Abstract
Despite improvements in survival among pediatric patients with acute lymphoblastic leukemia (ALL), survival outcomes for adolescents and young adults (AYAs) with ALL have lagged. The reasons for the inferior outcomes among AYAs are multifactorial, each presenting unique challenges and requiring novel solutions. First, adverse disease biology is more common among AYAs with ALL. Ongoing trials are investigating novel approaches to treatment, such as incorporating JAK inhibitors for Philadelphia chromosome–like ALL, menin inhibitors for KMT2A-rearranged ALL, and BCL2/BCLXL inhibition for T-cell ALL. Poorer adherence to therapy also impedes improvements in survival outcomes for AYAs with ALL, but early data suggest that technology, both for monitoring and interventions, may be useful in increasing adherence among this population. Finally, better access to clinical trials and collaboration between pediatric and adult centers is critical in advancing the care of AYAs with ALL. Significant improvements have been made over the past decade, but recognizing, understanding, and addressing each of these unique challenges provides hope that the outcomes for AYAs will continue to improve even further.
Learning Objectives
Identify challenges to improving outcomes in AYAs with ALL
Describe novel strategies to overcome adverse disease biology in AYAs with ALL
Evaluate measures and interventions to improve adherence among AYAs with ALL
Compare health care delivery models between pediatric and AYA patients with ALL
Identify challenges and potential solutions to clinical trial enrollment among AYAs with ALL
CLINICAL CASE
A 26-year-old woman presents with 4 weeks of fatigue. Peripheral blood laboratory analysis shows a white blood cell count of 6.5 K/uL, with 57% blasts, along with anemia (hemoglobin level, 5.8 g/dL) and thrombocytopenia (platelet count, 39 K/uL). A bone marrow biopsy is consistent with B-cell acute lymphoblastic leukemia (B-ALL). Karyotype is normal, but fluorescence in situ hybridization reveals a CRLF2 rearrangement, consistent with Philadelphia chromosome–like (Ph-like) ALL. She lives with her parents approximately 1 hour away from the hospital and is currently working part-time as a piano teacher, without health insurance. A clinical trial for young adults with ALL is discussed, but she and her family are concerned about potential side effects and how they will manage the frequency of hospital visits.
Introduction
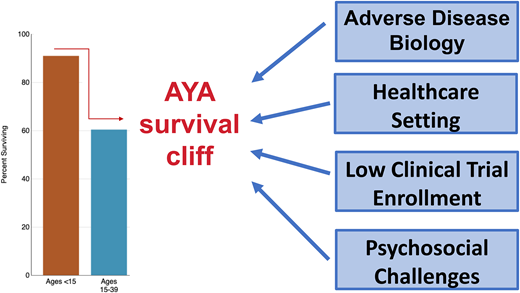
Over the past decade, significant gains have been made in the survival outcomes of adolescent and young adults (AYAs) with ALL. This progress has been due in large part to improved treatment approaches, including the adoption of pediatric-inspired regimens. Unfortunately, despite these improvements, outcomes remain significantly worse compared to pediatric patients, the so-called survival cliff for AYA patients.1 Unique challenges in the AYA population include increased frequency of adverse disease biology, differences in treatment setting, and psychosocial factors that can impact adherence to care and access to clinical trials. In this review we describe some of these barriers to improved outcomes and highlight potential solutions to overcome them.
Challenges with adverse disease biology
One of the key drivers of poorer outcomes in AYAs with ALL, compared to their pediatric counterparts, is the increasing frequency of adverse disease biology. While favorable-risk subtypes, such as ETV6/RUNX1 and hyperdiploidy, are more common among younger patients, adverse-risk subtypes, such as Ph-like ALL; KMT2A-rearranged (KMT2Ar) ALL, also known as mixed-lineage leukemia-rearranged ALL; and early T-cell precursor ALL, are more common in AYAs and adults.2 However, as our understanding of the mechanisms underlying these adverse-risk subtypes has increased, novel treatment strategies are emerging to improve outcomes for AYAs with ALL.
Ph-like ALL
Ph-like ALL is a high-risk subtype of B-ALL, defined by a gene expression profile similar to Ph+ ALL but lacking the hallmark BCR-ABL1 fusion.3 The prevalence of Ph-like ALL increases with age, from 10% to 15% of pediatric cases to 20% to 30% of AYA patients,4-6 and is associated with adverse outcomes, including increased rates of minimal residual disease (MRD) at end induction and higher rates of induction failure and relapse.4,5,7-9 Although a heterogenous disease, JAK/STAT pathway alterations are observed in the majority of AYAs with Ph-like ALL and have a particularly poor prognosis.2,6 Ongoing studies, such as the Children's Oncology Group (COG) study ALL1521, are investigating JAK/STAT pathway inhibition with ruxolitinib for AYAs with newly diagnosed Ph-like ALL.10 The phase 1 portion enrolled 40 patients and showed that the combination of ruxolitinib with intensive multiagent chemotherapy was well tolerated, with the predominant adverse effects being cytopenias and abnormal liver function tests.10 Another ongoing phase 1 trial for patients aged 18 to 39 years with Ph-like ALL and a JAK-targetable genetic signature is using ruxolitinib during postinduction phases of the C10403 chemotherapy regimen (NCT03571321), with efficacy data pending (Table 1).
Select trials in Ph-like ALL
| Target . | Agent . | Clinical trial # . | Phase . | Age (years) . | Disease status . | Results . |
|---|---|---|---|---|---|---|
| JAK | Ruxolitinib | NCT02723994 | 2 | 1-21 | Newly diagnosed | Tasian et al55 |
| JAK | Ruxolitinib | NCT03571321 | 1 | 18-39 | Newly diagnosed | N/A |
| JAK ABL | Ruxolitinib Dasatinib | NCT02420717 | 1/2 | 10+ | R/R | Jain et al56 |
While JAK inhibitors offer promise for Ph-like ALL, emerging data suggest there are alternative biologic dependencies capable of mediating resistance. For instance, preclinical data have demonstrated that PI3K, mTOR, and BCR pathway activation, along with BCL6 and MYC upregulation, may confer resistance to ruxolitinib monotherapy.11-14 In xenograft models, targeting these additional pathways prolongs survival and prevents resistance, but whether these combination approaches are more effective than ruxolitinib alone requires additional clinical studies. There is also promise in using recently approved therapies, such as blinatumomab and chimeric antigen recepter (CAR) T-cell therapy, for Ph-like ALL. A subgroup analysis of the TOWER study of blinatumomab for relapsed/refractory (R/R) B-ALL demonstrated an efficacy of blinatumomab comparable between patients with and without Ph-like ALL.15 Similarly, outcomes of anti-CD19 CAR T-cell therapy in pediatric and young adult patients did not differ significantly between Ph-like ALL and other subtypes.16 It remains to be determined whether these therapies sufficiently overcome the adverse prognostic effects of Ph-like ALL in the up-front setting.
KMT2A-rearranged ALL
KMT2Ar ALL is another high-risk subtype, characterized by rearrangements involving the long arm of chromosome 11 band q23.3 (11q23.3) and numerous fusion partners, with the most frequent being AFF1 on chromosome 4q21.17 KMT2Ar ALL follows a bimodal distribution, with a high incidence in infants, which declines during childhood and increases again in the AYA and adult population and is associated with increased chemotherapy resistance and higher relapse rates.2,18 Unfortunately, unlike Ph-like ALL, novel agents such as blinatumomab, CAR T cells, and inotuzumab ozogamicin appear to be less effective in KMT2Ar ALL. For CD19-directed therapies, this is partially due to the propensity for lineage switching (transformation into mixed phenotype or acute myeloid leukemia) following CD19 CAR T-cell therapy and blinatumomab.19,20 KMT2Ar ALL also has decreased CD22 expression and antigen density, resulting in lower response rates to CD22-directed therapy such as InO.21 As such, novel therapies are needed for KMT2Ar ALL (Table 2).
Select trials in KMT2Ar ALL
| Target . | Agent . | Clinical trial # . | Phase . | Age . | Disease status . | Results . |
|---|---|---|---|---|---|---|
| Menin | SNDX-5613 (revumenib) | NCT04065399 | 1/2 | >30 d | R/R | Issa et al22 |
| Menin | SNDX-5613 (revumenib) | NCT05326516 | 1 | >30 d | R/R | N/A |
| Menin | BMF-219 | NCT05153330 | 1 | 18 + y | R/R | Ravandi et al57 |
| Menin | DSP-5336 | NCT04988555 | 1/2 | 18 + y | R/R | Daver et al58 |
| Menin | JNJ-75276617 | NCT05521087 | 1/1b | 30 d–30 y | R/R | N/A |
| Target . | Agent . | Clinical trial # . | Phase . | Age . | Disease status . | Results . |
|---|---|---|---|---|---|---|
| Menin | SNDX-5613 (revumenib) | NCT04065399 | 1/2 | >30 d | R/R | Issa et al22 |
| Menin | SNDX-5613 (revumenib) | NCT05326516 | 1 | >30 d | R/R | N/A |
| Menin | BMF-219 | NCT05153330 | 1 | 18 + y | R/R | Ravandi et al57 |
| Menin | DSP-5336 | NCT04988555 | 1/2 | 18 + y | R/R | Daver et al58 |
| Menin | JNJ-75276617 | NCT05521087 | 1/1b | 30 d–30 y | R/R | N/A |
In KMT2Ar ALL, KMT2A and its cofactor menin bind to HOX gene promoters, leading to overexpression of HOX genes, and block in hematopoietic differentiation and leukemic transformation. As such, small-molecule inhibitors disrupting the KMT2A-menin interaction leukemias are currently being studied. In the phase 1 AUGMENT-101 trial of the menin inhibitor revumenib (SNDX-5613), 4 of 11 patients with R/R KMT2Ar ALL achieved morphologic remissions.22 Overall the treatment was well tolerated, with asymptomatic QT interval prolongation as the only dose- limiting toxicity, and the phase 1/2 study is ongoing (NCT04065399). However, the duration of remission (DOR) in this study was only 9.1 months,22 and recent reports have demonstrated that menin inhibitor monotherapy may induce mutations in the MEN1 gene that prevent drug binding and result in resistance.23 Thus, combination approaches are likely needed. Interestingly, KMT2Ar ALL exhibits elevated expression of antiapoptotic protein BCL2, and in preclinical studies, treatment with the BCL2 inhibitor venetoclax decreased leukemic growth.24 Whether BCL2 inhibition is synergistic with menin inhibition and will prolong remission in KMT2Ar ALL must be investigated in future trials.
T-cell ALL
T-cell ALL (T-ALL) accounts for only 10% to 15% of pediatric cases but represents up to 25% of diagnoses of ALL in adults, predominantly affecting young adults.25 Previous studies have demonstrated similar remission rates and overall survival between B-ALL and T-ALL, but patients with R/R T-ALL have poor outcomes, with limited treatment options.4 As such, there is a critical need for improvements in up-front therapy to prevent relapse and novel therapies for R/R T-ALL (Table 3).
Select trials in T-cell ALL
| Target . | Agent . | Clinical trial # . | Phase . | Age (years) . | Disease status . | Results . |
|---|---|---|---|---|---|---|
| N/A | Nelarabine | NCT02619630 | 2 | 18-59 | Newly diagnosed | |
| N/A | Nelarabine | NCT02881086 | 3 | 18-55 | Newly diagnosed | Goekbuget, et al59 |
| CD38 | daratumumab | NCT05289687 | 2 | 18+ | MRD+ CR/CRi | N/A |
| CD3-CD38 | XmAb18968 | NCT05038644 | 1 | 18+ | R/R | Murthy et al60 |
| BCL2/ BCLXL | LP-118 | NCT04771572 | 1 | 13+ | R/R | N/A |
| BCL2 + LCK | Venetoclax-ponatinib | NCT05268003 | 2 | 18+ | R/R | N/A |
| CD7 | WU-CART-007 | NCT04984356 | 1/2 | 12+ | R/R | Ghobadi et al34 |
| Target . | Agent . | Clinical trial # . | Phase . | Age (years) . | Disease status . | Results . |
|---|---|---|---|---|---|---|
| N/A | Nelarabine | NCT02619630 | 2 | 18-59 | Newly diagnosed | |
| N/A | Nelarabine | NCT02881086 | 3 | 18-55 | Newly diagnosed | Goekbuget, et al59 |
| CD38 | daratumumab | NCT05289687 | 2 | 18+ | MRD+ CR/CRi | N/A |
| CD3-CD38 | XmAb18968 | NCT05038644 | 1 | 18+ | R/R | Murthy et al60 |
| BCL2/ BCLXL | LP-118 | NCT04771572 | 1 | 13+ | R/R | N/A |
| BCL2 + LCK | Venetoclax-ponatinib | NCT05268003 | 2 | 18+ | R/R | N/A |
| CD7 | WU-CART-007 | NCT04984356 | 1/2 | 12+ | R/R | Ghobadi et al34 |
LCK, lymphocytic-specific kinase.
One strategy is to incorporate nelarabine, a soluble prodrug of ara-G (9-β-D-arabinofuranosyl-guanine) that is currently approved for treatment of R/R T-ALL, into up-front treatment.26 In a phase 3 COG trial (AALL0434) of 1596 pediatric patients with newly diagnosed T-ALL, the addition of nelarabine to a pediatric chemotherapy backbone significantly improved 5-year disease- free survival (88.2% vs 82.1%; P = .029), mainly related to a decrease in rates of central nervous system relapse (1.3% vs 6.9%; P = .0001).27 Although the UKALL14 trial in adults did not demonstrate similar benefits,28 ongoing studies (NCT02619630, NCT02881086) will help determine whether nelarabine should be incorporated into treatment of AYAs with newly diagnosed T-ALL.
Efforts to eradicate MRD are also being utilized in T-ALL to mitigate the increased risk of relapse.25 Preclinical studies have demonstrated that malignant T lymphoblasts express high levels of CD38 and that targeting CD38 with monoclonal antibodies, such as isatuximab and daratumumab, reduced tumor burden and improved MRD-negativity in patient-derived xenograft models.29,30 Clinical trials of daratumumab for MRD-positive T-ALL are underway, including EA9213, a single-arm phase 2 study administering 4 doses of weekly daratumumab to patients in complete remission/complete remission with incomplete count recovery (CR/CRi) with detectable residual disease (MRD ≥10−4) (NCT05289687). It is hoped that eradicating MRD early in the treatment of AYAs with T-ALL will result in decreased relapse rates and improved survival.
Another strategy exploits the dependence of T-ALL on antiapoptic pathways.31 In a phase 2 trial of 53 pediatric and adult patients with R/R ALL, dual BCL2/BCLXL inhibition with venetoclax and navitoclax demonstrated an overall response rate of 59.6%, although the DOR was only 10 months.32 Ongoing trials are investigating novel BCL2/BCLXL inhibitors, with the hope of improving response rates and increasing the DOR (NCT04771572). Future trials will investigate the combination of nelarabine and dual BCL2/BCLXL inhibition with venetoclax and navitoclax for AYAs with newly diagnosed T-ALL. Interestingly, recent work has demonstrated that tyrosine kinase inhibitors, such as dasatinib and ponatinib, may have activity in T-ALL through inhibition of lymphocytic-specific kinase and that this may be synergistic with BCL2/BCXL inhibition.33 Studies of these combinations are ongoing (NCT05268003). Numerous ongoing trials are also investigating the use of CAR T-cell therapy for R/R T-ALL, and the results appear promising, although more long-term data are needed.34,35
CLINICAL CASE (continued)
Concerned about the visits and travel involved, the patient chooses not to enroll in the clinical trial and to receive her care closer to home at a community-based center. She undergoes induction chemotherapy per C10403, a pediatric-based regimen, and achieves a complete remission at the end of induction therapy, although MRD is not measured.
She continues on treatment per C10403 but has to frequently reschedule appointments due to a lack of transportation, resulting in delays in treatment. She often forgets to take her oral chemotherapy. Eventually, tired of feeling that the treatment and ongoing clinic visits are interfering with her life, she stops coming to her appointments and is lost to follow-up.
Psychosocial challenges
Often diagnosed at a critical juncture between childhood and adulthood, AYAs with ALL face complex and unique psychosocial challenges. These issues are numerous and varied but include financial stressors such as a lack of health insurance and cost of health care, difficulty obtaining or maintaining employment and higher education, and fertility concerns, as well as long-term toxicities and survivorship. AYAs with ALL experience substantial psychological morbidities, which often go unrecognized by the providers.36 Research is ongoing to identify how to best address these complex challenges, but a multidisciplinary approach is likely required.
Adherence to therapy
Among AYAs with ALL, poor adherence to the prolonged oral maintenance therapy is common and associated with an increased risk of relapse.37-41 Tracking medication adherence can be challenging, with subjective methods of adherence monitoring, including self-report and provider surveys, tending to overestimate adherence.42 Medication Event Monitoring Systems cap technology, which tracks the date and time of pill bottle opening, has been increasingly used as an objective measure of adherence. Adherence is calculated as the ratio of the number of days with Medication Event Monitoring Systems cap opening to the prescribed days, with nonadherence typically defined as less than 90% or less than 95%.42 In several previous trials, this measure appeared to correlate well with pharmacologic drug-level monitoring and is currently being evaluated as a measure of adherence in AYAs with ALL (NCT03150693).39,40
Recently, several studies have leveraged technology to improve adherence.43 A pilot study of 18 AYAs with leukemia assessing adherence to oral chemotherapy demonstrated that using a daily text message survey to monitor adherence was feasible, although there was no significant correlation between the text message survey and electronic pill bottle adherence.44 In a study of 444 children and young adults with ALL, patients aged 12 and above who received personalized text message reminders to prompt directly supervised therapy had significantly higher adherence rates than those who received education alone (93.1% vs 90.0%; P = .04).45 While these approaches hold promise, other studies have demonstrated significant individual variability among barriers to adherence, suggesting that a personalized approach to improve adherence may be needed,46 in addition to ongoing study of novel adherence measures and interventions for AYAs.
Clinical trial enrollment
Another driving factor of poor outcomes among AYAs with ALL may be the “accrual cliff” in clinical trial enrollment. AYAs have superior survival outcomes when enrolled on clinical trials,47-49 yet there is a marked decrease in the proportion of AYA patients enrolled onto clinical trials above the age of 15 years.50 In a survey administered to providers by the COG, barriers to clinical trial enrollment are multifactorial, identified as logistical issues (45%), disparate enrollment practices (42%), lack of communication between pediatric and medical oncologists (27%), and limited trial availability (27%).51
Despite these challenges, there have been efforts to expand access to clinical trials through the National Cancer Institute Community Oncology Research Program (NCORP), with emphasis on clinical trials specifically for the AYA population. In a recent retrospective analysis of over 3000 AYAs with cancer, clinical trial enrollment increased significantly, from 14.8% to 17.9% between 2006 to 2012 and 2013, with the most significant improvements seen in patients aged 25 to 29 years, and notably doubled among uninsured patients, increasing from 5.7% to 12.9%.52 Unfortunately, clinical trial enrollment remained low for older patients and those treated by adult oncologists, suggesting that further interventions are needed.
Leveraging communication technology may be another strategy to improve clinical trial enrollment for AYAs. A consensus statement recently released by the COG recommends the use of remote patient eligibility screening, electronic informed consent, virtual tumor boards, remote study visits, and remote patient monitoring to increase AYA access to trials and decrease the burden of participation.53 Challenges to this approach include reimbursement, communication between electronic health record systems, and safeguards to maintain patient privacy and protect this vulnerable patient population. Future studies are needed to investigate whether these strategies are feasible and improve enrollment among AYAs.
Treatment setting
Several retrospective studies have demonstrated improved outcomes among AYAs with ALL treated at specialized, experienced centers. In a retrospective analysis of 1380 pediatric and AYA patients with ALL in the Los Angeles County Cancer Registry, patients treated at a National Cancer Institute–designated cancer center or COG center had significantly higher overall (HR, 0.80; 95% CI, 0.66-0.96) and leukemia-specific (HR, 0.80; 95% CI, 0.65-0.97) survival.54 A separate retrospective analysis of 1473 AYAs with ALL treated in California between 2004 and 2014 demonstrated that over two-thirds received care at an adult institution, with the majority (60%) at a community-based center,54 and that patients treated at a pediatric institution had significantly improved overall (HR, 0.53; 95% CI, 0.37-0.76) and leukemia-specific (HR, 0.51; 95% CI, 0.35-0.74) survival compared to those treated at adult institutions. Treatment preferences between institutions clearly contribute to these differences in outcome, with patients treated at community-based adult centers more likely to receive an adult-based regimen, but other facility and provider-related factors likely play a role.54 It has been speculated that these factors may include differences in utilization of MRD testing, less experience with complex pediatric-based regimens, or decreased psychosocial support, but to date limited data exist. Ongoing studies aim to identify specific facility and provider-related factors contributing to poorer outcomes and to propose solutions in hopes of improving the outcomes for AYAs with ALL treated in a wide range of settings (NCT03204916). Nonetheless, these studies highlight the importance of treatment of AYAs with ALL at experienced, specialized centers with multidisciplinary psychosocial support.
Conclusions
Significant progress has been made in the treatment of AYAs with ALL, but many challenges remain. These challenges are multifaceted, including differences in disease biology, psychosocial issues, variation in treatment approaches, and disparities in enrollment on clinical trials. Collaboration between pediatric and adult groups is critical to improving outcomes of AYAs with ALL.
Conflict-of-interest disclosure
Annabelle Anandappa: no competing financial interests to declare.
Emily Curran: advisory board member: Kite, Amgen, Incyte, Pfizer, Servier, Jazz Pharmaceuticals.
Off-label drug use
Annabelle Anandappa: nothing to disclose.
Emily Curran: nothing to disclose.