 15-21 of 28-day cycles up to 1 year | 15 | 1-21 | MTD |
| NCT04980404 | 1 | Recruiting | Decitabine/cedazuridine on days 1-3 Q42 days | 22 | ≥18 | DLT |
| Additional disease-based maintenance approaches | ||||||
| NCT03267186 | 2 | Active, not recruiting | Ibrutinib PO daily up to 18 months post-HCT | 8 | 18-70 | 18-month relapse rate |
| NCT03932643 | 1 | Recruiting | Onc201 at various dose levels weekly for up to 52 weeks | 20 | ≥19 | MTD |
| NCT04168502 | 3 | Recruiting | Glasdegib 100 mg daily for 1 year vs clinical observation | 414 | 18-60 | DFS |
| NCT03286530 | 2 | Recruiting | Ruxolitinib orally twice daily Q28 days for up to 24 cycles | 64 | 60-80 | 12-month GRFS rate |
| ClinicalTrials.gov identifier . | Phase . | Status . | Drug/schedule . | N . | Age . | Primary outcome . |
|---|---|---|---|---|---|---|
| Post-HCT FLT3-ITD maintenance | ||||||
| NCT02997202, CTN 1506 | 3 | Active, not recruiting | Gilteritinib 120 mg daily vs placebo for 2 years | 356 | >18 | 96-month RFS |
| NCT03690115, PONALLO trial | 2 | Active, not recruiting | Ponatinib 30 mg daily for 1 year | 77 | 18-70 | 24-month RFS |
| Post-HCT IDH 1/2 maintenance | ||||||
| NCT03728335 | 1 | Active, not recruiting | Enasidenib (dose unspecified) daily Q28 days for 24 cycles | 15 | >18 | Incidence of AEs |
| NCT03564821 | 1 | Active, not recruiting | Ivosidenib 500 mg daily dose escalation study | 18 | 18-75 | MTD |
| NCT04522895 | 2 | Active, not recruiting | Enasidenib 100 mg daily Q28 days for 12 cycles | 50 | >18 | Incidence of AEs |
| Post-HCT HMA-based maintenance | ||||||
| NCT01995578 | 2 | Active, not recruiting | Azacitadine 32 mg/m2 Q5 days every 28 days starting days +60-120 post-HCT up to 1 year post-HCT for ~8-10 cycles | 32 | 1-75 | 24-month relapse rate |
| NCT04173533 | 3 | Recruiting | Oral azacitidine (CC-486) 14 days for each 28-day cycle for 12 cycles | 324 | ≥16 | 12-month RFS |
| NCT03613532 | 1 | Recruiting | Azacitidine 5 doses with venetoclax 14 doses for 8-12 cycles or oral decitabine/cedazuridine 3 doses with venetoclax 14 doses for 8 cycles | 68 | ≥18 | MTD |
| NCT04128501 | 2 | Recruiting | Azacitidine days 1-5 + venetoclax days 1-7 Q4-8 weeks for up to 12 cycles. Dosing unspecified | 125 | 18-75 | RFS |
| NCT04161885, VIALE-T | 3 | Recruiting | Azacitidine days 1-5 + venetoclax days 1-28 Q28 for 6 cycles. Dosing unspecified | 424 | >18 | MTD and RFS |
| NCT03843528 | 1 | Recruiting | Azacitidine days 1-5 Q28 days. Vorinostat concurrently on days 1-7 and 15-21 of 28-day cycles up to 1 year | 15 | 1-21 | MTD |
| NCT04980404 | 1 | Recruiting | Decitabine/cedazuridine on days 1-3 Q42 days | 22 | ≥18 | DLT |
| Additional disease-based maintenance approaches | ||||||
| NCT03267186 | 2 | Active, not recruiting | Ibrutinib PO daily up to 18 months post-HCT | 8 | 18-70 | 18-month relapse rate |
| NCT03932643 | 1 | Recruiting | Onc201 at various dose levels weekly for up to 52 weeks | 20 | ≥19 | MTD |
| NCT04168502 | 3 | Recruiting | Glasdegib 100 mg daily for 1 year vs clinical observation | 414 | 18-60 | DFS |
| NCT03286530 | 2 | Recruiting | Ruxolitinib orally twice daily Q28 days for up to 24 cycles | 64 | 60-80 | 12-month GRFS rate |
Accessed on April 23, 2023, using search terms “maintenance post allogeneic transplant,” “maintenance post allo transplant,” “maintenance post allo aml or mds” and “relapse prevention after allo.”
AE, adverse events; DLT, dose limiting toxicity; DFS, disease free survival; MTD, maximum tolerated dose; Q, every.
Conclusion 1: Maintenance strategies are a promising approach for relapse prevention. Further randomized clinical trials are needed to characterize benefit and delineate the patient population best suited for such treatments with several ongoing clinical trials (Table 1).
CLINICAL CASE 2
A 68-year-old African American man was diagnosed with high-risk MDS. An unrelated registry donor search revealed no human leukocyte antigen (HLA)–matched donors. His son was identified as a haploidentical (haplo) donor, and he subsequently proceeded to HCT using reduced intensity conditioning (RIC) and received peripheral blood mobilized stem cells (PBSCs). GVHD prophylaxis includes posttransplant cyclophosphamide (PTCY) (days +3 and +4) along with tacrolimus (TAC) and mycophenolate mofetil (MMF). MMF was stopped on day +35. He had mild grade 1 skin acute GHVD that was controlled with topical steroids. TAC was ultimately able to be discontinued by day +180. At 12 months post-HCT, he had no evidence of MDS, with 100% donor chimerism, and no evidence of GVHD.
Approaches to GVHD prevention in HCT
One of the challenges of HCT is the interplay of disease relapse prevention and controlling GVHD. The usage of calcineurin inhibitors, such as TAC in combination with other GVHD prophylaxis therapies, including methotrexate (MTX), has been an established standard. Advances in GVHD prophylaxis with PTCY have led to its subsequent widespread adoption based on initial studies in the haplo setting.21-24 The monumental success of the PTCY platform to prevent GVHD in HLA disparate haplo transplants with promising survival outcomes changed the landscape for the transplant community at large. This approach paved the path for utilization of PTCY for mismatched unrelated donors and now more recently to well-matched donors, which will be discussed in this section (Table 2).
Pivotal studies using PTCY for GVHD prophylaxis
| Study . | Population . | Design . | Comparison . | Summary findings . |
|---|---|---|---|---|
| Haploidentical | ||||
| Luznik et al. (2008)20 | 0.5-70 years old receiving NMA BM HCT with haplo donors for hematologic disorders (n = 68) | Single-arm prospective multicenter | Single Arm PTCY/MMF/TAC | The CI of grades II-IV and III-IV acute GVHD by day +200 was 34% and 6%, respectively. Graft failure rate was 13%. CI of NRM and relapse at 1 year post-HCT was 15% and 51%, respectively. |
| Ciurea et al. (2015)21 | Adults with de novo or secondary AML who received their first HCT | Retrospective registry study | Haplo BM graft with PTCY prophylaxis (n = 192) vs PBSC mobilized or BMT graft 8/8 HLA-matched MUD receiving calcineurin inhibitor GVHD backbone (n = 1982) | CI of acute grades II-IV (16% vs 33%, P < .0001) and 3-year chronic GVHD (30% vs 53%, P < .0001) was lower after haplo vs MUD in patients who received MAC. Similarly, CI of acute grades II-IV GVHD was 19% vs 28% (P = .05) and 34% vs 52% (P = .002) for chronic GVHD for RIC. OS was similar for haplo and MUD. |
| Fuchs et al. (2021)22 | Adults aged 18-70 (n = 368) with lymphoma or acute leukemia receiving RIC with Flu/Cy/TBI | Two parallel phase 2 trials | Umbilical cord blood (n = 186) with GVHD prophylaxis with cyclosporine and MMF vs haplo (n = 182) with GVHD prophylaxis with PTCY/TAC/MMF | Two-year PFS was 35% (95% CI, 28%-42%) vs 41% (95% CI, 34%-48%) after UCB compared to haplo, respectively (P = .41). Two-year NRM was significantly higher for UCB at 18% (95% CI, 13%-24%) vs 11% (95% CI, 6%-16%) or haplo, P = .04. Two-year OS after UCB was lower at 46% (95% CI, 38%-53%) vs 57% (95% CI, 49%- 64%) for haplo, respectively (P = .04). |
| Related/unrelated | ||||
| Bolaños-Meade et al. (2019)2 | 18-75 years old RIC HCT with related and MUD donors | Randomized phase 2 | TAC/MMF/PTCY (n = 92) or TAC/MTX/bortezomib (n = 89) or TAC/MTX/ maraviroc (n = 92) vs TAC/MTX (n = 224 controls) | PTCY/TAC/MMF had the best GRFS benefit with an HR of 0.72 (90% CI, 0.54-0.94, P = .044) compared to TAC/MTX. Results from this study prompted launch of phase 3 BMT CTN 1703 comparison of PTCY with MTX and calcineurin inhibitor for GVHD prophylaxis. |
| Luznik et al. (2022)26 | ≤65 receiving 8/8 HLA-matched MAC HCT for AML in CR or MDS with <5% blasts | Randomized phase 3 | (1) Ex vivo CD34 selected T-cell–depleted PBSC graft (n = 114), (2) unmanipulated BM graft followed by single-agent Cy (n = 114), and (3) TAC/MTX (n = 118) | Intent-to-treat 2-year CRFS was 50.6% for CD34 selection (HR in comparison to TAC/MTX, 0.80; 95% CI, 0.56-1.15; P = .24), 48.1% for PTCY (HR, 0.86; 0.61-1.23; P = .41), and 41.0% for control. Calcineurin-free regimens did not translate to improved survival. |
| Bolaños-Meade et al. (2022)25 | ≥18 with hematologic malignancies receiving RIC using a 6/6 MRD, (n = 128), 8/8 MUD (n = 288), or 7/8 single mismatched (n = 15) PBSC donor | Randomized phase 3 | TAC/MMF/PTCY (n = 214) vs TAC/MTX (n = 217) | The PTCY group had significantly lower hazard GRFS than TAC/MTX arm (HR, 0.641; 95% CI, 0.492-0.835; P = .001). Day 100 grade III-IV acute GVHD was 6.3% vs 14.7% (P = .001), and chronic GVHD rate at 1 year was 21.9% vs 35.1% (P = .005) for PTCY vs TAC/MTX, respectively. No difference in risk of relapse 1-year post-HCT (20.8% vs 20.2%, P = .9) was noted. |
| MMUD | ||||
| Malki et al. (2021)29 | Adults ≤75 years old receiving PBSC MMUD HCT using RIC or MAC with MMUD HLA matched ≥6/8 (N = 38) | Single-arm prospective | None. Single-arm PTCY/MMF/TAC | One-year OS and GRFS were 87% (95% CI, 71%-94%) and 68% (95% CI, 51%-81%), respectively. CI of NRM at 100 days and 1 year was 0% and 11% (95% CI, 4%-27%), respectively. CI 100-day acute GVHD grades II-IV and III-IV and 1-year chronic GVHD were 50% (95% CI, 36%-69%), 18% (95% CI, 9%-36%), and 48% (95% CI, 34%-68%), respectively. |
| Shaw et al. (2021)30 | 15-71 with hematologic malignancies using a BM graft with either MAC or RIC, mismatched in at least at least 1 allele (4-6/8) (N = 80) | Prospective phase 2 | PTCY/MMF/sirolimus vs CIBMTR contemporary controls with PTCY | Nearly 50% enrollment of ethnic minorities. The 1-year OS of 76% (90% CI, 67.3%-83.3%) was for all patients in the cohort and 72% and 79% for MAC and RIC, respectively. In regard to GVHD, patients receiving MAC had grade III-IV acute and chronic GVHD of 18% and 36% at 1 year, respectively. For RIC, no grade III-IV acute GVHD was noted with 18% chronic GVHD at 1 year. |
| Study . | Population . | Design . | Comparison . | Summary findings . |
|---|---|---|---|---|
| Haploidentical | ||||
| Luznik et al. (2008)20 | 0.5-70 years old receiving NMA BM HCT with haplo donors for hematologic disorders (n = 68) | Single-arm prospective multicenter | Single Arm PTCY/MMF/TAC | The CI of grades II-IV and III-IV acute GVHD by day +200 was 34% and 6%, respectively. Graft failure rate was 13%. CI of NRM and relapse at 1 year post-HCT was 15% and 51%, respectively. |
| Ciurea et al. (2015)21 | Adults with de novo or secondary AML who received their first HCT | Retrospective registry study | Haplo BM graft with PTCY prophylaxis (n = 192) vs PBSC mobilized or BMT graft 8/8 HLA-matched MUD receiving calcineurin inhibitor GVHD backbone (n = 1982) | CI of acute grades II-IV (16% vs 33%, P < .0001) and 3-year chronic GVHD (30% vs 53%, P < .0001) was lower after haplo vs MUD in patients who received MAC. Similarly, CI of acute grades II-IV GVHD was 19% vs 28% (P = .05) and 34% vs 52% (P = .002) for chronic GVHD for RIC. OS was similar for haplo and MUD. |
| Fuchs et al. (2021)22 | Adults aged 18-70 (n = 368) with lymphoma or acute leukemia receiving RIC with Flu/Cy/TBI | Two parallel phase 2 trials | Umbilical cord blood (n = 186) with GVHD prophylaxis with cyclosporine and MMF vs haplo (n = 182) with GVHD prophylaxis with PTCY/TAC/MMF | Two-year PFS was 35% (95% CI, 28%-42%) vs 41% (95% CI, 34%-48%) after UCB compared to haplo, respectively (P = .41). Two-year NRM was significantly higher for UCB at 18% (95% CI, 13%-24%) vs 11% (95% CI, 6%-16%) or haplo, P = .04. Two-year OS after UCB was lower at 46% (95% CI, 38%-53%) vs 57% (95% CI, 49%- 64%) for haplo, respectively (P = .04). |
| Related/unrelated | ||||
| Bolaños-Meade et al. (2019)2 | 18-75 years old RIC HCT with related and MUD donors | Randomized phase 2 | TAC/MMF/PTCY (n = 92) or TAC/MTX/bortezomib (n = 89) or TAC/MTX/ maraviroc (n = 92) vs TAC/MTX (n = 224 controls) | PTCY/TAC/MMF had the best GRFS benefit with an HR of 0.72 (90% CI, 0.54-0.94, P = .044) compared to TAC/MTX. Results from this study prompted launch of phase 3 BMT CTN 1703 comparison of PTCY with MTX and calcineurin inhibitor for GVHD prophylaxis. |
| Luznik et al. (2022)26 | ≤65 receiving 8/8 HLA-matched MAC HCT for AML in CR or MDS with <5% blasts | Randomized phase 3 | (1) Ex vivo CD34 selected T-cell–depleted PBSC graft (n = 114), (2) unmanipulated BM graft followed by single-agent Cy (n = 114), and (3) TAC/MTX (n = 118) | Intent-to-treat 2-year CRFS was 50.6% for CD34 selection (HR in comparison to TAC/MTX, 0.80; 95% CI, 0.56-1.15; P = .24), 48.1% for PTCY (HR, 0.86; 0.61-1.23; P = .41), and 41.0% for control. Calcineurin-free regimens did not translate to improved survival. |
| Bolaños-Meade et al. (2022)25 | ≥18 with hematologic malignancies receiving RIC using a 6/6 MRD, (n = 128), 8/8 MUD (n = 288), or 7/8 single mismatched (n = 15) PBSC donor | Randomized phase 3 | TAC/MMF/PTCY (n = 214) vs TAC/MTX (n = 217) | The PTCY group had significantly lower hazard GRFS than TAC/MTX arm (HR, 0.641; 95% CI, 0.492-0.835; P = .001). Day 100 grade III-IV acute GVHD was 6.3% vs 14.7% (P = .001), and chronic GVHD rate at 1 year was 21.9% vs 35.1% (P = .005) for PTCY vs TAC/MTX, respectively. No difference in risk of relapse 1-year post-HCT (20.8% vs 20.2%, P = .9) was noted. |
| MMUD | ||||
| Malki et al. (2021)29 | Adults ≤75 years old receiving PBSC MMUD HCT using RIC or MAC with MMUD HLA matched ≥6/8 (N = 38) | Single-arm prospective | None. Single-arm PTCY/MMF/TAC | One-year OS and GRFS were 87% (95% CI, 71%-94%) and 68% (95% CI, 51%-81%), respectively. CI of NRM at 100 days and 1 year was 0% and 11% (95% CI, 4%-27%), respectively. CI 100-day acute GVHD grades II-IV and III-IV and 1-year chronic GVHD were 50% (95% CI, 36%-69%), 18% (95% CI, 9%-36%), and 48% (95% CI, 34%-68%), respectively. |
| Shaw et al. (2021)30 | 15-71 with hematologic malignancies using a BM graft with either MAC or RIC, mismatched in at least at least 1 allele (4-6/8) (N = 80) | Prospective phase 2 | PTCY/MMF/sirolimus vs CIBMTR contemporary controls with PTCY | Nearly 50% enrollment of ethnic minorities. The 1-year OS of 76% (90% CI, 67.3%-83.3%) was for all patients in the cohort and 72% and 79% for MAC and RIC, respectively. In regard to GVHD, patients receiving MAC had grade III-IV acute and chronic GVHD of 18% and 36% at 1 year, respectively. For RIC, no grade III-IV acute GVHD was noted with 18% chronic GVHD at 1 year. |
BM, bone marrow; CI, cumulative incidence; CIBMTR, Center for International Blood and Marrow Transplant Research; CR, complete remission; CRFS, chronic relapse-free survival; Cy, cyclophosphamide; Flu, fludarabine; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; NMA, nonmyeloablative; PFS, progression-free survival; TBI, total body irradiation; UCB, umbilical cord blood.
PTCY as a preventative measure for GVHD in matched related, matched unrelated, and mismatched unrelated donor HCT
The BMT CTN previously compared the efficacy of 3 GVHD prophylaxis regimens comparing TAC/MMF/PTCY (n = 92) or TAC/MTX/bortezomib (n = 89) or TAC/MTX/maraviroc to contemporaneous controls of TAC/MTX (n = 224 controls) in a phase 2 randomized study to determine the most promising approaching in the RIC setting using matched related and matched unrelated donors (BMT CTN 1203). The study demonstrated a benefit of using PTCY/TAC/MMF compared to TAC/MTX.25 Thus, the BMT CTN subsequently launched the 1703 study, a phase 3 study of standard TAC/MTX (n = 217) vs PTCY/TAC/MMF (n = 214) for RIC HCT using matched related or 7-8/8 matched unrelated PBSC donors. GVHD/relapse or progression-free survival (GRFS) at 1 year was improved in the PTCY group (HR, 0.641; 95% CI, 0.492-0.835; P = .001). The adjusted 1-year GRFS rate was 52.7% (95% CI, 45.8%-59.2%) for PTCY vs 34.9% (95% CI, 28.6%-41.3%) for TAC/MTX. The day 100 grade III to IV acute GVHD was 6.3% vs 14.7% (P = .001), and chronic GVHD rate at 1 year was 21.9% vs 35.1% (P = .005) for PTCY vs TAC/MTX, respectively. This landmark study supports a new standard of care for GVHD prophylaxis for matched donors receiving RIC.26
In the MAC setting, the BMT 1301 evaluated (1) ex vivo CD34+ selected T-cell depleted PBSC graft (n = 114), (2) unmanipulated bone marrow graft with single-agent cyclophosphamide 50 mg/kg on days +3 and +4 post-HCT (n = 114), or (3) unmanipulated bone marrow graft with TAC/MTX (n = 118). The intent-to-treat 2-year chronic GVHD (moderate or severe) and chronic relapse-free survival was 50.6% for CD34 selection (HR in comparison to TAC/MTX, 0.80; 95% CI, 0.56-1.15; P = .24), 48.1% for PTCY (HR, 0.86; 95% CI, 0.61-1.23; P = .41), and 41.0% for control. One of the most important findings from the study was that the 2-year cumulative incidence of NRM was surprisingly low at 7.9%, and 2-year OS for the TAX/MTX cohort was 76.1%. Despite the highest rate of chronic GVHD in this arm (33.7% at 2 years), the low NRM and relapse rates made it comparable to the other approaches. For example, the CD34+ selected arm had the lowest 2-year cumulative incidence of chronic GVHD at 8.9%, with the highest NRM at 2 years at 21.5%. Thus, study was not able to demonstrate that calcineurin-free prophylaxis as administered to that study population resulted in improved chronic relapse-free survival when compared with TAC/MTX. However, additional GVHD preventive therapy is likely helpful in conjunction with PTCY in preventing GVHD.27 Further studies to elucidate the efficacy of PTCY in patients receiving MAC with PBSC grafts are needed to address this knowledge gap. Combination of PTCY and novel agents such as abatacept and JAK-inhibition is additionally being explored to build on the efficacy of PTCY to improve GRFS.28,29
These successes have led investigators to evaluate PTCY in more HLA-disparate donors, including mismatched unrelated donors.30,31 In a phase 2 study done by the National Donor Marrow Program, using at least a single HLA allele mismatched donor (4-6/8) with PTCY/MMF and sirolimus for GVHD prophylaxis was evaluated in patients receiving either MAC or RIC for conditioning chemotherapy. All patients (n = 80) received fresh bone marrow grafts on day 0, PTCY on days +3 and +4 after HCT, and sirolimus with MMF starting on day +5. The 1-year OS was 76% (90% CI, 67.3%-83.3%) for all patients in the cohort and 72% and 79% for MAC and RIC, respectively. In regard to GVHD, patients receiving MAC had grade III to IV acute and chronic GVHD of 18% and 36% at 1 year, respectively. For RIC, no grade III to IV acute GVHD was noted with 18% chronic GVHD at 1 year. Notably, this study had 48% enrollment of racial or ethnic minorities.31 The ACCESS study (NCT04904588) is further expanding on this experience, evaluating the efficacy of mismatched unrelated donor PBSCs using MAC and RIC in adult patients and evaluating a pediatric stratum. This highly anticipated study addresses an unmet patient need to further mitigate risk of NRM and GVHD with expected accrual to be complete in 2023.32
Toxicity considerations with PTCY
Given that cyclophosphamide is a nitrogen mustard alkylating agent, it is historically associated with hemorrhagic cystitis, renal insufficiency, and notably cardiac toxicity, including cardiomyopathy, arrythmias, and pericarditis. With the expansion of PTCY usage, there have been some reports of early cardiac events after HCT, with the incidence of cardiac toxicity ranging from 7% to 19% in patients having received PTCY.33-35 Pre-HCT workup includes detailed cardiac history and function evaluation. Close monitoring of cardiac function should be considered in patients chosen to receive these medications with noted pre-HCT cardiac abnormalities. Studies to determine the lowest effective dose of PTCY that facilitates GVHD reduction but also minimizes toxicity are currently under way (NCT05436418). Immune dysregulations considerations resulting in infectious complications will be discussed later in this article.
Conclusion 2: Advances in GVHD prophylaxis with PTCY have improved GVHD outcomes, with several prospective studies showing promising outcomes. Given improvements in GVHD outcomes and survival, this broadens the curative potential of HCT to a wider patient population. Ongoing prospective studies are under way and give hope to the continued possibility to improve GVHD outcomes with novel combination therapies.
CLINICAL CASE 3
A 42-year-old woman presents for myeloablative conditioning followed by HCT using her haploidentical sibling donor (sister) for high-risk monosomal karyotype AML. Planned GVHD prophylaxis includes PTCY to be administered on days +3 and +4. Both recipient and donor are cytomegalovirus (CMV) seropositive. Along with standard prophylactic antimicrobials, she will begin letemovir daily beginning between day 0 and day +28 post-HCT and continue through day +100 for CMV prophylaxis.
Novels approaches to infection prevention
Detailed pretransplant evaluation includes planning for specific tailored antimicrobial prophylaxis post-HCT. Nonetheless, infections with CMV have remained clinically significant.36 Reactivation is influenced by both recipient and donor CMV serostatus prior to HCT, with recipients who are CMV seropositive being at the greatest risk for reactivation and occurrence of CMV disease after HCT. Notably, CMV infection risk is highest in PTCY recipients irrespective of donor type. In a Center for International Blood and Marrow Transplant Research analysis, CMV infection was highest for CMV-seropositive HCT recipients irrespective of donor type. When further evaluating risk of CMV infection in seropositive recipients by GVHD prophylaxis type, matched related siblings (n = 1065) who received calcineurin inhibitors had the lower risk (HR, 24.4), followed by matched related siblings (n = 279) who received PTCY having a higher risk (HR, 47.7) and haploidentical recipients (n = 545) who received PTCY having the highest risk (HR, 50.3) (P < .001).37
Given toxicities of agents like ganciclovir, CMV monitoring and preemptive therapy has been a standard of care. Investigational agents have been evaluated in the HCT setting, but both orally available brincidofovir and mirabavir did not confirm benefit in the phase 3 setting.38,39 More recently, a phase 3, double-blind, placebo-controlled trial in CMV-seropositive transplant recipients evaluated the efficacy of letemovir, a viral terminase complex inhibitor, for CMV prophylaxis. Patients on the letemovir treatment arm were found to have a significantly lower rate of infection (n = 122 of 325 treated [37.5%]) than recipients of placebo (n = 103 of 170 [60.6%]) at 24 weeks after HCT,40 had no excess toxicity, and had lower all-cause mortality compared to placebo.
PTCY can also increase the risk of non-CMV herpes viral (NCHV) infections. In the largest such study to date (over 1100 haploidentical and matched sibling recipients), NCHV-related infection was 11% with PTCY in comparison to 4% in matched siblings who received calcineurin-based GVHD prophylaxis (P < .001), with HHV-6 being the most common viral infection noted (P = .004).41 Development of NCHV infection was also associated with NRM. Current prospective studies are evaluating the efficacy of additional platforms beyond pharmaceuticals, including the usage of directed concentrated antibodies and adoptive cell immunotherapies for antiviral control for both CMV (NCT05370976; NCT04056533) and NCHV infections (NCT05305040) to address this ongoing need.
Conclusion 3: Patients who have received a T-cell–depleting GVHD preventative strategy are at risk for CMV reactivation and infection. Thus, usage of an efficacious aggressive CMV preventative strategy, letemovir, should be considered in such patients, as demonstrated by its efficacy in the PTCY-based setting. Additional prospective studies are needed to evaluate mechanisms to improve immune reconstitution and identify infectious risk in new transplant treatment platforms.
Summary
By identifying the most common causes of HCT treatment failure, risk mitigation strategies can be considered in individual HCT recipients to optimize HCT outcomes. In conjunction with pre-HCT workup, a treatment plan can be tailored to delineate a comprehensive treatment plan. Novel agents and combination therapies for patients in these high-risk scenarios are currently being developed to further improve HCT outcomes that will broaden the availability of this treatment option to a wider patient population based on underlying disease, age, racial disparities, comorbid conditions, and underlying risk factors such as infection.
Acknowledgments
The author thanks Joseph Pidala, MD, PhD, for discussion, feedback, and critical reading of the manuscript. The author also thanks Zhuoer Xie, MD, MSCR, for assistance with configuring the visual abstract.
Conflict-of-interest disclosure
Asmita Mishra: no competing financial interests to declare.
Off-label drug use
Asmita Mishra: cyclophosphamide, 5-azacytidine, decitabine, enasidenib, and ivosidenib are discussed.