Abstract
Allogeneic hematopoietic cell transplantation (alloHCT) requires the comprehensive evaluation of patients across multiple dimensions. Among the factors considered, comorbidities hold great significance in the pretransplant assessment. As many as 40% of alloHCT recipients will have a high burden of comorbidities in contemporary cohorts. To ensure a standardized evaluation, several comorbidity scores have been developed; however, they exhibit variations in properties and performance. This review examines the strengths and weaknesses associated with these comorbidity scores, critically appraising these models and proposing a framework for their application in considering the alloHCT candidate. Furthermore, we introduce the concept that comorbidities may have specific effects depending on the chosen transplantation approach and outline the findings of key studies that consider the impact of individual comorbidities on alloHCT outcomes. We suggest that a personalized transplantation approach should not rely solely on the overall burden of comorbidities but should also take into account the individual comorbidities themselves, along with other patient, disease, and transplantation-related factors.
Learning Objectives
Incorporate comorbidity data into a comprehensive framework for risk-informed decision-making
Identify the main comorbidity-based indices used to assess patients undergoing allogeneic HCT
Critically appraise comorbidity indices, identifying their strengths and weaknesses
Introduce the concept that the impact of comorbidities on alloHCT outcomes can vary according to transplantation platform
CLINICAL CASE
A 59-year-old man was diagnosed with de novo normal-karyotype acute myeloid leukemia (AML) 3 months ago. Molecular testing at diagnosis detected an fms-like receptor tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutation. He achieved complete remission without evidence of measurable residual disease after initial induction therapy with “7 plus 3” and midostaurin and went on to receive a consolidation cycle with high-dose cytarabine and midostaurin. He is now presenting for an evaluation to determine suitability for an allogeneic hematopoietic cell transplantation (alloHCT). His past medical history is notable for diabetes mellitus type 2 controlled with metformin, stage IIa colon adenocarcinoma resected 4 years ago without adjuvant treatment, and nonalcoholic fatty liver disease. He has a good performance status (Karnofsky Performance Status 90), and his physical exam is remarkable for a body mass index of 37. Blood laboratory measurements show slightly elevated liver enzymes (aspartate aminotransferase [AST] 74, alanine aminotransferase [ALT] 66), a creatinine level of 1.3 (estimated glomerular filtration rate [eGFR] of 63 mL/min/1.73 m2), a lactate dehydrogenase (LDH) level of 240 µL, and a platelet count of 112 K/µL. His predicted forced expiratory volume in 1 second (FEV1) and hemoglobin-adjusted diffusing capacity of the lungs for carbon monoxide (DLCo) are 92% and 89%, respectively. The left ventricular ejection fraction (LVEF) is 55%. He is cytomegalovirus immunoglobulin G–positive. He has an available 10 out of 10 human leukocyte antigen–matched unrelated donor who is also cytomegalovirus-seropositive.
Introduction
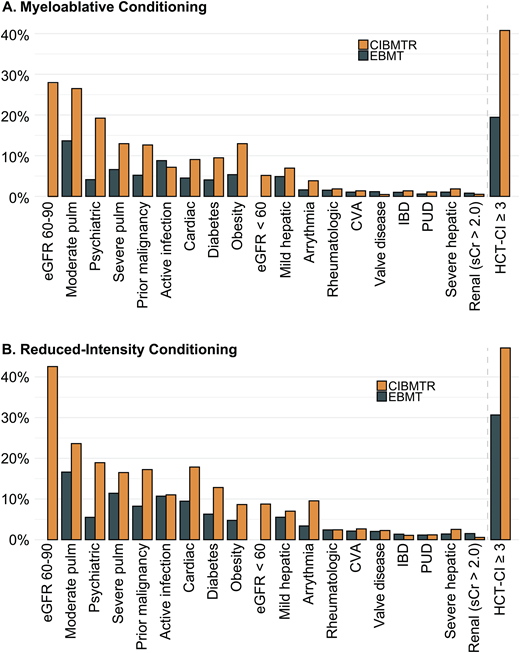
AlloHCT provides a potentially curative treatment for hematologic malignancies and disorders. However, despite improvements in transplantation outcomes over time, significant risks of morbidity and mortality persist.1 Careful consideration of benefits and risks is essential in candidate evaluation and transplantation planning. Pretransplantation comorbidities (ie, coexisting medical conditions) significantly impact alloHCT recipient prognosis.2-4 Individual comorbidities, as well as their cumulative burden, are integral factors that need to be considered when deciding if and how to perform the transplantation. The introduction of reduced-intensity conditioning and improvement in supportive care has made it possible and commonplace to transplant patients with high comorbidity burdens. Indeed, a survey of the US and European transplantation registries shows that among patients receiving myeloablative and reduced-intensity conditioning, 20% to 40% and 31% to 42% had a Hematopoietic Cell Transplantation-Specific Comorbidity Index (HCT-CI) score of 3 or higher, respectively (Figure 1A, B). Here we review current knowledge about the role of pretransplantation comorbidity-based indices and individual comorbidity in alloHCT.
Prevalence of comorbidities in myeloablative and reduced-intensity conditioning cohorts from the CIBMTR and EBMT. The CIBMTR cohort consists of 3685 patients, median age 57.5 (IQR, 50.5, 63.9); the EBMT cohort consists of 9323 patients with a median age of 51.5 years (42.8, 62.1). Both cohorts were selected to include only patients with AML in complete remission. CVA, cerebrovascular accident; IBD, inflammatory bowel disease; IQR, interquartile range; PUD, peptic ulcer disease; pulm, pulmonary disease; sCr, serum creatinine. Data adapted from Fein et al.3 and Farhadfar et al.46
Prevalence of comorbidities in myeloablative and reduced-intensity conditioning cohorts from the CIBMTR and EBMT. The CIBMTR cohort consists of 3685 patients, median age 57.5 (IQR, 50.5, 63.9); the EBMT cohort consists of 9323 patients with a median age of 51.5 years (42.8, 62.1). Both cohorts were selected to include only patients with AML in complete remission. CVA, cerebrovascular accident; IBD, inflammatory bowel disease; IQR, interquartile range; PUD, peptic ulcer disease; pulm, pulmonary disease; sCr, serum creatinine. Data adapted from Fein et al.3 and Farhadfar et al.46
Comorbidity-based prognostic models
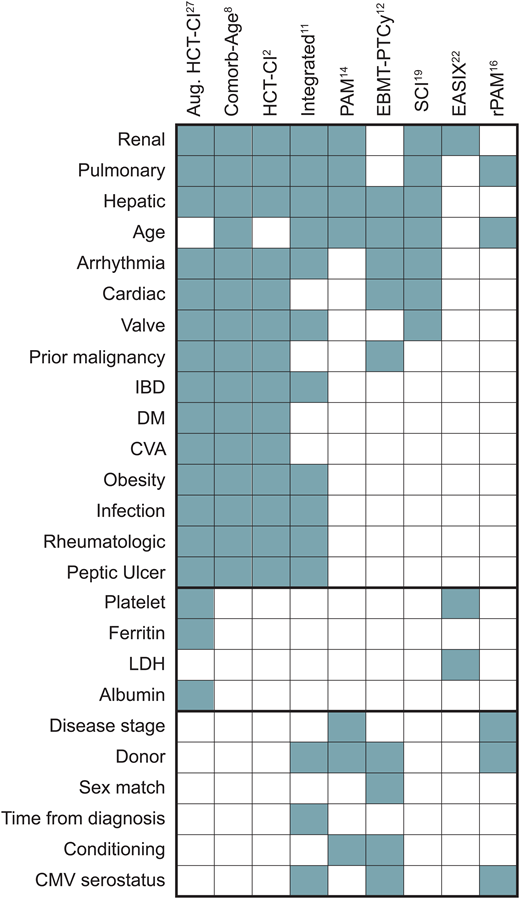
Comorbidity scoring systems standardize the approach to quantifying patients' burden of comorbid conditions and evaluating the risks associated with transplantation. These scores also facilitate adjustments for confounding factors in statistical analyses and serve as quality assurance benchmarks. We expect that higher scores, indicative of a greater pre-HCT comorbidity burden, correspond to recipients' lower capacity to withstand the physiological demands and complications associated with transplantation. Moreover, these scores may offer insights into the recipients' later ability to endure cancer maintenance therapy or receive “salvage” treatment in the event of disease recurrence after transplantation. An ideal comorbidity score serves as a decision-support tool for personalizing interventions. However, evidence-based application of these scores to optimize HCT is challenging since the bulk of our knowledge stems from retrospective studies with inherent biases. Prospective studies considering comorbidities as a pivot for therapeutic decisions are lacking. Nonetheless, insights from numerous retrospective analyses have guided management in the absence of large-scale clinical trials. Over the past 2 decades, various comorbidity-based prognostic models, or risk scores, have emerged, incorporating various comorbidities and other factors to quantify mortality risk following HCT (Figure 2). These scores diverge in the selection and weight of component comorbidities, consideration of determinants beyond comorbidities, and characteristics of the cohorts used for development (Table 1). Here we present a nonexhaustive overview of the predominant comorbidity-based scores.
Comorbidity and noncomorbidity components of individual scores. CMV, cytomegalovirus; CVA, cerebrovascular accident; DM, diabetes mellitus; IBD, inflammatory bowel disease.
Comorbidity and noncomorbidity components of individual scores. CMV, cytomegalovirus; CVA, cerebrovascular accident; DM, diabetes mellitus; IBD, inflammatory bowel disease.
Characteristics of the development cohort for individual scores
| . | . | HCT-CI2 . | Comorb-age8 . | Integrated score11 . | Aug-HCI26 . | PAM14 . | rPAM16 . | EASIX22 . | SCI19 . | EBMT-PTCy12 . |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of pts | 708 | 3033 | 812 | 3917 | 1401 | 1549 | 311 | 573 | 1,861 | |
| Median age | 45 (1-73) | n/a | 58 (20-76) | n/a | n/a | 51 (15-79) | 53 (17-70) | 56 (46-64)a | 51 (18-79) | |
| Years | 1997-2003 | 2000-2006 | 2000-2011 | n/a | 1990-2002 | 2003-2009 | 2001-2013 | 2008-2018 | 2010-2018 | |
| Donor type | Related | 612 (58%) | 1677 (55%) | 432 (53%) | 1962 (50%) | 663 (47%) | 584 (38%) | 109 (35%) | 195 (34%) | 254 (14%) |
| Unrel. | 443 (42%) | 1356 (45%) | 380 (47%) | 1942 (50%) | 738 (53%) | 886 (57%) | 189 (61%) | 378 (66%) | 1607 (86%) 56% haplo | |
| Stem cell source | Marrow | 306 (29%) | 660 (22%) | n/a | n/a | 1059 (76%) | n/a | 202 (6%) | 0 (0%) | 507 (27%) |
| PB | 749 (71%) | 2373 (78%) | n/a | n/a | 329 (23%) | n/a | 291 (94%) | 573 (100%) | 1354 (73%) | |
| Cord | 0 (0%) | 0 (0%) | n/a | n/a | 0 (0%) | n/a | 20a (6%) | 0 (0%) | 0 (0%) | |
| Regimen intensity | MAC | 760 (72%) | 1,889 (62%) | 0 (0%) | 2083 (53%) | 1314 (94%) | 940 (61%) | 72 (23%) | 573 (100%) | 561 (30%) |
| RIC | 295 (28%) | 295 (28%) | 812 (100%) | 1810 (46%) | 87 (6%) | 609 (39%) | 239 (77%) | 0 (0%) | 1300 (7%) | |
| T-cell depletion | None specified | 708 (100%) | 2790 (92%) | 472 (58%) | n/a | n/a | n/a | 122 (39%) | 0 (0%) | 0 (0%) |
| ATG | n/a | 243 (8%) | 340 (42%) | n/a | n/a | n/a | 189 (61%) | 0 (0%) | 0 (0%) | |
| PTCy | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0 (0%) | 1861 (100%) | |
| CD34 selection | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 573 (100%) | 0 (0%) |
| . | . | HCT-CI2 . | Comorb-age8 . | Integrated score11 . | Aug-HCI26 . | PAM14 . | rPAM16 . | EASIX22 . | SCI19 . | EBMT-PTCy12 . |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of pts | 708 | 3033 | 812 | 3917 | 1401 | 1549 | 311 | 573 | 1,861 | |
| Median age | 45 (1-73) | n/a | 58 (20-76) | n/a | n/a | 51 (15-79) | 53 (17-70) | 56 (46-64)a | 51 (18-79) | |
| Years | 1997-2003 | 2000-2006 | 2000-2011 | n/a | 1990-2002 | 2003-2009 | 2001-2013 | 2008-2018 | 2010-2018 | |
| Donor type | Related | 612 (58%) | 1677 (55%) | 432 (53%) | 1962 (50%) | 663 (47%) | 584 (38%) | 109 (35%) | 195 (34%) | 254 (14%) |
| Unrel. | 443 (42%) | 1356 (45%) | 380 (47%) | 1942 (50%) | 738 (53%) | 886 (57%) | 189 (61%) | 378 (66%) | 1607 (86%) 56% haplo | |
| Stem cell source | Marrow | 306 (29%) | 660 (22%) | n/a | n/a | 1059 (76%) | n/a | 202 (6%) | 0 (0%) | 507 (27%) |
| PB | 749 (71%) | 2373 (78%) | n/a | n/a | 329 (23%) | n/a | 291 (94%) | 573 (100%) | 1354 (73%) | |
| Cord | 0 (0%) | 0 (0%) | n/a | n/a | 0 (0%) | n/a | 20a (6%) | 0 (0%) | 0 (0%) | |
| Regimen intensity | MAC | 760 (72%) | 1,889 (62%) | 0 (0%) | 2083 (53%) | 1314 (94%) | 940 (61%) | 72 (23%) | 573 (100%) | 561 (30%) |
| RIC | 295 (28%) | 295 (28%) | 812 (100%) | 1810 (46%) | 87 (6%) | 609 (39%) | 239 (77%) | 0 (0%) | 1300 (7%) | |
| T-cell depletion | None specified | 708 (100%) | 2790 (92%) | 472 (58%) | n/a | n/a | n/a | 122 (39%) | 0 (0%) | 0 (0%) |
| ATG | n/a | 243 (8%) | 340 (42%) | n/a | n/a | n/a | 189 (61%) | 0 (0%) | 0 (0%) | |
| PTCy | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0 (0%) | 1861 (100%) | |
| CD34 selection | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 573 (100%) | 0 (0%) |
Provided in the original article as median and interquartile range.
described in the original article as “bone marrow or unrelated cord blood.”
ATG, antithymocyte globulin; Aug-HCI, augmented HCT-CI; Comorb-Age, comorbidity-age score; unrel, unrelated; PB, peripheral blood; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning.
Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI)
The Charlson Comorbidity Index pioneered comorbidity-based prognostic modeling in medicine, assigning weights to 19 medical conditions based on their impact on mortality. The Index proposed a standardized comorbidity classification scheme validated in various medical conditions. However, the score was not well fit to the role of comorbidity in the unique physiologic stressor of alloHCT, providing an incentive for the development of the HCT-CI by Mohamad Sorror and colleagues from the Fred Hutchinson Cancer Research Center (FHCRC).2 The HCT-CI was developed on a cohort of 708 patients transplanted in the late 1990s at FHCRC and came to include 15 comorbidities weighted according to their association with nonrelapse mortality (NRM). The HCT-CI is typically categorized into three intervals (0, 1-2, ≥3), corresponding with increasing NRM risk. The HCT-CI has been validated repeatedly across various transplantation indications, conditioning regimens, and donor types, demonstrating its utility in stratifying NRM and overall mortality risk.5-7 It has also been expanded to nontransplant settings and modified to include age, performance status, and disease features.8-10 While the HCT-CI remains the most widely used comorbidity score, it has important limitations in the current era. It was developed on a heterogeneous cohort of patients transplanted over 2 decades ago. The threshold for including comorbidities in the HCT-CI was not based on statistical significance, leading to the incorporation of less common comorbidities for which the association with mortality may be unstable. Finally, the HCT-CI prognostic capacity has been variably reproduced, with C statistic values ranging from approximately 0.55 to 0.65.5-7,11-13
Pretransplant assessment of mortality score
Similar to the HCT-CI, the Pretransplant Assessment of Mortality (PAM) score was developed in alloHCT patients (n = 1401) transplanted at the FHCRC between 1990 and 2002, largely with myeloablative conditioning.14 It includes measures of physiological reserve (age, serum creatinine level, serum alanine aminotransferase level, and pulmonary function metrics) as well as disease- and transplantation-related features selected based on their association with overall mortality in a multivariable Cox regression model. The score ranged from 9 to 44 and was broken into 4 categories (9-16, 17-23, 24-30, and 31-44), corresponding with increasing mortality risk across the development and validation cohorts. C statistic values ranged from 0.69 to 0.76 across validation cohorts.13-15 Due to evolving alloHCT strategies with more frequent application of nonmyeloablative conditioning regimens, the PAM score was reevaluated and streamlined on a cohort transplanted between 2003 and 2009.16 The revised PAM score includes only 5 elements, of which only 1 (FEV1) represents a comorbidity. It has been validated in multiple cohorts, with C indices ranging from 0.64 to 0.68.13,17,18
Simplified comorbidity index
The Simplified Comorbidity Index (SCI) was developed in a cohort of 573 patients who underwent CD34-selected alloHCT after myeloablative conditioning at the Memorial Sloan Kettering Cancer Center.19 It incorporates 4 comorbidities and age. Comorbidities were included if their prevalence exceeded 5% and showed a statistically significant association with NRM in multivariable analysis. The SCI score components differ from their HCT-CI counterpart definitions in several ways. The HCT-CI definition of renal disease, a creatinine of greater than 2 mg/dL, was replaced with the estimated glomerular filtration rate (eGFR) calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.20 None of the patients in the development cohort met the renal dysfunction criteria according to HCT-CI, while 24% and 5% met the SCI criteria for mild (eGFR, 60-89.9 mL/min per 1.73 m2) and moderate to severe (eGFR <60 mL/min per 1.73 m2) renal comorbidities, respectively. SCI utilizes a composite definition of cardiac comorbidity, encompassing cardiac disease, arrhythmia, and valve disease, each as defined by HCT-CI. Moderate to severe hepatic comorbidity was included, while mild hepatic disease was not. A higher SCI score was strongly associated with increased NRM risk, as validated in an independent data set from another center utilizing lower-intensity conditioning regimens in unmodified transplants. The score was also validated in an independent cohort of patients undergoing alloHCT following reduced-intensity conditioning.21 Notably, patients with an SCI score of 0 who had elevated HCT-CI scores did not have greater NRM risk, suggesting that the SCI effectively captures the most prognostically meaningful information.
Endothelial activation and stress index
Endothelial dysfunction significantly contributes to complications encountered in alloHCT, including graft-versus-host disease (GVHD), sinusoidal obstruction syndrome, and transplant-associated microangiopathy. Luft et al. developed the endothelial activation and stress index (EASIX) as a score for mortality prediction in patients with GVHD.22 This index includes laboratory tests ([creatinine × LDH]/platelets) intended to serve as indicators of endothelial dysfunction and complement activation.23 EASIX also demonstrated utility as a pre-alloHCT predictor of survival following transplantation.13,23 However, discrimination was only reported by one group (C statistic of approximately 0.65 for NRM).13 A notable advantage of EASIX is that it is composed of objective, readily available measurable markers. However, EASIX is a continuous metric with no consistent threshold for defining high-risk vs low-risk scores. Instead, studies have divided EASIX scores into quartiles, which are cohort dependent, and have demonstrated that patients in the highest-scoring quartiles experience excess mortality.
How to evaluate the performance of comorbidity scores
Extracting the most useful and actionable information from these prediction models in the clinical setting depends on a critical appraisal of their respective literature. Transparent reporting of the development and validation process allows us to evaluate each score's generalizability, risk of bias, and replication potential.24 The most common 2 metrics for describing the performance of a prediction model are calibration and discrimination. Calibration describes how well the predicted probabilities or risk estimates align with the actual outcomes observed in a given population. In other words, it assesses whether the risk score accurately reflects the true probabilities of an event occurring. Calibration is rarely reported in HCT scores.13 Discrimination describes the ability of a risk score to accurately distinguish between individuals who experience an event and those who do not. The most general and widely reported measure of discrimination is the concordance index (C index or C statistic), which ranges from 0.5 to 1. Values below 0.6 and above 0.9 represent poor and excellent discrimination, respectively.24,25 In HCT, most comorbidity-based indices have discrimination in the range of 0.6 to 0.7, underlining their limitations as clinical decision-making tools. Discrimination can be evaluated at different time landmarks after transplantation (ie, 100-day or 2-year overall survival), potentially revealing sets of predictors particularly relevant to shorter- or longer-term outcomes.13 Critically, c-statistic can only be directly compared within the same cohort of patients, though HCT scores tend to fall in this range across numerous studies.7
We ascribe the generally suboptimal discrimination with comorbidity scores to several important factors. First, comorbidities are a single aspect among many influencing transplant outcomes, including other patient characteristics, disease profile, and transplantation factors (eg, donor type and conditioning). However, it is noteworthy that even incorporating these additional dimensions only marginally improves predictive accuracy.11-13,26 Second, the subjective nature of defining a patient's comorbidities and the potential for interobserver variation may lead to less accurate risk prediction even when these indices are used correctly. For this reason, we believe that future scores based on objective biomarkers of organ function and physiological reserve may have improved performance and reproducibility. Third, the effects of individual comorbidities within an index might interact with the selected transplantation approach, such as conditioning or GvHD prophylaxis regimens.3,27 This potential interaction points to the need for indices validated within, or perhaps even designed for, specific transplant contexts rather than relying on universal scores. Lastly, transplantation may be inherently unpredictable over a long timescale, with random and unanticipatable events limiting precise prediction. Dynamic models that adjust predictions over time based on incorporating posttransplantation events such as GVHD and infections may mitigate this predictive uncertainty.
From composite scores to individual comorbidities
We have described the need for a single and comprehensive quantity to represent a patient's fitness for transplantation. The cumulative burden of comorbidities allows for risk-stratification across cohorts. However, the consideration of comorbidities in aggregate risks losing vital prognostic information which should bear upon clinical decision-making. In a recent study, we identified that in a low-conditioning-intensity comorbidity setting (ie, one enriched for patients preselected due to comorbidities and other adverse features) several but not all of the individual comorbidities included in the HCT-CI still contributed to an elevated hazard of NRM.3 Cardiac and psychiatric disease contributed meaningfully to further increased risk. In contrast, moderate pulmonary disease had a hazard ratio of approximately 1.0 despite a large sample size (n = 275/1663, 17%). This suggests that moderately diminished FEV1 or DLCo does not further increase risk beyond the baseline NRM anticipated in this low-conditioning-intensity setting. In another study from the European Society for Blood and Marrow Transplantation (EBMT) encompassing over 38 000 transplantations and including reduced-intensity and myeloablative approaches, the only comorbidities significantly associated with NRM were pulmonary, obesity, cardiac, infection, diabetes, and renal.4 With the exception of renal disease (defined in the HCT-CI primarily by a serum creatinine >2.0 g/dL, which applies to few HCT recipients), hazard ratios for these comorbidities ranged from 1.13 to 1.24, suggesting that the specific risk of any individual comorbidity remains modest. Results of selected publications describing the risk associated with common individual comorbidities are described in Table 2.
Selected studies of outcomes associated with individual comorbidities
| Article . | Brief summary . |
|---|---|
| Pulmonary disease | |
| Parimon 200529 | 2852 patients 1990-2001; decreasing FEV1 or DLCo, greater A-a gradient associated with early respiratory failure and overall mortality |
| Parimon 200614 | PAM score; FEV1 < 80% and DLCo <70% associated with increased risk of overall mortality |
| Tran 201130 | 845 adult patients 2005-2009; former or current smoking not associated with higher risk of NRM |
| Shibasaki 201631 | 101 adult patients 2005-2014; Dinakara equation preferred for adjusting DLCo for hemoglobin when compared to Cotes equation |
| Fein 201827 | 875 adult patients 2006-2015; “severe” but not “moderate” pulm disease associated with NRM; significantly worse outcomes in severe pulm disease if conditioned with Flu/Mel |
| Yang 202232 | 923 myeloablative recipients 2015-2018; decreased FEV1/FVC ratio associated with chronic pulmonary GVHD |
| Schierbeck 202333 | 663 adult patients 2012-2019; severe pulmonary disease associated with NRM |
| Penack 20224 | 38 760 patients treated 2010-2016; pulm disease associated with increased NRM risk |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; no significant association between moderate disease and NRM |
| Hermans 202312 | 1861 adult patients receiving PTCy 2010-2018; no association between pulm comorbidity and NRM |
| Alhomoud 202334 | 511 adult patients treated 2013-2020; abnormal chest computed tomograpy findings before transplantation associated with increased NRM and pulm morbidity |
| Hepatic disease | |
| Parimon 200614 | PAM score; ALT >49 but not total bilirubin >1.3 associated with increased risk of overall mortality |
| Barba 201135 | 455 adult patients 1998-2008; elevated bilirubin and GGT associated with 100-day NRM; elevated ALT associated with increased chronic GVHD |
| Fein 201827 | 875 adult patients 2006-2015; “severe” but not “mild” disease associated with worse NRM, especially in patients conditioned with Flu/Treo |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; neither mild nor moderate/severe hepatic disease significantly associated with increased NRM |
| Hermans 202312 | 1861 adult patients receiving PTCy 2010-2018; moderate/severe hepatic disease associated with increased NRM |
| Psychiatric disease | |
| El-Jawahri 201736 | 7433 CIBMTR patients 2008-2012; psychiatric comorbidity associated with overall mortality and severe acute GVHD |
| Solh 202037 | 556 adult patients 2003-2017; increased Transplant Evaluation Rating Scale associated with increased NRM and overall mortality |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; psychiatric disease associated with increased NRM |
| Prior solid malignancy | |
| Fein 201827 | 875 adult patients 2006-2015; prior solid malignancy not associated with increased NRM |
| Portuguese 202338 | 1193 adult patients 2010-2018; prior solid malignancy not associated with NRM or overall survival |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; prior solid malignancy not associated with increased NRM |
| Active infection | |
| Campbell 201539 | 458 patients with respiratory nasal wash collected pretransplant 2005-2010; ≥ 1 respiratory virus in 116 (23%) of adult patients; among asymptomatic patients, no difference in mortality but increased duration of hospitalization |
| Versluys 201840 | 179 pediatric patients who underwent routine BAL 2004-2013; asymptomatic URTI did not progress to lower respiratory tract infections; positive BAL associated with allo-mediated lung syndromes (see also Zinter 2021)47 |
| Ottaviano 202041 | 586 pediatric patients 2007-2016; 12% with positive nasal swab; 53 transplanted with active URTI. Increased NRM among patients with URTI who did not delay transplant, though these were potentially higher-risk patients for other reasons |
| Kim 202242 | 946 adult mostly myeloablative transplants 2010-2016; significantly lower day-100 survival with pretransplant lower respiratory disease including rhinovirus; URTI not significantly associated with NRM |
| Cardiac disease | |
| Qazilbash 200943 | 217 adult patients (25% with LVEF <45%) 2000-2006; no association between decreased LVEF and OS |
| Stillwell 201144 | 1178 adult patients 1999-2009; no association between pretransplantation coronary artery disease and mortality, ICU admission, length of stay, or posttransplant cardiac events |
| Fein 201827 | 875 adult patients 2006-2015; cardiac disease, arrhythmia, and valve disease associated with increased NRM; elevated risk seen in cardiac disease with Flu/Bu4 but not Flu/Bu2 or Flu/Treo conditioning |
| Penack 20224 | 38 760 patients treated 2010-2016; cardiac disease and arrhythmia significantly associated with increased NRM |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; combined cardiac disease (including arrhythmia and valve disease) associated with elevated NRM risk |
| Hermans 202312 | 1861 adult patients receiving PTCy 2010-2018; cardiac disease associated with significantly increased NRM |
| Renal disease | |
| Shouval 201845 | 1217 adult patients treated 2003-2015; eGFR <60 mL/min/1.73 m2 associated with increased NRM |
| Fein 201827 | 875 adult patients 2006-2015; eGFR <60 mL/min/1.73 m2 associated with increased NRM |
| Farhadfar 202146 | 13 305 adult patients ≥40 years 2008-2016 (in two groups); decreased eGFR associated with increased NRM |
| Shouval 202219 | SCI Score; 573 adult patients undergoing CD34+ selected transplant 2008-2018; eGFR <60 and 60-89.9 associated with increased NRM |
| Article . | Brief summary . |
|---|---|
| Pulmonary disease | |
| Parimon 200529 | 2852 patients 1990-2001; decreasing FEV1 or DLCo, greater A-a gradient associated with early respiratory failure and overall mortality |
| Parimon 200614 | PAM score; FEV1 < 80% and DLCo <70% associated with increased risk of overall mortality |
| Tran 201130 | 845 adult patients 2005-2009; former or current smoking not associated with higher risk of NRM |
| Shibasaki 201631 | 101 adult patients 2005-2014; Dinakara equation preferred for adjusting DLCo for hemoglobin when compared to Cotes equation |
| Fein 201827 | 875 adult patients 2006-2015; “severe” but not “moderate” pulm disease associated with NRM; significantly worse outcomes in severe pulm disease if conditioned with Flu/Mel |
| Yang 202232 | 923 myeloablative recipients 2015-2018; decreased FEV1/FVC ratio associated with chronic pulmonary GVHD |
| Schierbeck 202333 | 663 adult patients 2012-2019; severe pulmonary disease associated with NRM |
| Penack 20224 | 38 760 patients treated 2010-2016; pulm disease associated with increased NRM risk |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; no significant association between moderate disease and NRM |
| Hermans 202312 | 1861 adult patients receiving PTCy 2010-2018; no association between pulm comorbidity and NRM |
| Alhomoud 202334 | 511 adult patients treated 2013-2020; abnormal chest computed tomograpy findings before transplantation associated with increased NRM and pulm morbidity |
| Hepatic disease | |
| Parimon 200614 | PAM score; ALT >49 but not total bilirubin >1.3 associated with increased risk of overall mortality |
| Barba 201135 | 455 adult patients 1998-2008; elevated bilirubin and GGT associated with 100-day NRM; elevated ALT associated with increased chronic GVHD |
| Fein 201827 | 875 adult patients 2006-2015; “severe” but not “mild” disease associated with worse NRM, especially in patients conditioned with Flu/Treo |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; neither mild nor moderate/severe hepatic disease significantly associated with increased NRM |
| Hermans 202312 | 1861 adult patients receiving PTCy 2010-2018; moderate/severe hepatic disease associated with increased NRM |
| Psychiatric disease | |
| El-Jawahri 201736 | 7433 CIBMTR patients 2008-2012; psychiatric comorbidity associated with overall mortality and severe acute GVHD |
| Solh 202037 | 556 adult patients 2003-2017; increased Transplant Evaluation Rating Scale associated with increased NRM and overall mortality |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; psychiatric disease associated with increased NRM |
| Prior solid malignancy | |
| Fein 201827 | 875 adult patients 2006-2015; prior solid malignancy not associated with increased NRM |
| Portuguese 202338 | 1193 adult patients 2010-2018; prior solid malignancy not associated with NRM or overall survival |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; prior solid malignancy not associated with increased NRM |
| Active infection | |
| Campbell 201539 | 458 patients with respiratory nasal wash collected pretransplant 2005-2010; ≥ 1 respiratory virus in 116 (23%) of adult patients; among asymptomatic patients, no difference in mortality but increased duration of hospitalization |
| Versluys 201840 | 179 pediatric patients who underwent routine BAL 2004-2013; asymptomatic URTI did not progress to lower respiratory tract infections; positive BAL associated with allo-mediated lung syndromes (see also Zinter 2021)47 |
| Ottaviano 202041 | 586 pediatric patients 2007-2016; 12% with positive nasal swab; 53 transplanted with active URTI. Increased NRM among patients with URTI who did not delay transplant, though these were potentially higher-risk patients for other reasons |
| Kim 202242 | 946 adult mostly myeloablative transplants 2010-2016; significantly lower day-100 survival with pretransplant lower respiratory disease including rhinovirus; URTI not significantly associated with NRM |
| Cardiac disease | |
| Qazilbash 200943 | 217 adult patients (25% with LVEF <45%) 2000-2006; no association between decreased LVEF and OS |
| Stillwell 201144 | 1178 adult patients 1999-2009; no association between pretransplantation coronary artery disease and mortality, ICU admission, length of stay, or posttransplant cardiac events |
| Fein 201827 | 875 adult patients 2006-2015; cardiac disease, arrhythmia, and valve disease associated with increased NRM; elevated risk seen in cardiac disease with Flu/Bu4 but not Flu/Bu2 or Flu/Treo conditioning |
| Penack 20224 | 38 760 patients treated 2010-2016; cardiac disease and arrhythmia significantly associated with increased NRM |
| Fein 20233 | 1663 adults receiving low-conditioning intensity 2008-2018; combined cardiac disease (including arrhythmia and valve disease) associated with elevated NRM risk |
| Hermans 202312 | 1861 adult patients receiving PTCy 2010-2018; cardiac disease associated with significantly increased NRM |
| Renal disease | |
| Shouval 201845 | 1217 adult patients treated 2003-2015; eGFR <60 mL/min/1.73 m2 associated with increased NRM |
| Fein 201827 | 875 adult patients 2006-2015; eGFR <60 mL/min/1.73 m2 associated with increased NRM |
| Farhadfar 202146 | 13 305 adult patients ≥40 years 2008-2016 (in two groups); decreased eGFR associated with increased NRM |
| Shouval 202219 | SCI Score; 573 adult patients undergoing CD34+ selected transplant 2008-2018; eGFR <60 and 60-89.9 associated with increased NRM |
BAL, bronchoalveolar lavage; Bu2, 2 days of Busulfan; Bu4, 4 days of Busulfan; Flu/Mel, Fludarabine/Melphalan; FVC, forced vital capacity; GGT, gamma-glutamyl transferase; Treo, Treosulfan; URTI, upper respiratory tract infection.
One compelling approach is to consider whether the impact of individual comorbidities is mediated by conditioning regimen–specific toxicities. Such findings might guide the personalization of conditioning chemotherapy for each patient. This possibility was considered by Fein et al. in a single-center study of 875 patients, where the NRM risk of prevalent comorbidities was studied within conditioning-regimen cohorts.27 The authors noted a striking increase in NRM for patients with cardiac comorbidity conditioned with fludarabine and 4 days of busulfan; in contrast, no statistically significant difference was observed among patients who received fludarabine and treosulfan. Overall, weighing individual comorbidities when considering a transplantation approach may contribute to treatment personalization, whereas aggregating comorbidities in a score potentially leads to prognostic information loss.
CLINICAL CASE (continued)
We calculated several comorbidity indices for our patient. With the HCT-CI, he has a total score of 6 (3 points for prior malignancy and 1 each for obesity, diabetes mellitus, and mild hepatic disease). In comparison, by the SCI score he has a total of 2 points for reduced eGFR only. His revised PAM (rPAM) score is 13.75, in the second quartile of scores in 1 large external validation,13 and his EASIX score is 2.12, in the third quartile of scores.13 These indices stratify his risk range from the lower moderate (SCI, rPAM) to higher (EASIX) to very high (HCT-CI) given the stark differences in what factors they include and how they are weighted. Multiple discussions with the patient and his family, informed by NRM-predictive comorbidity scores and accounting for his individual comorbidities alongside the consideration of the relapse potential of MRD-negative intermediate-risk AML, lead to a shared decision to proceed with transplantation. Based on the results of the BMT CTN 0901 trial along with what is felt to be an intermediate comorbidity burden, conditioning with fludarabine and melphalan (140 mg/m2) is selected.28 GvHD prophylaxis with post-transplant cyclophosphamide is chosen, drawing on the results of the BMT CTN 1703, with consideration to the possible benefit of an earlier withdrawal of calcineurin inhibitors given his chronic renal disease. He experienced stage I gut and skin GVHD, which improved with steroids and topical therapies, and is alive and relapse-free at 14 months posttransplantation.
Conclusions
NRM remains a tenacious challenge in alloHCT, and the development of instruments that can help anticipate its occurrence is crucial. Physician intuition plays a vital role in evaluating patient suitability and eligibility for transplantation. Objective, reproducible measures such as comorbidity scores help to guard against anecdotal bias when estimating the likelihood of transplantation success. Nevertheless, caution is needed in using these scores for clinical decision-making—as we have shown, they do not fully account for variability in transplantation outcomes. Comorbidity scores account for one among many axes, together with other patient-, disease-, and transplant-related features, along which the pretransplantation evaluation is conducted and a transplantation approach selected. The extreme ends of these scores may often predict good or poor outcomes, and thus the scores are most valid when used for risk-stratification rather than individualized prediction. Decision-making should also be informed by considering the patient's individual comorbidities and how they interact with the chosen transplantation platform. In conclusion, a comprehensive approach that integrates multiple factors, including comorbidity scores and individual comorbidities, is necessary to enhance risk assessment and improve patient care in alloHCT.
Acknowledgments
The authors would like to extend their gratitude to the EBMT and the Center for International Blood and Marrow Transplantation Research (CIBMTR) and to Mohamad Mohty, Arnon Nagler, and Saurabh Chhabra for their assistance in obtaining data included in this review.
Roni Shouval received support from the Memorial Sloan Kettering Cancer Center Core grant (P30 CA008748) from the National Institutes of Health/National Cancer Institute and grants from the Long Island Sound Chapter, Swim across America, the Robert Hirschhorn Award, Memorial Sloan Kettering Steven Greenberg Lymphoma Research, and the Lymphoma Research Foundation Career Development Award.
Conflict-of-interest disclosure
Roni Shouval: no competing financial interests to declare.
Joshua A. Fein: no competing financial interests to declare.
Off-label drug use
Roni Shouval: nothing to disclose.
Joshua A. Fein: nothing to disclose.