Abstract
Richter transformation (RT) represents an uncommon (2% to 10%) but feared complication of chronic lymphocytic leukemia (CLL). The disease is characterized by rapid disease kinetics, a high-risk genetic mutational profile, chemoimmunotherapy resistance, and consequent poor survival. The typical overall survival (OS) from the pre-Bruton tyrosine kinase (BTK)/B-cell lymphoma 2 (BCL2) inhibitor CLL era is 6–12 months, and recent series of RT complicating progression on a BTK or BCL2 inhibitor in heavily pretreated relapsed CLL patients suggests an OS of only 3–4 months. Despite these sobering survival statistics, novel agents have the potential to impact the natural RT disease course. This article reviews recent therapeutic developments, focusing on inhibitors of BTK, BCL2, the PD1-PDL1 axis, and T-cell–activating/engaging therapies. Herein, I discuss the importance of randomized clinical trials in a disease where small single-arm studies dominate; industry engagement, including the role of registrational studies; and the need to integrate prospectively planned correlative biological studies embedded within future clinical trials to help discover which patient benefits most from each class or combination of novel targets.
Learning Objectives
To better understand the currently applied management strategy for patients with Richter transformation
To obtain knowledge of the key novel agents in development in Richter transformation management
CLINICAL CASE
A previously fit 57-year-old man with untreated chronic lymphocytic leukemia (CLL) presented with a large, rapidly growing right-sided cervical neck mass. A biopsy revealed a CD5-positive, CD20-positive diffuse large B-cell lymphoma (DLBCL). The immunohistochemical MIB-1 (a proliferation-related antigen) index was 80%, and immunohistochemical staining revealed a nongerminal center B-cell (non-GCB) phenotype by the Hans algorithm. Fluorescence in situ hybridization and next generation sequencing was performed. No myelocytomatosis (MYC) or B-cell lymphoma 2 (BCL2) rearrangements were noted by fluorescence in situ hybridization. A TP53 mutation/17 p deletion was observed in both the DLBCL biopsy and the peripheral blood CLL population. Given the patient's history of untreated CLL, the diagnosis of Richter transformation (RT) was made. Fluorodeoxyglucose–positron emission tomography staging demonstrated nonbulky stage III disease with a maximum standardized uptake value of 35 in the right side of the neck. The patient received 6 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) and achieved a partial metabolic remission at the end of treatment positron emission tomography/computed tomography response assessment. He received an unrelated reduced-intensity allogenic stem cell transplantation in first remission as consolidation and remained in remission for 9 months before relapsing with biopsy-confirmed stage IV non-GCB DLBCL. He was considered fit for clinical trial assessment and enrolled in a noncovalent Bruton tyrosine kinase inhibitor (BTKi) trial but unfortunately progressed after an initial partial response and died 4 months later with palliative care support.
The challenge of Richter transformation
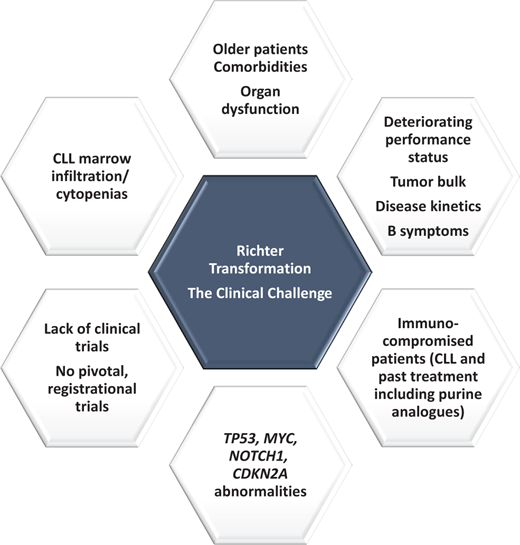
Richter transformation (RT) represents one of the most feared complications for individuals with CLL and occurs in 2% to −10% of patients. Three percent of a series of 2975 chemoimmunotherapy (CIT) treated CLL patients within German CLL Study Group (GCLLSG) front-line clinical trials developed RT.1 RT most commonly represents a large cell transformation from CLL to a DLBCL-type histology. Hodgkin-like transformation, T-cell transformation, and Burkitt-like/lymphoblastic transformations are all described but are rare. RT is characterized by rapid disease kinetics, a poor-risk genomic profile (TP53, MYC, NOTCH1, CDKN2A,2 and DNA damage response mutations3 ), chemotherapy-resistance and typically poor overall survival (OS) (see Figure 1). Recent comprehensive paired analysis of RT and CLL samples have improved understanding of the biological drivers of RT. RT is characterized by profound genomic instability, associated with chromothripsis/chromoplexy and whole genome duplication. Moreover, multiplexed in vivo CRISPR-Cas9 B-cell editing analysis has demonstrated tonic PI3K signaling and activation of MYC/ mTOR/PI3K as a key pathway in RT.4 For further detail on this topic, see the recent review by Parry et al. in Blood 2023.5
The median OS observed from nonrandomized studies of CIT from the pretargeted inhibitor CLL era typically ranged from 6 to 12 months.6 Intensification of treatment beyond standard CHOP (rituximab, doxorubicin, cyclophosphamide, vincristine, prednisolone) plus anti-CD20 monoclonal antibody (mab) therapy7,8 using infusional EPOCH-R9 (etoposide, doxorubicin, vincristine, cyclophosphamide, prednisolone, rituximab) or combinations including purine analogues,10 cytarabine,11 or platinum-based therapy12 did not improve survival outcomes but resulted in substantial treatment-related infectious morbidity and mortality. More intensive chemotherapy was clearly not better. Heavily pretreated CLL patients developing RT in the contemporary era following a targeted inhibitor such as a Bruton tyrosine kinase inhibitor (BTKi) have potentially an even worse outlook, with series demonstrating an OS of only 3–4 months.13,14
Although there are possible exceptions, such as patients with clonally unrelated DLBCL15 and TP53-intact patients with treatment-naïve CLL,16,17 these patients are generally in the minority, and routine, widespread testing of the clonal relationship of DLBCL to the underlying CLL by next generation sequencing or Sanger sequencing of the immunoglobulin heavy chain variable region (IGVH) gene or by B-cell polymerase chain reaction (PCR) clonality is limited. Most patients either do not respond to front-line CIT or progress early after an initial response, leading some to debate whether R-CHOP genuinely represents a de facto standard-of-care first-line therapy. Consolidation with allogenic stem cell transplantation (SCT) (as described in our clinical case) or autologous SCT represent standard options for patients achieving a first remission.18 A recent Center for International Blood and Transplant Research (CIBMTR) registry study evaluated outcomes for patients following autologous SCT (N = 53) or allogenic SCT (allo-SCT) (N = 118).19 The allo-SCT cohort was a higher-risk group compared with the autologous SCT cohort because a higher proportion had a 17 p deletion, more patients received prior targeted agents, and more individuals were less often in a complete remission pre-SCT. In the auto-SCT cohort, the 3-year relapse incidence, PFS, and OS were 37%, 48%, and 57%, respectively. In the allo-SCT cohort, the 3-year relapse incidence, PFS, and OS were 30%, 43%, and 52%, respectively. Depth of response prior to allo-SCT but not 17 p deletion status or prior novel agent exposure were associated with improved survival outcomes. Overall, these sizeable series suggest either approach remains valid for suitable patients obtaining a stable first remission. However, the broad applicability of allo- or auto-SCT is limited by the lack of durable disease control in first response and therefore an inherent selection in bias in published transplant series, and the patient's ability to withstand the well-documented toxicity risks with transplantation. Patient age, fitness, and comorbidity burden, and the history of CLL including prior CLL-directed treatment and its complications (eg, immunosuppression, infection, bronchiectasis), all impact these decisions.
Tight eligibility criteria for clinical trials,20 a relative lack of available RT-specific trials, a lack of histopathological diagnostic RT reporting concordance,21 RT kinetics, and a lack of RT cell lines and animal models have all impacted our ability to make progress in this disease. The sobering reality is that a high unmet medical need continues to exist for novel, efficacious, and well-tolerated treatment.
Light at the end of the tunnel?
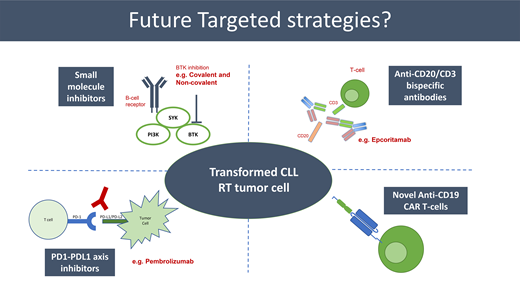
So, is there light at the end of this long and rather bleak tunnel? Despite the challenges described, broader therapeutic advances in hemato-oncology are starting to impact the RT space. This includes BTK inhibitors (reversible [covalent] and nonreversible [non-covalent]), PD1-PDL1 inhibition, BCL2 inhibition, bispecific antibodies, chimeric antigen receptor (CAR) T-cell therapy, and combination strategies. Key selected recently published data and ongoing clinical trials are presented in Tables 1 and 2, respectively.
Clinical trials: novel agents in development in Richter transformation (RT)
| Reference . | Treatment . | Number . | ORR . | Survival . |
|---|---|---|---|---|
| Eyre et al., Lancet Haem 2021 | Acalabrutinib | N = 25 | ORR 38% CR 14% | mPFS 3.2 m mDOR 5.7 m |
| Tsang et al., Blood 2015 | Ibrutinib | N = 4 | 2 PR, 1 CR, 1 clinical benefit | Median duration on treatment 6.1 m |
| Wierda et al., ASH 2022 | Pirtobrutinib | N = 75 | ORR 52% CR 13% | mPFS 3.7 m |
| Tam et al., HemaSphere 2023 | Zanubrutinib | N = 13 | ORR 61.5% CR 15.4% | mPFS 17.3 m |
| Tam et al., HemaSphere 2023 | Zanubrutinib-tislelizumab | N = 7 | ORR 42.9% CR 14.3% | mPFS 2.9 m |
| Jain et al., Blood Adv. 2022 | Ibrutinib-nivolumab | N = 24 | ORR 42% CR 34% | mOS 13 m |
| Ding et al., Blood 2017 | Pembrolizumab | N = 9 | ORR 44% CR 11% | mOS 10.7 m |
| Armand et al., BJH 2020 | Pembrolizumab | N = 23 | ORR 13% CR 4% | mOS 3.8 m |
| Davids et al., JCO 2017 | Venetoclax | N = 7 | ORR 43% No CRs | NK |
| Davids et al., Blood 2022 | Venetoclax-EPOCH-R | N = 12 evaluable N = 20 total | ORR 75% CR 67% | mPFS is 10 m mOS is 16.3 m |
| Davids et al., ICML 2023 | R-CHOP-venetoclax | N = 25 evaluable N = 27 total | ORR 68% CR 48% | mPFS 7.2 m mOS 19.5 m |
| Mato et al., ASH 2020 | Novel BTKi, DTRMWXHS-12 (DTRM-12), everolimus and pomalidomide | N = 11 | ORR 36% | NK |
| Kater et al., ASH 2022 | Epcoritamab | N = 10 | ORR 60% CR 50% | NK |
| Carlo-Stella et al., ICML 2023 | Glofitamab | N = 11 | ORR 63.6% CR 45.5% | NK |
| Reference . | Treatment . | Number . | ORR . | Survival . |
|---|---|---|---|---|
| Eyre et al., Lancet Haem 2021 | Acalabrutinib | N = 25 | ORR 38% CR 14% | mPFS 3.2 m mDOR 5.7 m |
| Tsang et al., Blood 2015 | Ibrutinib | N = 4 | 2 PR, 1 CR, 1 clinical benefit | Median duration on treatment 6.1 m |
| Wierda et al., ASH 2022 | Pirtobrutinib | N = 75 | ORR 52% CR 13% | mPFS 3.7 m |
| Tam et al., HemaSphere 2023 | Zanubrutinib | N = 13 | ORR 61.5% CR 15.4% | mPFS 17.3 m |
| Tam et al., HemaSphere 2023 | Zanubrutinib-tislelizumab | N = 7 | ORR 42.9% CR 14.3% | mPFS 2.9 m |
| Jain et al., Blood Adv. 2022 | Ibrutinib-nivolumab | N = 24 | ORR 42% CR 34% | mOS 13 m |
| Ding et al., Blood 2017 | Pembrolizumab | N = 9 | ORR 44% CR 11% | mOS 10.7 m |
| Armand et al., BJH 2020 | Pembrolizumab | N = 23 | ORR 13% CR 4% | mOS 3.8 m |
| Davids et al., JCO 2017 | Venetoclax | N = 7 | ORR 43% No CRs | NK |
| Davids et al., Blood 2022 | Venetoclax-EPOCH-R | N = 12 evaluable N = 20 total | ORR 75% CR 67% | mPFS is 10 m mOS is 16.3 m |
| Davids et al., ICML 2023 | R-CHOP-venetoclax | N = 25 evaluable N = 27 total | ORR 68% CR 48% | mPFS 7.2 m mOS 19.5 m |
| Mato et al., ASH 2020 | Novel BTKi, DTRMWXHS-12 (DTRM-12), everolimus and pomalidomide | N = 11 | ORR 36% | NK |
| Kater et al., ASH 2022 | Epcoritamab | N = 10 | ORR 60% CR 50% | NK |
| Carlo-Stella et al., ICML 2023 | Glofitamab | N = 11 | ORR 63.6% CR 45.5% | NK |
CHOP-R, doxorubicin, vincristine, cyclophosphamide, prednisolone, rituximab; CR, complete response; EPOCH-R, etoposide, doxorubicin, vincristine, cyclophosphamide, prednisolone, rituximab; m, month; mDOR, median duration of response; mOS, median overall survival; mPFS, median progression-free survival; NK, not known; ORR, overall response rate; PR, partial response.
Summary of key ongoing trials in Richter transformation (RT)
| Trial name and identifier . | Planned enrollment . | Trial design . | Novel treatment . |
|---|---|---|---|
| Bispecific antibody | |||
| EPCORE CLL-1, NCT04623541 | 102 (RT and CLL) | Single-arm phase 2 | Anti-CD20/CD3 bispecific antibody in recruiting patients with CLL or RT |
| Doublet/triplet combination | |||
| GCLLSG CLL-RT1, NCT04271956 | 52 | Single-arm phase 2 | Tislelizumab, a PD1 inhibitor, with zanubrutinib, a 2nd BTK inhibitor in R/R or 1 L RT |
| Israeli CLL Study Group, GIVeRS, NCT04939363 | 15 | Single-arm phase 2 | Obinutuzumab, ibrutinib, and venetoclax for 1 L or R/R RT |
| Acalabrutinib, Venetoclax and Durvalumab for the Treatment of RT, NCT05388006 | 33 | Single-arm phase 2 | Time-limited acalabrutinib, venetoclax, and durvalumab for patients with 1 L RT |
| Atezolizumab (PD-L1 mAb) in Combination With Obinutuzumab and Venetoclax for Patients With Chronic Lymphocytic Leukemia and Richter Transformation, NCT02846623 | 65 (RT and CLL) | Single-arm phase 2 | Time-limited atezolizumab, venetoclax, and obinutuzumab for patients with 1 L RT |
| Pirtobrutinib, Venetoclax, and Obinutuzumab, NCT05536349 | 60 (RT and CLL) | Single-arm phase 2 | Time-limited pirtobrutinib, venetoclax, and obinutuzumab for patients with 1 L CLL or RT |
| BTK inhibition | |||
| BRUIN, NCT03740529 | 82 | Single-arm phase 1/2 | Pirtobrutinib monotherapy in 1 L and R/R RT |
| Chemoimmunotherapy plus targeted inhibitor | |||
| NCRI STELLAR trial, NCT03899337 | 60 | Randomized phase 2 | R-CHOP versus R-CHOP-acalabrutinib in 1 L RT |
| Venetoclax Plus Dose-Adjusted R-EPOCH or R-CHOP for RT, NCT03054896 | 66 | Single-arm phase 2 | Venetoclax plus dose-adjusted R-EPOCH (N = 26) or R-CHOP (N = 40) for 1 L RT |
| CAR-T–based combinations | |||
| Lisocabtagene Maraleucel, Nivolumab and Ibrutinib for the Treatment of RT, NCT05672173 | 20 | Single-arm phase 2 | Lisocabtagene maraleucel, nivolumab, and ibrutinib |
| Trial name and identifier . | Planned enrollment . | Trial design . | Novel treatment . |
|---|---|---|---|
| Bispecific antibody | |||
| EPCORE CLL-1, NCT04623541 | 102 (RT and CLL) | Single-arm phase 2 | Anti-CD20/CD3 bispecific antibody in recruiting patients with CLL or RT |
| Doublet/triplet combination | |||
| GCLLSG CLL-RT1, NCT04271956 | 52 | Single-arm phase 2 | Tislelizumab, a PD1 inhibitor, with zanubrutinib, a 2nd BTK inhibitor in R/R or 1 L RT |
| Israeli CLL Study Group, GIVeRS, NCT04939363 | 15 | Single-arm phase 2 | Obinutuzumab, ibrutinib, and venetoclax for 1 L or R/R RT |
| Acalabrutinib, Venetoclax and Durvalumab for the Treatment of RT, NCT05388006 | 33 | Single-arm phase 2 | Time-limited acalabrutinib, venetoclax, and durvalumab for patients with 1 L RT |
| Atezolizumab (PD-L1 mAb) in Combination With Obinutuzumab and Venetoclax for Patients With Chronic Lymphocytic Leukemia and Richter Transformation, NCT02846623 | 65 (RT and CLL) | Single-arm phase 2 | Time-limited atezolizumab, venetoclax, and obinutuzumab for patients with 1 L RT |
| Pirtobrutinib, Venetoclax, and Obinutuzumab, NCT05536349 | 60 (RT and CLL) | Single-arm phase 2 | Time-limited pirtobrutinib, venetoclax, and obinutuzumab for patients with 1 L CLL or RT |
| BTK inhibition | |||
| BRUIN, NCT03740529 | 82 | Single-arm phase 1/2 | Pirtobrutinib monotherapy in 1 L and R/R RT |
| Chemoimmunotherapy plus targeted inhibitor | |||
| NCRI STELLAR trial, NCT03899337 | 60 | Randomized phase 2 | R-CHOP versus R-CHOP-acalabrutinib in 1 L RT |
| Venetoclax Plus Dose-Adjusted R-EPOCH or R-CHOP for RT, NCT03054896 | 66 | Single-arm phase 2 | Venetoclax plus dose-adjusted R-EPOCH (N = 26) or R-CHOP (N = 40) for 1 L RT |
| CAR-T–based combinations | |||
| Lisocabtagene Maraleucel, Nivolumab and Ibrutinib for the Treatment of RT, NCT05672173 | 20 | Single-arm phase 2 | Lisocabtagene maraleucel, nivolumab, and ibrutinib |
BTK, Bruton tyrosine kinase; CAR-T, chimeric antigen receptor T cell; CHOP-R, doxorubicin, vincristine, cyclophosphamide, prednisolone, rituximab; CLL, chronic lymphocytic leukemia; EPOCH-R, etoposide, doxorubicin, vincristine, cyclophosphamide, prednisolone, rituximab; GCLLSG, German chronic lymphocytic leukemia study group; NCRI, National Cancer Research Institute; R/R, relapsed/refractory; RT, Richter transformation.
BTK inhibitors
Covalent BTK inhibitors have been transformational in CLL, mantle cell lymphoma (MCL), and Waldenström macroglobulinemia and have demonstrated some efficacy as monotherapy in RT. The second-generation BTKi acalabrutinib was shown to be active in 25 RT patients (including relapsed RT), with an overall response rate (ORR) of 40% (complete response [CR] 8%) and a median duration of response (mDOR) of 6.2 months.22 Small case series (N = 4) have shown activity with ibrutinib (2 PR, 1 CR, 1 clinical response).23 Two small recently published series24 suggest activity with the second-generation BTKi zanubrutinib as monotherapy (ORR 61.5%, CR 15.4%) and combination with the PD1 inhibitor tislelizumab (ORR 42.9%, CR 14.3%). A relatively large phase 2 GCLLSG (NCT04271956) group cooperative CLL-RT1 trial (N = 52) of zanubrutinib in combination with the tislelizumab25 has fully enrolled and the results are eagerly awaited. The National Cancer Research Institute UK-wide first-line STELLAR trial is currently enrolling to test whether the addition of acalabrutinib to R-CHOP provides a progression-free survival (PFS) improvement compared with R-CHOP alone.26 This is the first and currently the only randomized clinical trial globally in RT.
Pirtobrutinib is a first-in-class, noncovalent, reversible BTKi. Pirtobrutinib inhibits both wildtype and C481-mutant BTK with equal low nanomolar potency and has a favorable oral pharmacology that enables continuous BTK inhibition throughout the dosing interval regardless of intrinsic rate of BTK turnover.27 Drug plasma exposures exceeds BTK IC90 throughout the 24-hour dosing interval. These favorable pharmacokinetic properties may enable enhanced therapeutic activity in more highly proliferative tumors that remain dependent on B-cell receptor signaling, such as MCL and RT. The phase 1/2 BRUIN trial has recruited 82 RT patients with efficacy data available for 75 patients to date and included 68 patients who had received prior RT treatment (median prior lines of RT treatment was 2 [0–8]).28 The ORR was 52% and CR rate 10%, with an ORR of 47% in patients who received a prior covalent BTKi and an ORR of 50% with RT who had received prior RT-directed therapy. The mDOR was 5.6 months, median PFS 3.7 months and median OS 13.1 months. The toxicity prolife for pirtobrutinib across all B-cell histologies (N = 773) and the RT cohort (N = 82) was favorable, with only 2.6% discontinuing due to treatment-related adverse events and only 4.5% requiring dose reductions, lending itself well to future combination strategies. The combination of time-limited pirtobrutinib-venetoclax-obinutuzumab is currently being studied in RT (NCT05536349).
PD1-PDL1 axis inhibitors
The PD1-PDL1 axis is known to be upregulated in the RT microenvironment, although the data regarding efficacy of PD1-PD1L axis inhibition are mixed. A small series (N = 9) provides proof of principle of the activity of PD1 inhibitors in RT. Pembrolizumab monotherapy delivered at 200 mg every 3 weeks has demonstrated an ORR of 44%, a CR rate of 11%, and a median PFS of 10.7 months.29 A trend of increased expression in PD1 was observed in the tumor microenvironment in RT patients who had confirmed responses. PD1 inhibitors are also well tolerated, and combination strategies have also been tested. The nivolumab-ibrutinib combination provided an ORR of 42%, with potentially deeper responses than with a PD1 inhibitor or BTKi alone (CR rate 34%).30 Less encouraging was a small nontrial cohort (n = 10) from The Ohio State who had only a 10% ORR with PD1 inhibitor combinations/monotherapy and a trial cohort of 23 patients in which the ORR was only 13%.31,32 Ongoing trials are testing triplet combinations including a PD1-PD-L1 axis inhibitor in RT, namely acalabrutinib, venetoclax, and durvalumab (PD-L1 mab) (NCT05388006) and obinutuzumab, venetoclax, and atezolizumab (PD-L1 mab) (NCT02846623).
B cell lymphoma 2 inhibitors
Promising early data of the BCL2 inhibitor venetoclax in RT patients has led to its exploration in combination with both targeted inhibitors and CIT. Initially, responses were seen in 3 of 7 patients receiving monotherapy in a B-cell malignancy basket phase 1 trial.33 The combination of venetoclax with standard CIT has been explored in a first-line single-arm phase 2 trial34 with the hypothesis that the BCL2 inhibitor may sensitize the RT tumor to CIT. Venetoclax was delivered with an accelerated daily ramp-up to the target dose of 400 mg after cycle 1 and continued across the following 5 cycles of CIT. The CR rate for 26 patients receiving venetoclax plus dose-adjusted EPOCH-R was 50%, with 11 achieving bone marrow minimal residual disease for the CLL disease component. The ORR was 62%, median PFS 10.1 months, and median OS 19.6 months. Hematological toxicity was notable in this study, with grade ≥3 neutropenia in 65%, febrile neutropenia in 38%, and a single fatal episode of sepsis observed with venetoclax-EPOCH-R. Daily venetoclax ramp-up was safe with no tumor lysis syndrome events reported. The encouraging deep and durable responses have led to an extension of this study with a total of 67 patients enrolled (NCT03054896), and the final results are awaited. The CIT backbone was deintensified from dose-adjusted EPOCH-R to R-CHOP because of excess toxicity (cytopenias, infection). Forty patients received R-CHOP-venetoclax, an approach that enabled outpatient delivery (personal communication), with initial results of the first 27 patients presented at ICML 2023 (ORR 68%, CR 48%).35 A real-world analysis36 from the Mayo Clinic and MD Anderson suggests that R-CHOP-venetoclax may improve PFS compared with standard CIT approaches or the BTKi-BCL2i-Obinutuzumab triplet, although a prospective randomized first-line trial is required to formally answer this question. BCL2 targeting has also formed part of a range of combination strategies in ongoing, enrolling clinical trials as already discussed (NCT05388006, NCT05536349, NCT02846623, NCT04939363).
Anti-CD20-CD3 bispecific antibodies
Early data with the anti-CD20-CD3 bispecific antibody epcoritamab have been recently presented.37 Epcoritamab and glofitamab bind to CD3 on T cells and CD20 on B cells to induce T-cell–mediated killing of CD20-positive malignant B cells. Bispecific antibody development in RT/CLL has been slow compared with DLBCL and follicular lymphoma (FL) in part because of the rarity of the phenomenon (RT) but also because of (a) the risk of severe cytokine release syndrome considering the circulating peripheral blood CLL component and (b) T cell dysfunction in CLL patients. Epcoritamab is delivered subcutaneously to progression or intolerance whereas glofitamab is delivered intravenously for a fixed duration. The initial results from the RT cohort in the ongoing phase 1b/2 EPCORE CLL-1 trial observed an ORR of 60% and CR of 50% with epcoritamab in 10 RT patients.37 This study (NCT04623541) continues to accrue patients (the aim is for 102 RT or CLL patients), and we await a mature larger data set with great interest. Recently, responses were reported in 63.6% (CR 45.5%) in 11 RT patients receiving glofitamab38 providing further early evidence of anti-CD20-CD3 bispecific antibody activity in RT.
Chimeric antigen receptor (CAR) T-cell therapy
Finally, anti-CD19–directed CAR-T therapy has changed the treatment paradigm of relapsed, refractory large B-cell lymphoma,39 MCL,40 and FL41 over recent years. Although data specifically in RT remain limited, the treatment approach is highly promising. Two recently published real-world series from the US42 and Israel43 have demonstrated ORRs of 89% with Axi-cel (N = 9, CR N = 5) and 71% (N = 8 including 1 “accelerated CLL” and 1 prolymphocytic transformation, CR N = 5), respectively. Global availability of anti-CD19 CAR-T-cell therapy for RT remains highly variable. At present, CAR-T therapy is considered a standard treatment option in the third-line setting in the United Kingdom for RT patients who have received ≥2 prior DLBCL treatments including R-CHOP.44 An important ongoing clinical trial is assessing the anti-CD19 CAR-T lisocabtagene maraleucel in combination with nivolumab and ibrutinib. Both nivolumab and ibrutinib have the potential to enhance the activity of CAR-T-cell therapy via independent mechanisms (PD1-PDL1 axis and upregulation of T-cell activity via ITK inhibition,45 respectively), and both also provide direct anti-RT tumor activity (NCT05672173).
Future targets?
Most current studies involve combinatorial approaches targeting BTK, BCL2, or PD1 or integrating T-cell engaging therapy. Examples of future targets beyond these approaches may include targeting the feto-embryonic antigen ROR1, the MAPK pathway,3 MYC/mTOR-PI3K signaling the cell cycle regulator CDK9, and the nuclear pore complex (XPO1).5
Future directions
The recent explosion in targeted therapeutics across hemato-oncology has started to impact RT management. Promising targets include inhibition of BTK, BCL2, the PD1-PDL1 axis, and T-cell–activating/engaging therapies. Many of these therapies are particularly well tolerated and lend themselves well to combinatory studies. Despite this promise, no agents have been licensed or reimbursed specifically for RT in the United States and Europe. Genuine progress in RT suffers from the relative rarity of the disease, the small commercial impact of any potential future drug approval, and the consequent lack of investment into registrational trials from major industry partners. This must change if we are to see the impact of novel agents in this catastrophic disease—the light at the end of the tunnel. Although the portfolio of agents studied over recent years is increasingly impressive, the relative lack of clinically meaningful, correlative biological sub-studies is also noteworthy. The role of precision medicine will become increasingly important in a disease where multiple agents have potential efficacy but typically modest response rates. Carefully designed, planned correlative biological embedded studies should be strongly encouraged within future clinical trials to help discover which patient benefits from which class or combination of novel targets.
Conflict-of-interest disclosure
Toby A. Eyre: Roche: education honorarium, advisory board honorarium, travel to scientific conferences; Gilead: honorarium; research support, travel to scientific conferences; KITE: education honorarium, advisory board honorarium; Janssen: honorarium; AbbVie: honorarium, travel to scientific conferences; AstraZeneca: honorarium, research funding, travel to scientific conferences; Loxo Oncology: advisory board honorarium, trial steering committee; Beigene: advisory board honorarium, research funding; Incyte: advisory board honorarium; Secura Bio: advisory board honorarium; Autolus: advisory board honorarium.
Off-label drug use
Toby A. Eyre: Nothing to disclose.