Abstract
Significant improvements have occurred for adolescent and young adult (AYA) B-cell acute lymphoblastic leukemia (B-ALL) patients following the widespread adoption of “pediatric-inspired” treatment regimens for AYA patients cared for in adult oncology settings. However, for AYA patients, aged 15 to 39, an outcomes gap remains in B-ALL, necessitating the incorporation of novel therapies into up-front treatment regimens. As a result, clinical trial enrollment remains the current standard of care for AYA B-ALL across disease subtypes when available and accessible. Currently, several up-front trials are looking to incorporate the use of inotuzumab, blinatumomab, and chimeric antigen receptor T-cell therapy into existing chemotherapy backbones for AYA patients, as well as tyrosine kinase inhibitors for both Philadelphia-positive (Ph+) and Ph-like B-ALL. In addition to ongoing attempts to improve up-front treatments by incorporating immunotherapy and targeted approaches, the increased use of next generation sequencing for measurable residual disease evaluation has led to superior risk-stratification and a decreased need to pursue consolidative hematopoietic stem cell transplantation during the first complete remission for many patients.
Learning Objectives
Evaluate pediatric and “pediatric-inspired” regimens for the treatment of AYA B-ALL
Compare current clinical trial approaches for AYA B-ALL subtypes in the up-front setting
Discuss changing standards for MRD assessment and the impact on consolidative HSCT in first CR
CLINICAL CASE
A 20-year-old man presents to an urgent care clinic for bruising and profound fatigue. Blood work shows anemia, thrombocytopenia, and a white blood cell count (WBC) of 32 × 109/L. Since he is over 18 years old, the patient is referred to the adult emergency room, where a repeat complete blood count shows 68% lymphoblasts on manual differential with morphology suggestive of ALL (acute lymphoblastic leukemia). The patient is admitted to the Adult Leukemia Service for a further work-up and initiation of treatment.
“Pediatric-inspired” regimens for adolescent and young adult B-cell ALL
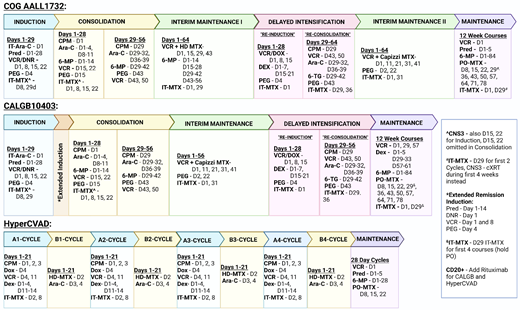
Up-front treatment for adolescent and young adult (AYA) patients with B-cell ALL (B-ALL) at pediatric centers consists of a 4-drug induction, augmented Berlin-Frankfurt-Münster (BFM) consolidation, interim maintenance with high-dose methotrexate, delayed intensification, a second interim maintenance with Capizzi methotrexate, and, finally, an extended maintenance therapy (Figure 1). This treatment backbone has experienced only minor changes over the last few decades, including the shortening of maintenance to 2 years for males and a decrease in frequency of steroid/vincristine pulses to every 12 weeks.1 These modifications were adapted for the Children's Oncology Group (COG) current up-front high-risk trial AALL1732 (NCT03959085). While variations on the COG backbone exist, like the Associazone Italiana Ematologia Oncologia Pediatrica-BFM (eg, AIEOP-BFM ALL 2017, NCT03643276) or St Jude Total Therapy (eg, St Jude Total XVII, NCT03117751), many of the same general treatment principles persist. Compared to adult ALL regimens, pediatric approaches are less myelosuppressive and include more asparaginase with greater total doses of steroids, vincristine, and intrathecal chemotherapy.2 Despite the ability of many pediatric hospitals to treat older adolescent B-ALL patients, even up to the age of 21 to 24 years, the majority of AYA patients over 18 (87.1%) are managed by adult oncologists, often in community settings, which has traditionally limited exposure to pediatric regimens.3
Up-front AYA B-ALL treatment schemas. Ara-C, cytarabine; CNS3, central nervous system; CPM, cyclophosphamide; cXRT, cranial radiation; DEX, dexamethasone; DNR, daunorubicin; DOX, doxorubicin; HD-MTX, high-dose methotrexate; IT-MTX, intrathecal methotrexate; IT-Arac, intrathecal cytarabine; PEG, pegaspargase; PO-MTX, oral methotrexate; Pred, prednisone; 6-MP, mercaptopurine; 6-TG, thioguanine; VCR, vincristine.
Up-front AYA B-ALL treatment schemas. Ara-C, cytarabine; CNS3, central nervous system; CPM, cyclophosphamide; cXRT, cranial radiation; DEX, dexamethasone; DNR, daunorubicin; DOX, doxorubicin; HD-MTX, high-dose methotrexate; IT-MTX, intrathecal methotrexate; IT-Arac, intrathecal cytarabine; PEG, pegaspargase; PO-MTX, oral methotrexate; Pred, prednisone; 6-MP, mercaptopurine; 6-TG, thioguanine; VCR, vincristine.
Following a large retrospective study showing a doubling of survival for AYA patients treated on US pediatric cooperative group trials compared to US adult cooperative group trials (7-year event-free survival [EFS], 63% vs 34%; P < .001),4 a prospective phase 2 trial evaluated the use of a “pediatric-inspired” regimen by adult oncology providers. Based on the pediatric AALL0232 backbone, CALGB10403 showed both an improved EFS of 59% and overall survival (OS) of 73% when compared to historical controls. The regimen also had acceptable toxicity rates in the treatment population, and delivery was feasible in the adult oncology setting.5 Superior outcomes using pediatric- inspired regimens have also been demonstrated across multiple prospective trials by other cooperative groups (Table 1),2 resulting in the pediatric-inspired approach becoming the new standard of care for AYA B-ALL patients treated in adult centers.6
“Pediatric-inspired” B-ALL trials
| Trial . | No. of patients . | Age (years), median (range) . | CR (%) . | OS (%) . | EFS (%) . | DFS (%) . | Duration of follow-up . | AlloHSCT . |
|---|---|---|---|---|---|---|---|---|
| JALSG 202-U | 139 | 19 (16-24) | 97 | 74 | - | 71 | 4 year | t(4;11) |
| DFC1 01-1756 | 92 | 28 (18-50) | 86 | 70 | - | 71 | 4 year | t(4;11), +8, Ph+ |
| DFCI 06-254 | 110 | 32 (18-50) | 89 | 75 | - | 73 | 3 year | t(4;11), +8, Ph+ |
| UKALL 2003 | 229 | 16-24 | 97 | 76.4 | 72.3 | - | 5 year | |
| MDACC aBFM | 106 | 22 (13-39) | 93 | 53 | - | 60 | 5 year | t(4;11) or MRD+ |
| NOPHO ALL 2008 | 221 | 26 (18-45) | - | 78 | 74 | - | 5 year | D29 MRD >5% or D79 > 0.1% |
| GRAALL 2005 | 787 | 36 (18-60) | 92 | 58.5 | 52 | - | 5 year | High risk or MRD+ |
| CALGB10403 | 295 | 24 (17-39) | 89 | 73 | 59 | 66 | 3 year | |
| ALLRE08 PETHEMA | 89 | 20 (15-29) | 95 | 74 | 62 | 65 | 5 year | MRD+ |
| ALL06 | 86 | 22 (15-39) | 90.2 | 74.9 | - | 72.8 | 3 year | t(4;11), MRD+, poor steroid response or WBC >100 |
| GIMEMA LAL-1308 | 76 | 23 (18-35) | 92 | 60.3 | - | 60.4 | 4 year | No CR at D33, pro-B ALL or WBC >100, t(4;11), MRD+ |
| Trial . | No. of patients . | Age (years), median (range) . | CR (%) . | OS (%) . | EFS (%) . | DFS (%) . | Duration of follow-up . | AlloHSCT . |
|---|---|---|---|---|---|---|---|---|
| JALSG 202-U | 139 | 19 (16-24) | 97 | 74 | - | 71 | 4 year | t(4;11) |
| DFC1 01-1756 | 92 | 28 (18-50) | 86 | 70 | - | 71 | 4 year | t(4;11), +8, Ph+ |
| DFCI 06-254 | 110 | 32 (18-50) | 89 | 75 | - | 73 | 3 year | t(4;11), +8, Ph+ |
| UKALL 2003 | 229 | 16-24 | 97 | 76.4 | 72.3 | - | 5 year | |
| MDACC aBFM | 106 | 22 (13-39) | 93 | 53 | - | 60 | 5 year | t(4;11) or MRD+ |
| NOPHO ALL 2008 | 221 | 26 (18-45) | - | 78 | 74 | - | 5 year | D29 MRD >5% or D79 > 0.1% |
| GRAALL 2005 | 787 | 36 (18-60) | 92 | 58.5 | 52 | - | 5 year | High risk or MRD+ |
| CALGB10403 | 295 | 24 (17-39) | 89 | 73 | 59 | 66 | 3 year | |
| ALLRE08 PETHEMA | 89 | 20 (15-29) | 95 | 74 | 62 | 65 | 5 year | MRD+ |
| ALL06 | 86 | 22 (15-39) | 90.2 | 74.9 | - | 72.8 | 3 year | t(4;11), MRD+, poor steroid response or WBC >100 |
| GIMEMA LAL-1308 | 76 | 23 (18-35) | 92 | 60.3 | - | 60.4 | 4 year | No CR at D33, pro-B ALL or WBC >100, t(4;11), MRD+ |
alloHCT, allogeneic hematopoietic stem cell transplantation; MRD, measurable residual disease.
Adapted from Carobolante et al.2
Alternative AYA treatment approaches do exist, including MD Anderson Cancer Center's hyperCVAD regimen (Figure 1), as well as its augmented hyperCVAD that adds blocks of pegylated asparaginase. This regimen was shown in its single-center retrospective analysis to produce similar results to the BFM-based approach in AYA patients.7 However, in other retrospective analyses it was found to be inferior compared to pediatric-inspired regimens in regard to 3-year OS (48.5% vs 72.6%; P = .4), mean OS (41.5 ± 6.4 months vs 53.9 ± 5.4 months; P = .012), mean relapse-free survival (RFS; 39.1 ± 6.8 months vs 53.9 ± 5.4 months; P = .009), and 3-year disease-free survival (DFS; 54.7% vs 76.4%; P = .44).8 An additional comparison of 2 pediatric-inspired regimens with hyperCVAD found superior complete remission (CR) rates (79.5% vs 64.2%; P = .02), lower relapse rates (44.1% vs 60.0%; P = .04), and improved 24-month OS (41.5% vs 28.1%; P = .012) with the pediatric-inspired regimens. Treatment per CALGB10403 was also the only independent prognostic factor for OS in patients older than 20 years (hazard ratio, 0.44; 95% CI, 0.20-0.97; P = .04).9
CLINICAL CASE (continued)
Following admission to the Adult Leukemia Service, the patient is confirmed on multiparameter flow cytometry (MFC) to have B-ALL that is CD20+. He is initiated on treatment per CALGB10403 with fluorescence in situ hybridization, mutational profile, and next generation sequencing (NGS) testing pending.
Philadephia-negative B-ALL
Regardless of the up-front treatment utilized in the AYA population (Figure 1), a survival gap exists in AYA B-ALL when compared to pediatric outcomes. Following up-front therapy, children aged 1 to 14 years now achieve a 5-year OS of greater than 93%, whereas the OS for AYA patients ranges from 60% to 78% despite CR rates of 85% to 95%.10 The cause of this outcome difference is multifactorial, including nonrelapse mortality from an increased risk of treatment-related toxicities compared to pediatric patients and the prevalence of chemoresistant disease due to the particular B-ALL mutational profile seen in AYA patients.11,12 Among early efforts to improve B-ALL outcomes was the introduction of the CD20 monoclonal antibody rituximab to up-front therapy based on the GRAALL-2005/R randomized trial showing improved EFS and decreased rates of relapse in CD20+ adult B-ALL patients.13 This is not standard practice in pediatric regimens, which do not incorporate rituximab into up-front treatment due to a lack of significant difference in measurable residual disease (MRD) response rates in patients receiving rituximab and those who do not receive it.14
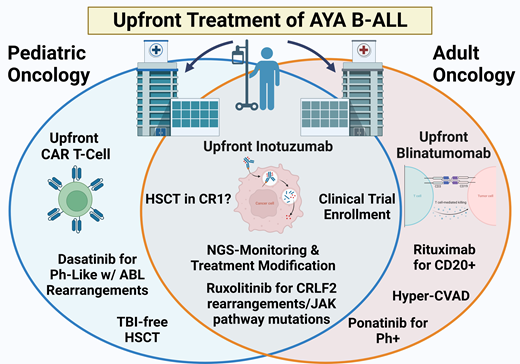
Currently, given the emergence of novel agents (Figure 2) and the success of inotuzumab, blinatumomab, and CD19 chimeric antigen receptor (CAR) T cells in the relapsed/refractory (R/R) B-ALL setting,15 the preferred up-front treatment for Ph− B-ALL is clinical trial enrollment, when available and accessible, investigating the incorporation of these treatment modalities.
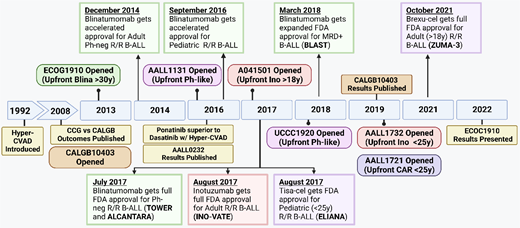
Time line of treatment approvals in B-ALL. Brexu-cel, brexucabtagene autoleucel; Car, chimeric antigen receptor; MRD, measurable residual disease; Ph-neg, Philadelphia negative; R/R, relapsed/refractory; Tisa-cel, tisagenlecleucel.
Time line of treatment approvals in B-ALL. Brexu-cel, brexucabtagene autoleucel; Car, chimeric antigen receptor; MRD, measurable residual disease; Ph-neg, Philadelphia negative; R/R, relapsed/refractory; Tisa-cel, tisagenlecleucel.
Inotuzumab
For AYA patients treated in pediatric centers, the up-front COG trial AALL1732 randomizes Ph− B-ALL patients who are end of consolidation (EOC) MRD-negative to receive 2 cycles of the anti-CD22 antibody drug conjugate inotuzumab (NCT03959085). A comparable study at adult centers is the successor to CALGB10403, Alliance trial A041501, which is investigating the use of 2 cycles of inotuzumab postinduction for MRD eradication (NCT03150693). Patients aged 18 to 25 would be eligible for either trial depending on treatment location.
Inotuzumab was chosen for these trials based on the results of the adult phase 3 INO-VATE trial showing both an improved CR rate (73.8% vs 30.9%; P < .0001) and MRD-negative CR (78.4% vs 28.1%; P < .001) in R/R B-ALL compared to chemotherapy,16 as well as an acceptable safety profile from the R/R pediatric B-ALL trial COG AALL1621.17 While the most frequent grade 3 adverse event in prior inotuzumab trials was hepatic toxicity, including cases of veno-occlusive disease, both AALL1732 and A041501 have noted increased rates of sepsis during subsequent blocks of chemotherapy, requiring protocol modifications.18 Although not temporally associated with inotuzumab infusion, concern exists for both prolonged myelosuppression with chemotherapy following inotuzumab exposure and inotuzumab-induced hypogammaglobinemia leading to increased risk of bacterial infection.
In addition to AALL1732 and A041501, numerous ongoing trials including AYA patients are also exploring the role of inotuzumab as part of postinduction therapy and its efficacy for MRD eradication in up-front therapy (Table 2).
Up-front inotuzumab and blinatumomab trials
| Trial . | Planned patients . | Ages . | Intervention . | Status . | Clinical trials . |
|---|---|---|---|---|---|
| Inotuzumab | |||||
| COG AALL1732 | 4772 | 1-25 | 2 cycles of Ino in consolidation | Recruiting | NCT03959085 |
| ALLIANCE A041501 | 310 | 18-39 | 2 cycles of Ino in consolidation | Suspended | NCT03150693 |
| ALLTogether | 6430 | 0-45 | 2 cycles of Ino prior to maintenance | Recruiting | NCT04307576 |
| GRAALL (B-2022) | 600 | 18-65 | 1 cycle of Ino in delayed intensification | Planned | Pending9 |
| Blinatumomab | |||||
| Jiangsu Institute of Hematology | 35 | 15-59 | Reduced-intensity induction + blina | Recruiting | NCT05557110 |
| HOVON146ALL | 71 | 18-70 | Steroid + blina prephase; blina for 2 cycles in consolidation | Closed to accrual | NCT03541083 |
| Blina-CELL (CZECRIN) | 45 | 18-65 | 1 cycle of blina + chemo for induction | Closed to accrual | NCT04554485 |
| GIMEMA (LAL2317) | 149 | 18-65 | 2 cycles of blina added to chemo | Closed to accrual | NCT03367299 |
| GRAALL (B/2014-QUEST) | 95 | 18-59 | 5 cycles of blina added to consolidation/ maintenance for MRD+ | Closed to accrual | NCT03709719 |
| GMALL (MolAct-1) | 84 | 18+ | Up to 2 cycles of blina added to chemo | Completed, results pending | NCT03109093 |
| Inotzumab + blinatumomab | |||||
| MDACC | 80 | 14+ | Blina cycles 5-8 + Ino in cycles 6 and 8 | Recruiting | NCT02877303 |
| Trial . | Planned patients . | Ages . | Intervention . | Status . | Clinical trials . |
|---|---|---|---|---|---|
| Inotuzumab | |||||
| COG AALL1732 | 4772 | 1-25 | 2 cycles of Ino in consolidation | Recruiting | NCT03959085 |
| ALLIANCE A041501 | 310 | 18-39 | 2 cycles of Ino in consolidation | Suspended | NCT03150693 |
| ALLTogether | 6430 | 0-45 | 2 cycles of Ino prior to maintenance | Recruiting | NCT04307576 |
| GRAALL (B-2022) | 600 | 18-65 | 1 cycle of Ino in delayed intensification | Planned | Pending9 |
| Blinatumomab | |||||
| Jiangsu Institute of Hematology | 35 | 15-59 | Reduced-intensity induction + blina | Recruiting | NCT05557110 |
| HOVON146ALL | 71 | 18-70 | Steroid + blina prephase; blina for 2 cycles in consolidation | Closed to accrual | NCT03541083 |
| Blina-CELL (CZECRIN) | 45 | 18-65 | 1 cycle of blina + chemo for induction | Closed to accrual | NCT04554485 |
| GIMEMA (LAL2317) | 149 | 18-65 | 2 cycles of blina added to chemo | Closed to accrual | NCT03367299 |
| GRAALL (B/2014-QUEST) | 95 | 18-59 | 5 cycles of blina added to consolidation/ maintenance for MRD+ | Closed to accrual | NCT03709719 |
| GMALL (MolAct-1) | 84 | 18+ | Up to 2 cycles of blina added to chemo | Completed, results pending | NCT03109093 |
| Inotzumab + blinatumomab | |||||
| MDACC | 80 | 14+ | Blina cycles 5-8 + Ino in cycles 6 and 8 | Recruiting | NCT02877303 |
Blina, blinatumomab; Ino, inotuzumab; MRD+, MRD-positive.
Blinatumomab
In addition to inotuzumab, the CD3-CD19 bispecific T-cell engager blinatumomab is being evaluated in the up-front treatment setting based on its efficacy in R/R (TOWER19 ) and MRD-positive (BLAST20 ) adult B-ALL trials. Unlike inotuzumab, blinatumomab response and outcomes are superior with low pretreatment tumor burden.19,20 This has led to its superior efficacy as a consolidative treatment rather than salvage therapy, as illustrated by the results of 2 pediatric phase 3 trials in patients up to the age of 30 years.21,22 Based on the initial results of the randomized phase 3 ECOG-ACRIN E1910 trial (which included patients as young as 30 years), the standard of care going forward for patients achieving an MRD-negative remission following induction will likely include the incorporation of blinatumomab.
Utilizing a BFM-based regimen adapted from the E2993/ UKALLXII that incorporated an extended remission induction, rituximab for CD20+ patients, and pegaspargase for patients younger than 55 years, E1910 randomized patients achieving MRD-negativity at the end of induction (EOI) to receive 4 cycles of blinatumomab. With a median follow-up of 43 months, a significant improvement in median OS was seen in the blinatumomab arm (not reached vs 71.4 months; P = .003).23 Currently, only AYA patients with Down syndrome are included in the up-front pediatric blinatumomab trial AALL1731 (NCT03914625), but several other ongoing trials are assessing the efficacy of blinatumomab with or without the addition of inotuzumab in the up-front setting (Table 2).
CAR T-cell products
Similar to blinatumomab, the 2 US Food and Drug Administration (FDA)–approved CAR T-cell products for R/R B-ALL, tisagenlecleucel (tisa-cel) and brexucabtagene autoleucel (brexu-cel), create a cytotoxic T-cell response against CD19-expressing B-ALL cells, resulting in high rates of MRD-negative CR (Table 3).24 Tisa-cel is FDA approved for patients up to 25 years of age with refractory disease or those in a second or later relapse. It is being evaluated in the up-front setting for AYA patients younger than 25 years who are EOC-positive as part of COG AALL1721 (NCT03876769). The use of CAR T cells earlier in therapy may be more efficacious at this time point given the effects of high disease burden and greater number of prior lines of therapy on outcomes.25 Tisa-cel, which utilizes a 4-1BB costimulatory domain, also has the potential for durable remission with a single CAR T-cell infusion.26,27 Based on the ability of NGS MRD assessment and loss of B-cell aplasia to predict relapse post CAR T cell, the proposed CAR-CURE BMT-CTN trial will seek to establish if CAR therapy alone is sufficient or whether a subset of patients may benefit from post-CAR consolidative hematopoietic stem cell transplantation (HSCT) (NCT05621291). Currently, no equivalent up-front trials exist for brexu-cel, which utilizes a CD28 costimulatory domain,28 or other CAR T-cell products.
CAR T-cell therapies FDA approved for AYA B-ALL
| Trial . | No. of patients . | Median age (range), years . | CR/CRi (%) . | MRD-negativeCR in responders (%) . | Median RFS (mo) . | Median OS (mo) . | Unique toxicity . |
|---|---|---|---|---|---|---|---|
| Tisa-cel | |||||||
| ELIANA26 | 75 | 11 (3-23) | 81 | 100 | EFS at 12 months = 50% | 19.1 | CRS (any): 77% Gr. ≥3: 35% ICANS (any): 40% Grade ≥3: 13% |
| Brexu-cel | |||||||
| ZUMA-3, phase 1/228 | 78 | 42.5 (18-84) | 73 | 97 | 11.7 | 25.4 | CRS (any): 93% Gr. ≥3: 31% ICANS (any): 78% Gr. ≥3: 38% |
| Trial . | No. of patients . | Median age (range), years . | CR/CRi (%) . | MRD-negativeCR in responders (%) . | Median RFS (mo) . | Median OS (mo) . | Unique toxicity . |
|---|---|---|---|---|---|---|---|
| Tisa-cel | |||||||
| ELIANA26 | 75 | 11 (3-23) | 81 | 100 | EFS at 12 months = 50% | 19.1 | CRS (any): 77% Gr. ≥3: 35% ICANS (any): 40% Grade ≥3: 13% |
| Brexu-cel | |||||||
| ZUMA-3, phase 1/228 | 78 | 42.5 (18-84) | 73 | 97 | 11.7 | 25.4 | CRS (any): 93% Gr. ≥3: 31% ICANS (any): 78% Gr. ≥3: 38% |
CRi, complete remission with incomplete count recovery; CRS, cytokine release syndrome; Gr, grade, ICANS, immune effector cell-associated neurotoxicity syndrome.
Philadelphia-positive B-ALL
Compared to pediatric B-ALL, where it accounts for 1% to 2% of cases, BCR-ABL1 rearrangement or Philadelphia-positive (Ph+) B-ALL is the most frequent genetic subcategory in adults, including an incidence in young adult patients of approximately 25% to 30%. Although previously carrying a dismal prognosis with an OS of less than 25% in adults and less than 50% in pediatric patients, the introduction of tyrosine kinase inhibitors (TKIs) has significantly altered overall outcomes.29 Ongoing trials including AYA patients seek to understand both the ideal TKI selection and overall treatment intensity, as well as the need for postremission HSCT following the incorporation of third-generation TKIs and/or blinatumomab into up-front therapy.
Despite remission rates of 95% to 100% with the integration of TKIs into pediatric-inspired or hyperCVAD regimens,29 relapse is common and is associated with kinase domain mutations that can be detected at the time of diagnosis.30 Compared to the first- and second-generation TKIs, ponatinib can overcome mutations like T315I and was shown to have a 3-year OS superior to dasatinib when combined with hyperCVAD for frontline therapy (83% vs 56%; P = .03).31 While it may become the preferred TKI in adult B-ALL, ponatinib is currently used in pediatrics only for relapsed disease or if a resistance mutation is detected. The efficacy of ponatinib in younger patients is being tested in the R/R setting as part of the phase 1/2 COG trial AALL1922, given the lack of pharmacokinetic and safety data for patients less than 18 years (NCT04501614).
Similar to Ph− B-ALL, current up-front trials are incorporating blinatumomab in combination with TKIs in hopes of improving EFS/OS and allowing chemotherapy dose reduction (Table 4).
Newly diagnosed Ph+ and Ph-like clinical trials
| Trial . | Planned patients . | Ages . | Intervention . | Status . | Clinical trials . |
|---|---|---|---|---|---|
| Ph+ | |||||
| EsPhALL2017/ COG AALL1631 | 475 | 1-21 | Standard risk: imatinib with EsPhALL vs COG high-risk pre-B ALL backbone High risk: alloBMT with imatinib maintenance | Recruiting | NCT03007147 |
| EA9181 | 330 | 18-75 | Dasatinib or ponatinib/blinatumomab vs dasatinib or ponatinib/Hyper-CVAD | Recruiting | NCT04530565 |
| GIMEMA ALL2820 | 236 | 18+ | Ponatinib/blinatumomab vs imatinib/ chemotherapy | Recruiting | NCT04722848 |
| U Chicago | 25 | 18+ | Inotuzumab + dasatinib + steroid Induction | Recruiting | NCT04747912 |
| Ph-like | |||||
| COG AALL1131 | 22 | 1-31 | Dasatanib with chemotherapy for ABL-class fusions | Closed to accrual | NCT02883049 |
| COG AALL1521 | 171 | 1-21 | Ruxolitinib with chemotherapy for CRLF2 rearrangements and other JAK pathway alterations | Closed to accrual | NCT02723994 |
| Total Therapy XVII | 790 | 1-18 | Dasatinib: patients with Ph+ and those with ABL-class fusion Ruxolitinib: patients with activation of JAK/STAT signaling | Closed to accrual | NCT03117751 |
| UCCC 1920 | 15 | 18-39 | Ruxolitinib: patients with activation of JAK/STAT signaling | Recruiting | NCT03571321 |
| Trial . | Planned patients . | Ages . | Intervention . | Status . | Clinical trials . |
|---|---|---|---|---|---|
| Ph+ | |||||
| EsPhALL2017/ COG AALL1631 | 475 | 1-21 | Standard risk: imatinib with EsPhALL vs COG high-risk pre-B ALL backbone High risk: alloBMT with imatinib maintenance | Recruiting | NCT03007147 |
| EA9181 | 330 | 18-75 | Dasatinib or ponatinib/blinatumomab vs dasatinib or ponatinib/Hyper-CVAD | Recruiting | NCT04530565 |
| GIMEMA ALL2820 | 236 | 18+ | Ponatinib/blinatumomab vs imatinib/ chemotherapy | Recruiting | NCT04722848 |
| U Chicago | 25 | 18+ | Inotuzumab + dasatinib + steroid Induction | Recruiting | NCT04747912 |
| Ph-like | |||||
| COG AALL1131 | 22 | 1-31 | Dasatanib with chemotherapy for ABL-class fusions | Closed to accrual | NCT02883049 |
| COG AALL1521 | 171 | 1-21 | Ruxolitinib with chemotherapy for CRLF2 rearrangements and other JAK pathway alterations | Closed to accrual | NCT02723994 |
| Total Therapy XVII | 790 | 1-18 | Dasatinib: patients with Ph+ and those with ABL-class fusion Ruxolitinib: patients with activation of JAK/STAT signaling | Closed to accrual | NCT03117751 |
| UCCC 1920 | 15 | 18-39 | Ruxolitinib: patients with activation of JAK/STAT signaling | Recruiting | NCT03571321 |
alloBMT, allogeneic bone marrow transplantation.
Philadelphia-like B-ALL
Philadelphia-like (Ph-like) B-ALL expresses a gene signature that is quite similar to Ph+ B-ALL but portends significantly worse outcomes due to its increased resistance to asparaginase and daunorubicin and poor sensitivity to glucocorticoids.32 Ph-like B-ALL is more prevalent in AYA patients (25-30%) compared to children (10-15%), with an increased prevalence in the Hispanic/Latino and Native American population.33-35 Patients with Ph-like disease have high rates of persistent MRD-positivity and OS rates of only 20% to 30%, necessitating HSCT for long-term remissions.36 Ongoing efforts to improve outcomes in AYA patients with Ph-like B-ALL have focused on targeting the JAK/STAT signaling pathway and ABL-class fusions (Table 3) and/or incorporating immunotherapy approaches.
Phase 2 studies exploring the efficacy of incorporating the JAK inhibitor ruxolitinib into induction regimens for Ph-like ALL are still ongoing (NCT03117751 and NCT03571321), including COG AALL1521 (NCT02723994) for patients with CRLF2 rearrangements or JAK pathway mutations. Although ruxolitinib in combination with a traditional chemotherapy backbone has been shown to be safe and tolerable, it is still unknown whether outcomes are better than historical controls.32 A trial adding ruxolitinib to hyperCVAD was terminated early due to low accrual and lack of efficacy (NCT02420717).
For Ph-like patients with ABL rearrangements, prior reports have suggested the benefit of BCR-ABL–specific TKIs for patients with rearrangements of PDGFRB, which are associated with a high risk of induction failure.37 COG AALL1131 (NCT02883049) was amended to test the benefit of dasatinib in patients with ABL class fusions, but results are pending.34 An interim data analysis of MDACC's phase 1/2 trial of hyper-CVAD plus dasatinib showed safety and efficacy in R/R, suggesting overall tolerability of the regimen.38
CLINICAL CASE (continued)
Fluorescence in situ hybridization testing reveals Ph+ B-ALL, and the patient is found to be IKFZpos on mutational profiling. Dasatinib is added to his induction therapy as well as rituximab for CD20+ disease. His EOI MRD testing on day 29 is positive by BCR-ABL polymerase chain reaction (PCR) and NGS.
Postinduction management of AYA patients
MRD monitoring and treatment modification
Regardless of B-ALL subtype or frontline regimen used, the most important prognostic factor for long-term outcomes in ALL remains the persistence of MRD following treatment.40 While acceptable MRD testing should consist of a standardized, validated assay with a sensitivity of 10−4, the optimal time point (EOI vs EOC, or prior to HSCT) and preferred method (MFC, reverse transcriptase PCR, or NGS) remains a point of debate.41
Compared to pediatric patients, the GRAALL-2003 and 2005 trials showed a delayed rate of MRD clearance by MFC in adult B-ALL patients. While only 36% of patients achieved MRD- negative CR at EOI, 76% of patients were in MRD-negative CR by the EOC.42 Despite the potential for later MRD clearance, postinduction MRD-positivity in Ph− AYA patients treated on CALGB10403 was an independent predictor of OS, suggesting that earlier MRD clearance may be more important for durable remissions in AYA patients.5 Currently, for both pediatric and adult treatment approaches for Ph− B-ALL, end consolidation MRD- positivity necessitates treatment modification and remains an indication for HSCT in patients able to obtain a first CR.43,44 Future studies incorporating up-front cellular and immunotherapy approaches may redefine the acceptable timing of MRD clearance.
In addition to the need to determine the importance of when MRD clears, there has been a shift from MFC to utilizing the more sensitive immunoglobulin or T-cell receptor clonotype based NGS, which has a sensitivity of up to 10−6. A recent analysis of discordant MFC and NGS outcomes showed a 5-year cumulative incidence of relapse of 0% in patients MRD-negative by both NGS and MFC compared to 36% in MRD-negative by MFC only. Patients found to be NGS negative at CR had a 5-year OS of 89% compared to 63% for NGS-positive patients, and achieving NGS MRD-negativity at a later time point did not predict low relapse risk.45 For Ph+ ALL, NGS may be superior to reverse transcriptase PCR and allow identification of patients with detectable BCR-ABL1 but low risk for relapse.46 In regard to HSCT, NGS-based MRD both pre- and post-HSCT has been shown to be predictive of transplant outcomes with a high concordance rate between peripheral blood and bone marrow sampling.47
Consolidative HSCT in the first CR
The introduction of pediatric-inspired regimens, the use of second- and third-generation TKIs for Ph+ disease, and the advent of improved MRD monitoring for treatment response have created a shift away from HSCT in the first CR (CR1) for the many AYA B-ALL patients. This is more in line with the pediatric approach where consolidative HSCT in CR1 is no longer indicated for several previously defined high-risk subtypes—hypodiploid ALL, Ph+, IKZFplus—if patients achieve an MRD-negative CR.48
While once considered the standard of care for Ph− AYA patients based on results from the MRC UKALL XII/ECOG E2993 trial,49 recent retrospective analysis comparing continued treatment per CALGB10403 to consolidative HSCT in CR1 showed a superior 5-year OS (66% chemo vs 47% HCT; P < .001) and DFS (58% chemo vs 44% HCT; P < .004) with chemotherapy alone.50 HSCT also had higher nonrelapse mortality (29% HCT vs 8% chemo; P < .001), and a separate analysis found HSCT to be the only factor associated with decreased OS on multivariable analysis.51
For Ph+ B-ALL, HSCT is no longer the standard of care for pediatric patients with chemotherapy plus TKI alone and has shown to be noninferior to HSCT.52 While transplant remains a recommendation for AYA patients in CR1 with Ph+ B-ALL with a suitable donor in adult settings,53 the addition of second- or third-generation TKIs to intensive chemotherapy can produce deep molecular responses for which HSCT has not been shown to provide additional benefit.31 For those patients proceeding to HSCT, maintenance therapy with a TKI remains the standard of care, although its benefit remains unknown with EsPhALL2017/COGAALL1631 (NCT03007147) prospectively evaluating the feasibility, toxicity, and outcomes of post-HCT TKI maintenance in the pediatric and young adult population.
Currently, proceeding to HSCT for AYA patients in CR1 is predominately reserved for patients with specific high-risk mutations (ie, Ph-like and KM2TAr) or individuals with persistent MRD with induction therapy able to achieve a remission.
CLINICAL CASE (continued)
The patient's TKI is switched to ponatinib, and he achieves a complete molecular remission at the EOC evaluation with negative MRD by MFC and NGS. He does not proceed to HSCT in CR1, and the patient remains in an ongoing remission during maintenance therapy with serial monitoring by BCR-ABL PCR.
Conclusion
Significant advances in the up-front treatment of AYA B-ALL have occurred with the adoption of pediatric-inspired regimens as a standard of care. Hope remains to further close the survival gap with the incorporation of immunotherapy and targeted agents in the up-front setting. Time will tell if the current, ongoing up-front trials in combination with more sensitive MRD testing can further improve OS/EFS, avoid the need for HSCT in CR1 for the majority of AYA patients, and lead to decreased treatment-associated toxicity through further dose reductions of chemotherapy.
Despite this optimism with improved treatment approaches, AYA B-ALL patients remain affected by significant financial and treatment-related toxicities that may lead to excessive long-term mortality and the negation of improvement in 5-year OS.54-57 Outcomes also remain disproportionately worse for minority patients,11 and substantial barriers remain for AYA clinical trial enrollment.58 In addition to improved up-front treatment regimens, attention must remain on the unique needs of this patient population, which often remain underrecognized or overlooked.59
Conflict-of-interest disclosure
John C. Molina: no competing financial interests to declare.
Seth Rotz: no competing financial interests to declare.
Off-label drug use
John C. Molina: The off-label use of blinatumomab, inotuzumab ozogamicin, tisagenlecleucel, ponatinib, ruxolitinib, dasatinib is discussed.
Seth Rotz: The off-label use of blinatumomab, inotuzumab ozogamicin, tisagenlecleucel, ponatinib, ruxolitinib, dasatinib is discussed.