Abstract
Adolescents and young adults (AYAs; ages 15-39 years) with acute lymphoblastic leukemia (ALL) have worse outcomes than pediatric patients with ALL. Multiple factors contribute to this differential survival. AYAs are more likely to have higher-risk leukemia biology than children with ALL. AYA patients have more choices for treatment facility and treatment protocol, as well as barriers to clinical trial enrollment, both of which can affect survival. AYAs must also navigate psychosocial factors inherent to their unique developmental stage. Furthermore, AYAs typically sustain more treatment-related toxicities than pediatric patients. Treatment on pediatric or pediatric-inspired ALL protocols at pediatric cancer centers has been associated with improved outcomes for AYAs with ALL, but there is still variation in the treatment that AYAs with ALL receive. Clinical trials focused on AYAs with ALL and individualized decision-making regarding choice of treatment facility and treatment protocol are needed to optimize the survival and long-term outcomes of this patient population.
Learning Objectives
To identify key factors contributing to relatively worse outcomes in adolescents and young adults with ALL as compared to pediatric patients with acute lymphoblastic leukemia
To compare and contrast treatment toxicities faced by adolescents and young adults vs pediatric patients with acute lymphoblastic leukemia
To describe barriers to clinical trial enrollment faced by adolescents and young adults with acute lymphoblastic leukemia
Acute lymphoblastic leukemia (ALL) is the most common cancer in children ages 0 to 14 years of age and has a survival rate above 90%.1-3 However, adolescents and young adults (AYAs; ages 15-39 years) with ALL have worse outcomes than younger children, with 5-year overall survival (OS) rates for AYAs ranging between 54% and 74%.4,5 Younger AYAs often fare better than older AYAs.4-6 Moreover, when including patients up to 29 years of age, AYA patients with diagnoses of any leukemia have the highest mortality rate of any cancer.4 This discrepant survival between pediatric and AYA patients with ALL is similar to AYAs with many other cancers as well.2,5,7-9 Additionally, survival for AYAs with cancer has not improved over time at the same pace as that for other age groups, causing what has been termed the “AYA gap.”5 This AYA gap has been an area of increasing concern in the pediatric and adult oncology communities over the past several decades. Reasons for this gap are multifactorial and include clinical, biological, and psychosocial factors, as well as barriers to clinical trial enrollment. Further, AYAs with ALL are particularly unique because while ALL is the most common cancer in children, it is relatively rare in adults. The treatment of AYAs with ALL therefore requires careful consideration of protocol type and treatment center.9-11 This review will use 2 clinical cases to explore discrepancies in survival, risks, and challenges with clinical trial enrollment for AYAs with ALL and to describe areas for optimization of care and quality of life of this unique population.
CLINICAL CASES
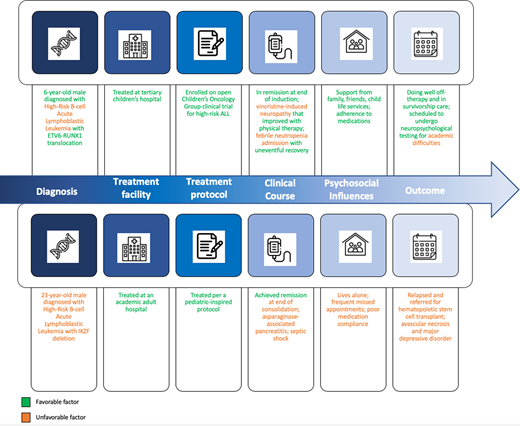
Case 1: A 10-year-old boy presents to an off-therapy oncology clinic for his annual follow-up appointment. He presented to the emergency room at 6 years of age with 2 weeks of fever and leg pain. He had a white blood cell (WBC) count of 60 × 109/L, hemoglobin of 7 g/dL, and platelet count of 55 × 109/L. He was diagnosed with B-cell ALL with ETV6-RUNX1 translocation. His cerebral spinal fluid was negative for leukemia. He was treated at a children's hospital and was enrolled onto the currently open Children's Oncology Group (COG) clinical trial for high-risk ALL, as his WBC count of ≥50 × 109/L classified him as high risk. His end of induction bone marrow aspirate testing was negative for minimal residual disease (MRD) assessed through flow cytometry, with negative MRD defined as <0.01. His treatment complications included mild vincristine-induced neuropathy that improved with physical therapy and 1 admission for febrile neutropenia in delayed intensification, during which he was diagnosed with influenza A and his blood culture was negative. He recovered uneventfully from this. He was well supported by his family, school, and the hospital's child life team. His parents diligently ensured that he adhered to oral maintenance therapy. He is overall doing well off-therapy. His parents and teacher have concerns about academic difficulties, especially in math, for which he is scheduled to undergo neuropsychological testing.
Case 2: A 28-year-old man presents to an oncology clinic. He initially presented at 23 years of age with fatigue and fevers. He had a WBC count of 80 × 109/L, hemoglobin of 11 g/dL, and platelet count of 40 × 109/L. He was diagnosed with B-cell ALL with IKZF1 deletion. His cerebral spinal fluid was negative for leukemia. He was treated at an academic adult hospital per a pediatric-inspired protocol (not on study) as there were no ALL trials open for his age. His bone marrow aspirate studies showed MRD positivity at end of induction (course I) and MRD negativity at end of consolidation (course II) assessed through flow cytometry, with negative MRD defined as <0.01. His treatment complications included asparaginase-associated pancreatitis in induction and delayed presentation for fever in delayed intensification (course IV) due to insurance concerns regarding mounting hospital bills, which resulted in intensive care unit admission for Escherichia coli septic shock. He has lived alone since time of diagnosis and has minimal psychosocial support, frequently missing appointments due to worries about losing his job. He had poor compliance to oral antimetabolite therapy and antimicrobial prophylaxis throughout treatment. At his most recent appointment 15 months off-therapy, he was found to have relapsed ALL and was referred for allogeneic hematopoietic stem cell transplantation. He has developed avascular necrosis of his knees and depression.
Introduction
Survival
Rates of remission for AYAs with ALL are similar to those for pediatric patients at greater than 90%. However, OS for AYAs is 54% to 74% as compared to greater than 90% in children.3-6,8,9,11-14 Underlying differences that increase risk and contribute to the differential long-term survival include the unique biology of ALL in AYAs, choice of treatment protocol and center, increased susceptibility to therapy-related toxicities, and psychosocial challenges.
Risks
Unique cancer biology
AYAs have unique cancer biology, with different prevalence of genetic mutations compared to pediatric patients.7,15 In ALL, AYAs are less likely to have leukemias with favorable features such as hyperdipoidy or ETV6-RUNX1 translocation; ETV6-RUNX1 translocation, which our pediatric patient had, has been identified in 10% of AYAs compared to nearly half of pediatric patients.7,8 Further, AYAs with ALL, as compared to pediatric patients, are more likely to have high-risk features, including T-cell ALL with the unfavorable HOX subtype, KMT2A/MLL or BCR-ABL translocations, CRLF2 mutations, and hypodiploidy (Table 1).7,8,16
Common genetic changes in pediatric and adolescent and young adult acute lymphoblastic leukemia
| Genetic change . | Prognostic significance . | ALL patient population in which genetic change is commonly found . |
|---|---|---|
| ETV6-RUNX1 | Favorable | Pediatric |
| Hyperdiploidy* | Favorable | Pediatric |
| Philadelphia chromosome-like | Unfavorable | AYA |
| IKZF1 | Unfavorable | AYA |
| BCR-ABL1 | Unfavorable | AYA |
| Hypodiploidy† | Unfavorable | AYA |
| Genetic change . | Prognostic significance . | ALL patient population in which genetic change is commonly found . |
|---|---|---|
| ETV6-RUNX1 | Favorable | Pediatric |
| Hyperdiploidy* | Favorable | Pediatric |
| Philadelphia chromosome-like | Unfavorable | AYA |
| IKZF1 | Unfavorable | AYA |
| BCR-ABL1 | Unfavorable | AYA |
| Hypodiploidy† | Unfavorable | AYA |
Leukemia blasts containing >50 chromosomes.
Leukemia blasts containing ≤45 chromosomes.
Choice of treatment protocol and treatment center
Differences in survival between AYA and pediatric patients can be addressed in part by using pediatric-inspired regimens in AYAs with ALL.2,9,10,14 In 2008, Stock and colleagues9 performed a retrospective cohort study of 321 AYAs (ages 16-20 years) with ALL and found that while the AYAs treated on pediatric and adult protocols both had remission rates of 90%, AYAs treated on a Children's Cancer Group (now COG) protocol had improved 7-year event-free survival (EFS) and OS. AYAs treated on pediatric protocols had 7-year EFS and OFS of 63% and 67%, respectively, as compared to 7-year EFS and OS of 34% and 46% for AYAs treated on the Cancer and Leukemia Group B (CALGB) adult protocol. This paved the way for CALGB 10403, a prospective study of 295 AYAs with ALL aged 17 to 39 years that mirrored the control arm of COG protocol AALL0232. CALGB 10403 demonstrated superior outcomes for AYAs treated on the pediatric-inspired protocol as compared to a standard adult ALL protocol. Median EFS was 78.1 months for those treated on CALGB 10403 vs 30 months for historic controls. Treatment- related mortality was 3%.2 Together, these studies, along with several other national and international studies, demonstrated that the pediatric backbones are effective and generally well tolerated in AYAs.2,17-20 Importantly, AYAs treated on pediatric- inspired protocols continued to have poorer outcomes than their pediatric counterparts, which may be due to inherent differences in leukemia biology and treatment response. A notable difference between pediatric and adult protocols is the types of chemotherapies used.14 Pediatric protocols include extended durations of high-dose glucocorticoids, higher doses of asparaginase and vincristine, and earlier and repeated administrations of frequent central nervous system prophylaxis. Conversely, adult ALL protocols typically use significantly myelosuppressive agents and administer central nervous system prophylaxis later during therapy and less frequently.2,9,14,21 Table 2 summarizes key differences in treatment protocol approaches.
Treatment approaches in pediatric and adolescent and young adult acute lymphoblastic leukemia by representative protocols
| . | Pediatric protocol for ALL* . | Pediatric-inspired ALL protocol for AYAs . | Adult ALL protocol for AYAs . |
|---|---|---|---|
| Representative protocol used | AALL1732†,37 | CALG B10403‡,2 | Hyper-CVAD38,39 |
| Induction chemotherapy agents used | DXM (<10 years old)/PDN (≥10 years old) VCR DNR ASP | PDN VCR DNR ASP | Hyperfractionated CPM VCR DOX DXM HD-MTX ARAC ±Rituximab if CD20+ |
| Approach to CNS prophylaxis | IT ARAC at diagnosis, then IT MTX throughout treatment 18 Gy CRT only if CNS3 | IT ARAC at diagnosis, then IT MTX throughout treatment Prophylactic CRT in any patient with T-ALL 18 Gy CRT if CNS leukemia at diagnosis | Alternating IT MTX and IT ARAC in induction and consolidation 30 Gy CRT to whole brain (frank leukemia) or to skull base (cranial nerve involvement) |
| Use of HSCT | EOC MRD >0.01 | If persistent MRD at EOI; if high-risk cytogenetics in CR1 | If persistent MRD at EOI; if high-risk cytogenetics in CR1 |
| Immunotherapy | InO given postconsolidation in experimental arm | Not used | Rituximab if CD20+ |
| Key regimen differences compared to a pediatric protocol | — | Extended remission induction (PDN, DNR, VCR, ASP) One IM phase (uses escalating-dose MTX) while pediatric protocol has 2 IM phases (HD-MTX, then escalating-dose MTX) Patients with T-ALL who were CNS negative at presentation receive prophylactic CRT, which is not done in pediatric protocols for most patients with T-ALL More likely to proceed to HSCT Does not use novel immunotherapy agents | ASP not used in induction Less frequent and less aggressive IT CNS prophylaxis Higher doses of myelosuppressive drugs More likely to proceed to HSCT Use of rituximab if CD20+ |
| . | Pediatric protocol for ALL* . | Pediatric-inspired ALL protocol for AYAs . | Adult ALL protocol for AYAs . |
|---|---|---|---|
| Representative protocol used | AALL1732†,37 | CALG B10403‡,2 | Hyper-CVAD38,39 |
| Induction chemotherapy agents used | DXM (<10 years old)/PDN (≥10 years old) VCR DNR ASP | PDN VCR DNR ASP | Hyperfractionated CPM VCR DOX DXM HD-MTX ARAC ±Rituximab if CD20+ |
| Approach to CNS prophylaxis | IT ARAC at diagnosis, then IT MTX throughout treatment 18 Gy CRT only if CNS3 | IT ARAC at diagnosis, then IT MTX throughout treatment Prophylactic CRT in any patient with T-ALL 18 Gy CRT if CNS leukemia at diagnosis | Alternating IT MTX and IT ARAC in induction and consolidation 30 Gy CRT to whole brain (frank leukemia) or to skull base (cranial nerve involvement) |
| Use of HSCT | EOC MRD >0.01 | If persistent MRD at EOI; if high-risk cytogenetics in CR1 | If persistent MRD at EOI; if high-risk cytogenetics in CR1 |
| Immunotherapy | InO given postconsolidation in experimental arm | Not used | Rituximab if CD20+ |
| Key regimen differences compared to a pediatric protocol | — | Extended remission induction (PDN, DNR, VCR, ASP) One IM phase (uses escalating-dose MTX) while pediatric protocol has 2 IM phases (HD-MTX, then escalating-dose MTX) Patients with T-ALL who were CNS negative at presentation receive prophylactic CRT, which is not done in pediatric protocols for most patients with T-ALL More likely to proceed to HSCT Does not use novel immunotherapy agents | ASP not used in induction Less frequent and less aggressive IT CNS prophylaxis Higher doses of myelosuppressive drugs More likely to proceed to HSCT Use of rituximab if CD20+ |
Protocols described are for Philadelphia chromosome-negative acute lymphoblastic leukemia.
ARAC, cytarabine; ASP, asparaginase; CNS, central nervous system; CPM, cyclophosphamide; CR1, first complete remission; CRT, cranial radiation therapy; CVAD, cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, dexamethasone; DNR, daunorubicin; DOX, doxorubicin; DXM, dexamethasone; EOC, end of consolidation; EOI, end of induction; Gy, Gray; HD, high dose; HSCT, hematopoietic stem cell transplant; IM, interim maintenance; InO, inotuzumab ozogamicin; IT, intrathecal; MTX, methotrexate; PDN, prednisone; VCR, vincristine.
A high-risk pediatric protocol is used as the representative pediatric regimen, as adolescents treated on pediatric protocols are considered high risk at time of diagnosis due to age.
COG trial AALL1732 was selected for use in this table for the high-risk pediatric protocol as this is the current ongoing pediatric trial in the COG. CALGB 10403 is based on the COG trial AALL0232 control arm.40 AALL0232 included 2 randomizations (dexamethasone vs prednisone for induction steroids and high-dose MTX vs Capizzi MTX in IM 1) while AALL1732 uses dexamethasone in induction if <10 years of age and prednisone in induction if ≥10 years of age, high-dose MTX in IM 1 with Capizzi MTX in IM 2, and randomization of the novel agent InO.37,40
Protocol CALGB 10403 was selected for the pediatric-inspired representative regimen for adult ALL as this is the trial discussed throughout the article. Current ongoing Alliance (formerly CALGB) trial A041501 is also testing the novel agent InO.
The type of treatment center is also closely linked to also closely linked both to outcome and to procotol selected to be used.10 AYAs with ALL may be treated at a children's hospital on a pediatric protocol, an adult hospital (academic or community) on a pediatric-inspired protocol, or an adult hospital (academic or community) on an adult protocol. Gupta et al14 reviewed 271 AYAs aged 15 to 21 years treated between 1992 and 2011. They found that from 1992 to 2005, when most AYAs at adult hospitals received adult protocols, 56% of AYAs were treated at an adult hospital with 5-year EFS and OS of 56% and 64%, respectively. For AYAs treated at pediatric centers, 5-year EFS and OFS were 72% and 82%, respectively. From 2006 to 2011, however, 66% of AYAs treated at adult hospitals received pediatric-inspired ALL protocols. Outcomes were better than those for AYAs treated at adult hospitals from 1992 to 2005 but worse than those for AYAs treated at children's hospitals from 2006 to 2011. The authors concluded that survival differences are driven by both lack of universal use of pediatric ALL protocols and factors inherent to children's hospitals. For example, as seen in case 1, there is typically more supervision in pediatric settings by pediatric oncology care teams and parents to ensure patients are compliant with medications and appointments, as well as more psychosocial support, such as child life services. Further, adult oncologists in both community and academic centers may be less familiar with pediatric-inspired ALL protocols and may favor the use of adult protocols when selecting treatment regimens.1,22
Susceptibility to toxicities
Even when AYAs are treated with pediatric-inspired protocols, there are differences in successful receipt of protocol-directed therapy. The rapid physical growth and hormonal changes of puberty alter drug metabolism, which may render AYAs more sensitive than pediatric patients to pediatric regimens.7,15 Pediatric protocols employ higher doses of glucocorticoids and asparaginase than adult protocols.2,8 While these drugs are not as myelosuppressive as the agents used in adult ALL protocols, they can still cause toxicities. Asparaginase is of specific concern. Alacacioglu et al.23 showed that adult patients with ALL aged 18 to 50 years treated on pediatric asparaginase-containing Berlin-Frankfurt-Munster regimens had similar rates of complete remission but higher 5-year OS and relapse-free survival as compared to patients aged 18 to 59 years on non-asparaginase-containing combination chemotherapy regimens with cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, and dexamethasone (hyper-CVAD). While crucial to therapy, there is age-dependent susceptibility to asparaginase toxicity. AYAs incur more frequent and higher grades of asparaginase-related toxicities, specifically hepatotoxicity, pancreatitis, and venous thromboembolism.21,24 Furthermore, when rates of adverse events during induction were compared between children and adolescents treated on pediatric protocols for high-risk ALL (Table 3), AYAs had higher rates of multiple toxicities, including hyperglycemia, hepatotoxicity, and thromboembolism.25 While this single-institution study did not demonstrate higher rates for all toxicities, the cohort included only induction and patients aged 1.0 to 19.8 years, thus not capturing the full range of AYA ages. Advani et al26 also compared toxicities during induction for AYAs treated on CALGB 10403 and COG study AALL0232 and found a direct association between toxicities and increasing age. More grade 3 to 4 toxicities were experienced by those on the CALGB 10403 protocol, which had an older overall age than the AALL0232 cohort. Studies evaluating all courses have demonstrated similar results, including describing higher rates of avascular necrosis (AVN) in AYAs.8 Our AYA patient developed bilateral AVN as a late effect of therapy and also had acute asparaginase-related pancreatitis. While use of adult protocols might mitigate these toxicity risks, this may result in undertreatment and poorer outcomes, as adult protocols often incorporate dose reductions that account for comorbidities found in older patients that AYAs may not have.7,9,15
Proportion of patients with high-risk acute lymphoblastic leukemia with adverse events in induction on pediatric protocols by age25
| . | Cohort, No. (%)* . | P value . | ||
|---|---|---|---|---|
| Overall (N = 235) . | <15 years (n = 176) . | ≥15 years (n = 59) . | ||
| Any adverse event | 190 (80.9) | 139 (78.9) | 51 (86.4) | .21 |
| Infection† | 83 (35.3) | 62 (35.2) | 21 (35.6) | .96 |
| Hypertension | 72 (30.6) | 52 (29.6) | 20 (33.9) | .53 |
| Hepatotoxicity | 72 (30.6) | 45 (25.6) | 27 (45.8) | <.01 |
| Fever† | 58 (24.7) | 44 (25.0) | 14 (23.7) | .84 |
| Hypoxia | 46 (19.6) | 35 (19.9) | 11 (18.6) | .84 |
| Hyperglycemia† | 42 (17.9) | 26 (14.8) | 16 (27.1) | .03 |
| Sepsis | 28 (11.9) | 21 (11.9) | 7 (11.9) | .98 |
| Hypotension | 27 (11.5) | 18 (10.2) | 9 (15.3) | .29 |
| Thromboembolism† | 21 (8.9) | 12 (6.8) | 9 (15.3) | .04 |
| Neuropathy | 11 (4.7) | 6 (3.4) | 5 (8.5) | .11 |
| Hyponatremia | 8 (3.4) | 6 (3.4) | 2 (3.4) | 1 |
| Pancreatitis | 8 (3.4) | 6 (3.4) | 2 (3.4) | 1 |
| Seizure | 6 (2.6) | 4 (2.3) | 2 (3.4) | .64 |
| Ileus | 5 (2.1) | 4 (2.3) | 1 (1.7) | 1 |
| Constipation | 3 (1.3) | 3 (1.7) | 0 (0.0) | .6 |
| ARDS | 3 (1.3) | 1 (1.7) | 2 (1.1) | 1 |
| Stroke | 2 (0.9) | 1 (0.6) | 1 (1.7) | .44 |
| Anaphylaxis | 2 (0.9) | 1 (0.6) | 1 (1.7) | .44 |
| . | Cohort, No. (%)* . | P value . | ||
|---|---|---|---|---|
| Overall (N = 235) . | <15 years (n = 176) . | ≥15 years (n = 59) . | ||
| Any adverse event | 190 (80.9) | 139 (78.9) | 51 (86.4) | .21 |
| Infection† | 83 (35.3) | 62 (35.2) | 21 (35.6) | .96 |
| Hypertension | 72 (30.6) | 52 (29.6) | 20 (33.9) | .53 |
| Hepatotoxicity | 72 (30.6) | 45 (25.6) | 27 (45.8) | <.01 |
| Fever† | 58 (24.7) | 44 (25.0) | 14 (23.7) | .84 |
| Hypoxia | 46 (19.6) | 35 (19.9) | 11 (18.6) | .84 |
| Hyperglycemia† | 42 (17.9) | 26 (14.8) | 16 (27.1) | .03 |
| Sepsis | 28 (11.9) | 21 (11.9) | 7 (11.9) | .98 |
| Hypotension | 27 (11.5) | 18 (10.2) | 9 (15.3) | .29 |
| Thromboembolism† | 21 (8.9) | 12 (6.8) | 9 (15.3) | .04 |
| Neuropathy | 11 (4.7) | 6 (3.4) | 5 (8.5) | .11 |
| Hyponatremia | 8 (3.4) | 6 (3.4) | 2 (3.4) | 1 |
| Pancreatitis | 8 (3.4) | 6 (3.4) | 2 (3.4) | 1 |
| Seizure | 6 (2.6) | 4 (2.3) | 2 (3.4) | .64 |
| Ileus | 5 (2.1) | 4 (2.3) | 1 (1.7) | 1 |
| Constipation | 3 (1.3) | 3 (1.7) | 0 (0.0) | .6 |
| ARDS | 3 (1.3) | 1 (1.7) | 2 (1.1) | 1 |
| Stroke | 2 (0.9) | 1 (0.6) | 1 (1.7) | .44 |
| Anaphylaxis | 2 (0.9) | 1 (0.6) | 1 (1.7) | .44 |
Bold values indicate statistically significant results.
Permission to use data was obtained from the authors of the primary manuscript. All patients in the cohort were treated on pediatric protocols for high-risk acute lymphoblastic leukemia. Patients ranged from age 1.0 to 19.8 years. Adverse events are grade ≥3 unless otherwise specified.
ARDS, acute respiratory distress syndrome.
Percentages represent column percentages.
Clinically significant grade 2 to 5 adverse event.
Psychosocial challenges
The psychosocial changes of adolescence and young adulthood can be similarly challenging and lead to barriers to treatment adherence and clinic attendance in AYAs compared to children. The autonomy of the AYA period can be threatened by a cancer diagnosis; some AYAs need to rely more on their parents/ guardians during a developmental stage that typically includes assertion of independence. Other AYAs may strive to remain autonomous by rebelling against treatment recommendations or inadvertently missing medication doses due to difficulty with properly self-managing complicated regimens. Bhatia and colleagues27 demonstrated lower adherence to oral 6-mercaptopurine chemotherapy in patients aged 12 and older. Furthermore, AYAs may be concerned about treatment negatively affecting their fertility, which may also lead to declining treatment or treatment nonadherence.28 These scenarios can all increase the risk of relapse.29 AYAs with cancer are also faced with significant financial burdens as they balance attendance at appointments with pressures of maintaining employment. These financial pressures are exacerbated by AYAs transitioning onto their own insurance plans and by potential expenses of fertility preservation.30 Our AYA patient faced several of these challenges while our pediatric patient benefited from significant psychosocial support and optimal medication adherence, with his parents' help.
Importantly, some these challenges disproportionately affect certain racial/ethnic minorities. For example, the poor-prognosis CRLF2 mutation has higher a prevalence in AYAs and Hispanic patients.2 Wolfson et al11 found that in 1870 patients with ALL and acute myeloid leukemia, AYAs aged ≥22 years who had either public or no insurance (odds ratio, 0.1; P = .004) or were African American or Hispanic (odds ratio, 0.3; P = .03) were less likely to receive treatment at a pediatric or academic adult site. This may exacerbate underlying health disparities.
Barriers to enrollment
There has been a historical paucity of available and accessible trials for AYAs, and despite attempts at addressing these barriers, availability remains an ongoing challenge.5,15,31 This is concerning because in addition to providing data for future patients, some trials demonstrate improved outcomes for enrolled patients.32-35 In 2006, only 14% of AYAs were enrolled on a trial, while 20% to 38% of pediatric patients were enrolled on a trial. These discrepancies have persisted over time.5 Jacob and Shaw31 aimed to determine if AYA enrollment at a large children's hospital would improve after inception of a formalized AYA program in 2006. From 2001 to 2006, pediatric and AYA enrollment rates were 38% and 27%, respectively. Between 2010 and 2014, rates of pediatric and AYA trial enrollment remained significantly different (34% and 24%, P = .0017), primarily due to a lack of open trials for AYAs. Unfortunately, AYAs may be too old for pediatric trials and too young for older adult trials. Furthermore, even when a trial exists, AYAs are often not eligible. AYAs may not have had adequate, required pretrial studies if they were referred from, and began treatment at, community- based cancer centers that do not participate in the trials.31 Further, misdiagnosis, such as steroid pretreatment for presumed asthma rather than the mediastinal mass, and delays to diagnosis can affect trial eligibility.31 AYAs may provide vague descriptions of symptoms that challenge timely diagnosis. Additionally, AYAs may be aging out of their insurance plans or between insurance providers, which can cause delays to care, subsequent clinical decline, and associated low performance scores, rendering these AYAs ineligible for trial enrollment.5,30,31 Finally, even when an open age-matched trial exists, there are unique reasons why AYAs may not opt to enroll, including desire to choose a treatment rather than be randomized, competing activities such as school or work, and disinterest in research.36
Conclusion
AYAs with ALL are a unique subpopulation of patients with ALL and AYAs with cancer, as they can be treated at either pediatric or adult centers. Most AYAs benefit from treatment at a pediatric center or at least on pediatric-inspired protocols. The contrasting clinical cases illustrate the challenges AYAs must navigate. AYA patients have an increased likelihood of high-risk clinical factors that portend worse outcomes and increased toxicities, encounter psychosocial challenges that threaten therapy adherence, and face barriers to enrollment on trials. The disparate outcomes between children and AYAs with ALL have garnered significant attention, and efforts to address these are under way. Areas of focus include creation of AYA-specific biorepositories to facilitate improved AYA-specific research, advocating for use of pediatric-inspired protocols at adult centers, and education to empower providers to consider referral to pediatric centers to optimize survival and health outcomes for these patients.
Acknowledgments
The authors thank Nicholas P. DeGroote, MPH, for assistance with analyses for this manuscript.
Conflict-of-interest disclosure
Sanyukta K. Janardan: no competing financial interests to declare.
Tamara P. Miller: no competing financial interests to declare.
Off-label drug use
Sanyukta K. Janardan: The authors have nothing to disclose.
Tamara P. Miller: The authors have nothing to disclose.