Abstract
Patients with relapsed and refractory (R/R) aggressive B-cell non-Hodgkin lymphomas have historically poor survival outcomes, with chimeric antigen receptor T-cell (CAR-T) therapy now presenting a curative option for a subset of those patients. However, with the approval of several novel bispecific monoclonal antibody (BsAb) therapies with considerable activity in R/R aggressive large B-cell lymphomas (LBCL), patients and oncologists will be faced with decisions regarding how to sequence CAR-T and BsAb therapies based on patient- and disease-related factors. In this review, we compare CAR-T and BsAb therapies for R/R LBCL, highlighting data on the efficacy and toxicity of each treatment paradigm, and provide a roadmap for sequencing these highly effective therapies.
Learning Objectives
Understand the efficacy and safety of chimeric antigen receptor T-cell (CAR-T) and bispecific antibody therapies for aggressive large B-cell lymphoma in the third-line setting
Examine the real-world efficacy and safety of CAR-T therapies in patients who were not eligible for the initial registrational trials
Compare advantages and disadvantages of initial sequencing of CAR-T first vs bispecific antibodies first in relapsed and refractory large B-cell lymphomas
Introduction
Survival of patients with relapsed and refractory (R/R) aggressive large B-cell lymphomas (LBCLs) was historically dismal in the chemotherapy era. However, the regulatory approval of chimeric antigen receptor T-cell (CAR-T) therapy has not only improved outcomes for heavily pretreated patients with R/R LBCL but also heralded a new epoch of immunotherapeutic trials in non-Hodgkin lymphomas (NHLs). Leading these trials are CD20-directed bispecific monoclonal antibody (BsAb) therapies, which have shown excellent efficacy and safety in R/R LBCL. With new regulatory approval of several BsAb agents, it is imperative to conceptualize a roadmap for sequencing these agents with CAR-T therapy in the treatment paradigm of aggressive LBCL. In this review, we will compare CAR-T and BsAb therapies for R/R LBCL and propose a treatment algorithm to guide practicing clinicians, highlighting data and arguments for the use of CAR-T first vs BsAb first in R/R LBCL.
CLINICAL CASE
A 70-year-old man with a good performance status and history of atrial fibrillation was diagnosed with stage IV diffuse large B-cell lymphoma (DLBCL) (germinal center cell of origin, MYC-amplified) 3 years ago and received 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone with a complete response (CR) at the end of treatment. He relapsed 15 months later and subsequently received second-line chemotherapy followed by autologous stem cell transplantation (ASCT). He did well for 11 months when he unfortunately developed a biopsy-proven relapse of DLBCL.
Third-line CAR-T therapy for aggressive LBCL
There are 3 CAR-T constructs that are approved by the US Food and Drug Administration (FDA) in the third-line setting for LBCL: axicabtagene ciloleucel (axi-cel) in 2017, tisagenlecleucel (tisa-cel) in 2018, and lisocabtagene maraleucel (liso-cel) in 2021. These FDA approvals were based on the ZUMA-1, JULIET, and TRANSCEND trials (Table 1). The long-term curative potential for CAR-T therapy has been demonstrated with axi-cel, with 5-year follow-up data demonstrating a progression-free survival (PFS) plateau of 32% and a disease-specific survival of 51%,1 as well as with CTL019 (tisa-cel) with a 5-year PFS of 31%.2 Although clearly with curative potential, CAR-T therapy can be associated with unique toxicities, with cytokine release syndrome (CRS) and neurotoxicity noted with all CAR-T constructs, as well as prolonged cytopenias in up to 45% of cases which can impact post–CAR-T outcomes.3 The TRANSCEND trial included more broad inclusion criteria than the other trials, including patients with older age, secondary central nervous system (CNS) involvement, and mild renal and cardiac insufficiency, and with no minimum absolute lymphocyte count requirement for apheresis. The efficacy and toxicity of CAR-T products in the third-line setting have not been directly compared. Indirect comparisons between the ZUMA-1 and TRANSCEND trials have demonstrated comparable efficacy between axi-cel and liso-cel with lower rates of toxicities with liso-cel, underscoring differing functions of the constructs' costimulatory domains (CD28 vs 4-1BB).4
Clinical trials of CAR T-cell and bispecific antibodies therapies in the third-line setting for aggressive LBCL
| Clinical trial . | Construct . | Patients . | Histologies . | Response rates . | Survival outcomes . | Longest median follow-up . | Duration of response . | Rates of CRS . | Rates of neurotoxicity . | Treatment-related mortality . | FDA approved . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAR-T therapies for third-line LBCL | |||||||||||
| ZUMA-11,5 | Axi-cel | 101 | DLBCL, PMBCL, tFL | ORR 82% (CR 54%) | mPFS 5.9 mo 12 mo: PFS 44%, OS 59% 5 y: PFS 32%, OS 43% | 63.1 mo | mDOR 11.1 mo mDOCR 62.2 mo | 93% (13% grade 3+) | 64% (28% grade 3+) | 3 patients (3%), 1 death due to CRS, 1 death due to HLH | Yes |
| JULIET2,6 | tisa-cel | 115 | DLBCL, tFL, HGBL | ORR 53% (CR 39%) | mPFS 2.9 mo 12 mo: RFS 65%, OS 49% 40 mo: PFS 38%, OS 39% 5 y: PFS 31% | 60.7 mo | mDOR NR | 58% (22% grade 3+) | 21% (12% grade 3+) | 0 patients | Yes |
| TRANSCEND7 | Liso-cel | 256 | DLBCL, tFL, HGBL, PMBCL, FL 3B | ORR 73% (CR 53%) | mPFS 6.8 mo 1 y: PFS 44%, OS 58% | 18.8 mo | mDOR 17 mo | 42% (2% grade 3+) | 30% (10% grade 3+) | 7 patients (3%) | Yes |
| Bispecific antibody therapies for third-line LBCL | |||||||||||
| NCT030756968,9 | Glofitamab | 154 51 (33%) with prior CAR-T exposure | DLBCL, tFL, HGBL, PMBCL | ORR 52% (CR 39%) Prior CAR-T: CR 35% | mPFS 4.9 mo 1 y: PFS 37%, OS 50% | 12.6 mo | mDOR 18.4 mo mDOCR NR (74% remained in CR at median of 18.1 mo of follow-up) | 63% (4% grade 3+) | 8% (3% grade 3+) | 8 patients (5%) | Yes (June 2023) for LBCL after at least 2 lines of therapy |
| EPCORE NHL-1 (NCT03625037)10,11 | Epcoritamab (dose escalation) | 68 46 with LBCL | Any relapsed or refractory CD20+ mature B-cell NHL | ORR 68% (CR 45%) for LBCL | mPFS for LBCL: 9.1 mo | 9.3 mo | 75% of LBCL responders in ongoing remission at 6 mo | 59% (no grade 3+) | 6% (3% grade 3+) | 0 patients | Yes (May 2023) for LBCL after at least 2 lines of therapy |
| Epcoritamab (dose expansion) | 157 61 (39%) with prior CAR-T exposure | DLBCL, PMBCL, HGBL, FL 3B | ORR 63% (CR 39%) Prior CAR-T: ORR 54% (CR 34%) | mPFS 4.4 mo 6 mo: PFS 44% mPFS NR among patients with CR | 10.7 mo | mDOR 12 mo (9.7 mo if prior CAR-T) mDOCR NR (NR if prior CAR-T) | 50% (2.5% grade 3+) | 6% (0.6% grade 3+) | 9 patients (6%), 1 death due to ICANS | ||
| NCT0250040712,13 | Mosunetuzumab (phase I data) | 197 129 with aggressive NHL | Any relapsed or refractory B-cell NHL | ORR 35% (CR 19%) | mPFS 1.4 mo | 11.9 mo | mDOR 7.6 mo mDOCR 23 mo | 27% (1% grade 3+) | 10-18% (4% grade 3+) | 3 patients (1.5%) | Yes, for relapsed or refractory FL |
| Mosunetuzumab (phase II data) | 88 | DLBCL, tFL, HGBL | ORR 42% (CR 24%) Prior CAR-T: ORR 23% (CR 12%) | mPFS 3.2 mo mOS 11.5 mo Prior CAR-T: mPFS 1.4 mo | 10.1 mo | mDOR 7.0 mo mDOCR NR | 26% (2.3% grade 3+) | 1% (no grade 3+) | 3 patients (3.4%) | ||
| ELM-1 (NCT02290951)14 | Odronextamab | 85 with LBCL 33 (39%) of LBCL with prior CAR-T exposure | Any relapsed or refractory B-cell NHL | No prior CAR-T: ORR 53% (CR 53%) Prior CAR-T: ORR 33% (CR 27%) | No prior CAR-T: mPFS 11.5 mo Prior CAR-T: mPFS 2.0 mo | 4.2 mo | No prior CAR-T: mDOR NR Prior CAR-T: mDOR NR | 54% (7% grade 3+) | 12% (3% grade 3+) | 7 patients (5%) | No |
| Clinical trial . | Construct . | Patients . | Histologies . | Response rates . | Survival outcomes . | Longest median follow-up . | Duration of response . | Rates of CRS . | Rates of neurotoxicity . | Treatment-related mortality . | FDA approved . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAR-T therapies for third-line LBCL | |||||||||||
| ZUMA-11,5 | Axi-cel | 101 | DLBCL, PMBCL, tFL | ORR 82% (CR 54%) | mPFS 5.9 mo 12 mo: PFS 44%, OS 59% 5 y: PFS 32%, OS 43% | 63.1 mo | mDOR 11.1 mo mDOCR 62.2 mo | 93% (13% grade 3+) | 64% (28% grade 3+) | 3 patients (3%), 1 death due to CRS, 1 death due to HLH | Yes |
| JULIET2,6 | tisa-cel | 115 | DLBCL, tFL, HGBL | ORR 53% (CR 39%) | mPFS 2.9 mo 12 mo: RFS 65%, OS 49% 40 mo: PFS 38%, OS 39% 5 y: PFS 31% | 60.7 mo | mDOR NR | 58% (22% grade 3+) | 21% (12% grade 3+) | 0 patients | Yes |
| TRANSCEND7 | Liso-cel | 256 | DLBCL, tFL, HGBL, PMBCL, FL 3B | ORR 73% (CR 53%) | mPFS 6.8 mo 1 y: PFS 44%, OS 58% | 18.8 mo | mDOR 17 mo | 42% (2% grade 3+) | 30% (10% grade 3+) | 7 patients (3%) | Yes |
| Bispecific antibody therapies for third-line LBCL | |||||||||||
| NCT030756968,9 | Glofitamab | 154 51 (33%) with prior CAR-T exposure | DLBCL, tFL, HGBL, PMBCL | ORR 52% (CR 39%) Prior CAR-T: CR 35% | mPFS 4.9 mo 1 y: PFS 37%, OS 50% | 12.6 mo | mDOR 18.4 mo mDOCR NR (74% remained in CR at median of 18.1 mo of follow-up) | 63% (4% grade 3+) | 8% (3% grade 3+) | 8 patients (5%) | Yes (June 2023) for LBCL after at least 2 lines of therapy |
| EPCORE NHL-1 (NCT03625037)10,11 | Epcoritamab (dose escalation) | 68 46 with LBCL | Any relapsed or refractory CD20+ mature B-cell NHL | ORR 68% (CR 45%) for LBCL | mPFS for LBCL: 9.1 mo | 9.3 mo | 75% of LBCL responders in ongoing remission at 6 mo | 59% (no grade 3+) | 6% (3% grade 3+) | 0 patients | Yes (May 2023) for LBCL after at least 2 lines of therapy |
| Epcoritamab (dose expansion) | 157 61 (39%) with prior CAR-T exposure | DLBCL, PMBCL, HGBL, FL 3B | ORR 63% (CR 39%) Prior CAR-T: ORR 54% (CR 34%) | mPFS 4.4 mo 6 mo: PFS 44% mPFS NR among patients with CR | 10.7 mo | mDOR 12 mo (9.7 mo if prior CAR-T) mDOCR NR (NR if prior CAR-T) | 50% (2.5% grade 3+) | 6% (0.6% grade 3+) | 9 patients (6%), 1 death due to ICANS | ||
| NCT0250040712,13 | Mosunetuzumab (phase I data) | 197 129 with aggressive NHL | Any relapsed or refractory B-cell NHL | ORR 35% (CR 19%) | mPFS 1.4 mo | 11.9 mo | mDOR 7.6 mo mDOCR 23 mo | 27% (1% grade 3+) | 10-18% (4% grade 3+) | 3 patients (1.5%) | Yes, for relapsed or refractory FL |
| Mosunetuzumab (phase II data) | 88 | DLBCL, tFL, HGBL | ORR 42% (CR 24%) Prior CAR-T: ORR 23% (CR 12%) | mPFS 3.2 mo mOS 11.5 mo Prior CAR-T: mPFS 1.4 mo | 10.1 mo | mDOR 7.0 mo mDOCR NR | 26% (2.3% grade 3+) | 1% (no grade 3+) | 3 patients (3.4%) | ||
| ELM-1 (NCT02290951)14 | Odronextamab | 85 with LBCL 33 (39%) of LBCL with prior CAR-T exposure | Any relapsed or refractory B-cell NHL | No prior CAR-T: ORR 53% (CR 53%) Prior CAR-T: ORR 33% (CR 27%) | No prior CAR-T: mPFS 11.5 mo Prior CAR-T: mPFS 2.0 mo | 4.2 mo | No prior CAR-T: mDOR NR Prior CAR-T: mDOR NR | 54% (7% grade 3+) | 12% (3% grade 3+) | 7 patients (5%) | No |
FL 3B, grade 3B follicular lymphoma; HGBL, high-grade B-cell lymphoma; HLH, hemophagocytic lymphohistiocystosis; ICANS, immune effector cell-associated neurotoxicity syndrome; mDOCR, median duration of complete response; mDOR, median duration of response; mOS, median overall survival; mPFS, median progression-free survival; NR, not reached; PMBCL, primary mediastinal large B-cell lymphoma; tFL, transformed follicular lymphoma.
Second-line CAR-T therapy for aggressive LBCL
ASCT is curative in less than 30% of patients with transplant-eligible R/R LBCL in the second-line setting, and its efficacy is particularly poor for patients with primary refractory or early-relapsed LBCL.15 Given the considerable activity of CAR-T therapy in LBCL in the third-line setting, especially for patients with relapse after ASCT, it was intuitive to assess the efficacy of CAR-T in the second- line setting in patients with high-risk R/R LBCL. There have been 3 large randomized controlled trials of CAR-T vs standard of care (SOC) in the second-line setting, with SOC consisting of second- line chemoimmunotherapy followed by ASCT in transplant- eligible high-risk primary refractory or early-relapsed LBCL within 12 months of initial therapy: ZUMA-7, BELINDA, and TRANFORM. ZUMA-7 and TRANSFORM met their primary end points of event-free survival, while BELINDA did not. In ZUMA-7, 359 patients with high-risk R/R LBCL were randomized to axi-cel vs SOC.16 Median event-free survival (EFS) was significantly higher with axi-cel at 8.3 months compared to 2 months for standard care, with 2-year EFS of 41% and 16% for axi-cel and standard care, respectively. Improved outcomes with axi-cel were also observed in patients aged ≥65 years.17 Although there was no significant difference in overall survival (OS) initially reported, updated statistically significant OS results have now been reported in favor of axi-cel.18 The TRANSFORM trial of 184 patients randomized to liso-cel vs SOC utilized a similar trial design as ZUMA-7, although crossover to the liso-cel arm was allowed per protocol. Similarly, median EFS was superior with liso-cel (not reached vs 2.4 months) with 18-month EFS of 53% and 21% for liso-cel and standard care, respectively.19 In a prespecified OS analysis adjusting for crossover, there was a trend toward improvement in OS in the liso-cel arm, with 18-month OS rates of 73% for liso-cel and 54% for SOC. In both ZUMA-7 and TRANSFORM, there were no deaths due to CRS or neurotoxicity.
Given the favorable efficacy and toxicity profile of liso-cel in transplant-eligible R/R LBCL, PILOT was a phase 2 single-arm trial that investigated the efficacy of liso-cel in 74 transplant- ineligible patients with R/R LBCL in the second-line setting.20 The overall response rate (ORR) was 80% (CR 54%) with a median PFS and OS of 9 months and not reached, respectively; for patients who achieved a CR, median PFS and OS were 23 months and not reached, respectively.
Based on the positive results of the ZUMA-7, TRANSFORM, and PILOT trials, the FDA approved both axi-cel and liso-cel for patients with high-risk LBCL in first relapse, as well as liso-cel in transplant-ineligible patients after failure of 1 line of therapy.
Third-line CD20-directed bispecific antibody therapy for aggressive LBCL
BsAb therapies cotarget tumor antigens and endogenous immune effector cells to induce tumor killing by recruiting and activating peripheral and intratumoral T cells, natural killer cells, and/or macrophages to the tumor microenvironment. In B-cell lymphomas, several off-the-shelf IgG-type BsAb agents have been developed that cotarget CD20 and CD3 for T-cell–mediated cytotoxicity—namely, mosunetuzumab, glofitamab, epcoritamab, and odronextamab (Table 1). Both glofitamab and epcoritamab have been studied in third-line LBCL and received FDA approval in mid-2023. In a phase 2 study of 154 patients with LBCL who received up to 12 cycles of fixed-duration intravenous glofitamab after at least 2 prior lines of therapy, ORR was 52% (CR 39%) with 1-year PFS and OS of 37% and 50%, respectively.8 Importantly, the 33% of patients in the study who had previously received CAR-T had a similar CR rate of 35%. Most patients who achieved a CR had durable remissions, with a median duration of CR not reached at 18.1 months of follow-up.9 Although CRS was common (63%), only 4% were high-grade events and none were grade 5; any-grade neurotoxicity occurred in 8% of patients with only 3% of high grade.
In a phase 1/2 study of 157 patients with LBCL after at least 2 lines of previous therapy who received subcutaneous epcoritamab until disease progression or unacceptable toxicity, ORR was 63% (CR 39%) with a median duration of response (DOR) of 12 months and 12-month PFS of 38%.10,11 Thirty-nine percent of patients had previously received CAR-T with an ORR of 54% (CR 34%) with median DOR of 9.7 months. Similar to glofitamab, CRs were durable after epcoritamab with median duration of CR not reached at 10.7 months of follow-up, including in post–CAR-T patients. CRS was also common with epcoritamab (50%), and although only 2.5% of events were high-grade CRS with no grade 5 events, there was 1 treatment-related death due to neurotoxicity in the study.
Although mosunetuzumab was FDA approved in December 2022 for R/R follicular lymphoma, activity was significantly lower in LBCL in a phase 1 study, with ORR 35% (CR 19%) as compared to ORR 66% (CR 49%) in indolent NHL, with a median PFS of 1.4 months in aggressive NHL.12 Follow-up phase 2 data from this study in 88 patients with R/R LBCL demonstrated ORR 42% (CR 24%) with a median PFS of 3.2 months, with a lower CR rate of 12% in patients who had previously received CAR-T.13 Combinations of other agents with mosunetuzumab have been investigated to improve efficacy, including polatuzumab vedotin with mosunetuzumab in R/R LBCL, which has demonstrated an ORR of 72% (CR 56%) in older patients who may not be candidates for CAR-T or who are relapsed after CAR-T.21
The durable remissions achieved in patients with heavily pretreated and poor-prognosis LBCL, including after CAR-T and with fixed-duration regimens such as glofitamab, have generated considerable interest in BsAb therapies, particularly given the low incidence of high-grade CRS and neurotoxicity. Further, both glofitamab and epcoritamab demonstrated a relatively fast time to response of 1.4 months, which is particularly important for an off-the-shelf therapeutic that does not require manufacturing. Other novel CD20xCD3 BsAb agents are under development, including odronextamab and plamotamab with ORR 33% to 53% (CR 27%-53%) and ORR 47% (CR 26%) in LBCL, respectively.14,22 Given the encouraging results of these therapies in heavily pretreated LBCL, several trials with BsAb therapies are ongoing in earlier lines of therapy either as single agents or in combination with chemotherapy. These results are awaited to clarify how BsAb therapies may be best sequenced after first-line therapy.23
Case for CAR-T therapy first
The primary advantage of sequencing CAR-T therapy before BsAb therapy in the third-line LBCL setting is the potential for cure with CAR-T in approximately one-third of patients at 5 years of follow-up, which has not yet been demonstrated with BsAb agents. However, given the higher risk of toxicities with CAR-T compared to BsAb therapies, particularly CRS and neurotoxicity (Table 1) as well as prolonged cytopenias and infections, there is concern that administering CAR-T to patients who did not meet the inclusion criteria of the initial trials may incur excess toxicity and mortality. Several retrospective, “real-world” studies of CAR-T outcomes have demonstrated comparable survival and safety outcomes when CAR-T was administered to patients who would have been ineligible for the original clinical trials due to advanced age, comorbidities, or performance status (Table 2). These real-world studies also demonstrated durable remissions in patients who achieved CR, suggesting the possibility for cure in trial-ineligible patients.24,25 Toxicities also appear to be comparable outside of the trial setting, with preserved survival rates despite higher use of tocilizumab and steroids in the real-world setting.24 Longitudinal improvements in quality of life and patient-reported outcomes after CAR-T have been demonstrated in both clinical trials and real-world studies,26,27 further supporting the tolerability of CAR-T outside of the trial setting.
Real-world CAR-T outcomes in third-line LBCL
| Real-world study design . | Constructs . | Patients . | Percent of patients ineligible for CAR-T clinical trials . | Response rates . | Survival outcomes . | Median duration of response . | Rates of CRS . | Rates of neurotoxicity . | Treatment-related mortality . | Conclusions . |
|---|---|---|---|---|---|---|---|---|---|---|
| Locke et al.28 Postapproval safety observational study | Axi-cel | 1343 | 38% aged ≥65, 4% with ECOG PS 2+, 13% cardiac comorbidities, 2% hepatic comorbidities, 2% renal comorbidities, 15% double/ triple-hit lymphoma, 66% refractory disease | ORR 74% (CR 56%) ORR 78% (CR 62%) for patients aged ≥65 ORR 57% (CR 29%) for hepatic comorbidities ORR 70% (CR 43%) for renal comorbidities ORR 47% (CR 20%) for ECOG 2-3 | 18 mo: PFS 42%, OS 52% | 18 mo: DOR 61% | 83% | 55% | Not assessed | Patients aged ≥65 with moderate to severe pulmonary disease and ECOG 2-3 had inferior ORR. Age ≥65 was not associated with inferior survival, but was associated with higher rates of CRS and ICANS. ECOG PS significantly affected all efficacy outcomes. |
| Jacobson et al.25 Postauthorization safety study through the CIBMTR registry | Axi-cel | 1297 | 57% were ineligible for ZUMA-1 | ORR 73% (CR 56%) | mPFS 8.6 mo mOS 21.8 mo | mDOR NR 24-mo DOR 57% | 83% (8% grade 3+) | 55% (24% grade 3+) | 3%, 2% died from CRS, 1% died from ICANS | Real-world response rates were similar to ZUMA-1, with similar DOR in patients who were and were not eligible for ZUMA-1. High-grade CRS and ICANS were lower in the real-world cohort. ECOG PS of 2 or greater was associated with inferior response rates, PFS, and OS. Patients age ≥65 had a higher ORR despite higher risk of CRS and ICANS as compared to younger patients. |
| Landsburg et al.29 Observational study through the CIBMTR registry | Tisa-cel | 1159 | 31% ineligible for JULIET | ORR 60% | 2 y: PFS 28%, OS 44% | 2 y: DOR 53% | 58% (6% grade 3+) | 23% (7% grade 3+) | Not assessed | Real-world outcomes were similar to JULIET. Patients with comorbidities (who were not eligible for JULIET) had similar efficacy outcomes. Patients with ECOG PS 2-4 had higher rates of high-grade CRS and ICANS but similar efficacy outcomes. |
| Bachy et al.30 Retrospective French DESCAR-T registry study with propensity score matching between axi-cel and tisa-cel | Axi-cel Tisa-cel | 452 axi-cel 277 tisa-cel | Not assessed | Axi-cel: ORR 80% (CR 60%) Tisa-cel: ORR 66% (CR 42%) | 1 y: PFS 47% axi-cel, 33% tisa-cel OS 64% axi-cel, 49% tisa-cel | 1 y: DOR 54% axi-cel, 42% tisa-cel | Axi-cel: 86% (5% grade 3+) Tisa-cel: 76% (9% grade 3+) | Axi-cel: 49% (14% grade 3+) Tisa-cel: 22% (3% grade 3+) | Axi-cel: no grade 5 CRS, 1 patient grade 5 ICANS Tisa-cel: 2 grade 5 CRS, no grade 5 ICANS No other treatment-related grade 5 AEs | Real-world response and survival rates were similar to clinical trials. Real-world ORR, CR, PFS, and OS better with axi-cel compared to tisa-cel, although axi-cel with more toxicity. |
| Chihara et al.31 Retrospective cohort from Medicare claims data | All CAR-T | 551 | 31% of patients in this cohort were age ≥75 | Not assessed | Age ≥75: mPFS 160 d mOS 403 d Age 70-74: mPFS 379 d mOS 603 d Age 65-69: mPFS 194 mOS 518 1 y PFS: Age ≥75 34% Age 70-74 52% Age 65-69 43% | Not assessed | Not assessed | Not assessed | Not assessed | Older patients, particularly those age ≥75, had significantly worse PFS and OS compared to younger patients as well as compared to clinical trials. |
| Nastoupil et al.32 Retrospective cohort from the US Lymphoma CAR-T Consortium | Axi-cel | 298 | 43% were ineligible for ZUMA-1 due to comorbidities | ORR 82% (CR 64%) | 1 y: PFS 47% OS 68% PFS 34% for ZUMA-1 ineligible patients | mDOR NR (median follow-up of 12.9 mo) | 91% (7% grade 3+) | 69% (31% grade 3+ | 4.4%, 1 death due to HLH, 1 death due to ICANS | Real-world outcomes and safety were comparable to ZUMA-1, although ECOG PS 2-4 had worse PFS, OS, and toxicities. Patients ineligible for ZUMA-1 had inferior PFS and OS. |
| Sano et al.33 Retrospective cohort from the US Lymphoma CAR-T Consortium | Axi-cel | 272 30% were aged ≥65 | Not assessed | Age ≥65 ORR 84% (CR 71%) Age ≤65 ORR 82% (CR 51%) | Age ≥65 mPFS 9.2 mo, mOS not assessable Age ≤65 mPFS 7.4 mo, mOS 18.7 mo | Not assessed | Age ≥65 92% (7% grade 3+) Age ≤65 91% (7% grade 3+) | Age ≥65 78% (35% grade 3+) Age ≤65 65% (31% grade 3+) | 2 deaths (1 in age ≥65 and 1 age <65) | Rate of complete response was higher in patients aged ≥65 compared to age <65. There was no difference in PFS, OS, and toxicities between patients aged <65 and ≥65. |
| Bethge et al.34 Retrospective cohort from the German Registry for Stem Cell Transplantation | Axi-cel Tisa-cel | 173 axi-cel 183 tisa-cel | 13% eligible for ZUMA-1 89% eligible for JULIET | Axi-cel: ORR 74% (CR 42%) Tisa-cel: ORR 53% (CR 32%) | Axi-cel: 1 y PFS 35%, OS 55% Tisa-cel: 1 y PFS 24%, OS 53% | Not assessed | Axi-cel: 81% (10% grade 3+) Tisa-cel: 65% (13% grade 3+) | Axi-cel: 44% (16% grade 3+) Tisa-cel: 22% (7% grade 3+) | Axi-cel: 2 y 10.4% Tisa-cel: 2 y 3.5% | Real-world ORR, CR, and OS were comparable to clinical trials, but real-world PFS was worse. Higher rates of delayed infection-related NRM in real-world cohort. |
| Riedell et al.35 Retrospective cohort of 8 US centers | Axi-cel Tisa-cel | 168 axi-cel 92 tisa-cel | 61% axi-cel ineligible for ZUMA-1 43% tisa-cel ineligible for JULIET | Axi-cel: ORR 52% (CR 44%) Tisa-cel: ORR 41% (CR 35%) | Axi-cel: 1 y PFS 42%, OS 62% Tisa-cel: 1 y PFS 32%, OS 59% | Axi-cel: 1 y DOR 70% Tisa-cel: 1 y DOR 75% | Axi-cel: 85% (9% grade 3+) Tisa-cel: 39% (1% grade 3+) mDOR NR for either cohort | Axi-cel: 56% (38% grade 3+) Tisa-cel: 11% (1% grade 3+) | Axi-cel: 9%, 4 deaths from ICANS Tisa-cel: 7% | Real-world outcomes were slightly lower compared to ZUMA-1 and JULIET, although safety outcomes were comparable. Axi-cel and tisa-cel had comparable efficacy, although less CRS and ICANS with tisa-cel. |
| Pasquini et al.36 Postauthorization safety study through the CIBMTR registry | Tisa-cel | 155 | Not assessed | ORR 62% (CR 40%) | 1 y: EFS 52% OS 77% | 1 y DOR 61% | 45% (4.5% grade 3+) | 18% (5.1% grade 3+) | 1.2% | Similar efficacy and improved safety compared to JULIET. |
| Kittai et al.37 Retrospective cohort from 4 centers | Axi-cel Tisa-cel | 94 axi-cel 36 tisa-cel | 57% of patients had high comorbidity based on CIRS score | ORR 68% (CR 42%) | mPFS 6.7 mo mOS NR 1 y OS 60% | Not assessed | 79% | 58% | 12.3%, 2 deaths due to CRS, 1 death due to ICANS | Worse ECOG PS and comorbidities by CIRS score were associated with worse survival. Presence of more comorbidities was not associated with worse CRS or ICANS. |
| Cook et al.38 Meta-analysis of prospective and retrospective studies of CAR-T in primary and secondary CNS lymphoma | Multiple CAR-T products | 30 patients with primary CNS lymphoma (PCNSL) 98 patients with secondary CNS lymphoma (SCNSL) | Not assessed | PCNSL: ORR 64% (CR 56%) SCNSL: ORR 57% (CR 47%) | PCNSL: 6-mo PFS 37% SCNSL: 6-mo PFS 37% | PCNSL: mDOR 9 mo SCNSL: mDOR 4.6 mo | PCNSL: 70% (13% grade 3+) SCNSL: 72% (11% grade 3+) | PCNSL: 53% (18% grade 3+) SCNSL: 48% (26% grade 3+) | Not assessed | Incidence of CRS and ICANS in real-world cohort of PCNSL and SCNSL is comparable to clinical trials. Approximately one-third of patients with PCNSL and SCNSL had 6-mo complete responses, which could be durable with longer follow-up. |
| Lin et al.50 Single-center retrospective cohort of older adults who did and did not receive CAR-T | Axi-cel and tisa-cel | 24 older adults received CAR-T 18 older adults did not receive CAR-T 25 younger adults (age <65) received CAR-T | Not assessed | 100-d CR 51% (for 49 patients who received CAR-T) | 6-mo PFS 48%, OS 71% (for 49 patients who received CAR-T) Older adults who received CAR-T had a significantly lower risk of death (HR 0.31) compared to older adults who did not receive CAR-T No difference in PFS or OS between older or younger adults who received CAR-T by chronological age, functional limitations, cognitive impairment, or comorbidity burden | Not assessed | 83% (8% grade 2±) | 54% (25% grade 2±) | 2 deaths in older adults after CAR-T | Older adults who receive CAR-T have better postrelapse OS compared to patients who instead receive chemotherapy and/or supportive care. PFS, OS, and rates of CRS and ICANS are not different between older and younger adults who receive CAR-T. |
| Real-world study design . | Constructs . | Patients . | Percent of patients ineligible for CAR-T clinical trials . | Response rates . | Survival outcomes . | Median duration of response . | Rates of CRS . | Rates of neurotoxicity . | Treatment-related mortality . | Conclusions . |
|---|---|---|---|---|---|---|---|---|---|---|
| Locke et al.28 Postapproval safety observational study | Axi-cel | 1343 | 38% aged ≥65, 4% with ECOG PS 2+, 13% cardiac comorbidities, 2% hepatic comorbidities, 2% renal comorbidities, 15% double/ triple-hit lymphoma, 66% refractory disease | ORR 74% (CR 56%) ORR 78% (CR 62%) for patients aged ≥65 ORR 57% (CR 29%) for hepatic comorbidities ORR 70% (CR 43%) for renal comorbidities ORR 47% (CR 20%) for ECOG 2-3 | 18 mo: PFS 42%, OS 52% | 18 mo: DOR 61% | 83% | 55% | Not assessed | Patients aged ≥65 with moderate to severe pulmonary disease and ECOG 2-3 had inferior ORR. Age ≥65 was not associated with inferior survival, but was associated with higher rates of CRS and ICANS. ECOG PS significantly affected all efficacy outcomes. |
| Jacobson et al.25 Postauthorization safety study through the CIBMTR registry | Axi-cel | 1297 | 57% were ineligible for ZUMA-1 | ORR 73% (CR 56%) | mPFS 8.6 mo mOS 21.8 mo | mDOR NR 24-mo DOR 57% | 83% (8% grade 3+) | 55% (24% grade 3+) | 3%, 2% died from CRS, 1% died from ICANS | Real-world response rates were similar to ZUMA-1, with similar DOR in patients who were and were not eligible for ZUMA-1. High-grade CRS and ICANS were lower in the real-world cohort. ECOG PS of 2 or greater was associated with inferior response rates, PFS, and OS. Patients age ≥65 had a higher ORR despite higher risk of CRS and ICANS as compared to younger patients. |
| Landsburg et al.29 Observational study through the CIBMTR registry | Tisa-cel | 1159 | 31% ineligible for JULIET | ORR 60% | 2 y: PFS 28%, OS 44% | 2 y: DOR 53% | 58% (6% grade 3+) | 23% (7% grade 3+) | Not assessed | Real-world outcomes were similar to JULIET. Patients with comorbidities (who were not eligible for JULIET) had similar efficacy outcomes. Patients with ECOG PS 2-4 had higher rates of high-grade CRS and ICANS but similar efficacy outcomes. |
| Bachy et al.30 Retrospective French DESCAR-T registry study with propensity score matching between axi-cel and tisa-cel | Axi-cel Tisa-cel | 452 axi-cel 277 tisa-cel | Not assessed | Axi-cel: ORR 80% (CR 60%) Tisa-cel: ORR 66% (CR 42%) | 1 y: PFS 47% axi-cel, 33% tisa-cel OS 64% axi-cel, 49% tisa-cel | 1 y: DOR 54% axi-cel, 42% tisa-cel | Axi-cel: 86% (5% grade 3+) Tisa-cel: 76% (9% grade 3+) | Axi-cel: 49% (14% grade 3+) Tisa-cel: 22% (3% grade 3+) | Axi-cel: no grade 5 CRS, 1 patient grade 5 ICANS Tisa-cel: 2 grade 5 CRS, no grade 5 ICANS No other treatment-related grade 5 AEs | Real-world response and survival rates were similar to clinical trials. Real-world ORR, CR, PFS, and OS better with axi-cel compared to tisa-cel, although axi-cel with more toxicity. |
| Chihara et al.31 Retrospective cohort from Medicare claims data | All CAR-T | 551 | 31% of patients in this cohort were age ≥75 | Not assessed | Age ≥75: mPFS 160 d mOS 403 d Age 70-74: mPFS 379 d mOS 603 d Age 65-69: mPFS 194 mOS 518 1 y PFS: Age ≥75 34% Age 70-74 52% Age 65-69 43% | Not assessed | Not assessed | Not assessed | Not assessed | Older patients, particularly those age ≥75, had significantly worse PFS and OS compared to younger patients as well as compared to clinical trials. |
| Nastoupil et al.32 Retrospective cohort from the US Lymphoma CAR-T Consortium | Axi-cel | 298 | 43% were ineligible for ZUMA-1 due to comorbidities | ORR 82% (CR 64%) | 1 y: PFS 47% OS 68% PFS 34% for ZUMA-1 ineligible patients | mDOR NR (median follow-up of 12.9 mo) | 91% (7% grade 3+) | 69% (31% grade 3+ | 4.4%, 1 death due to HLH, 1 death due to ICANS | Real-world outcomes and safety were comparable to ZUMA-1, although ECOG PS 2-4 had worse PFS, OS, and toxicities. Patients ineligible for ZUMA-1 had inferior PFS and OS. |
| Sano et al.33 Retrospective cohort from the US Lymphoma CAR-T Consortium | Axi-cel | 272 30% were aged ≥65 | Not assessed | Age ≥65 ORR 84% (CR 71%) Age ≤65 ORR 82% (CR 51%) | Age ≥65 mPFS 9.2 mo, mOS not assessable Age ≤65 mPFS 7.4 mo, mOS 18.7 mo | Not assessed | Age ≥65 92% (7% grade 3+) Age ≤65 91% (7% grade 3+) | Age ≥65 78% (35% grade 3+) Age ≤65 65% (31% grade 3+) | 2 deaths (1 in age ≥65 and 1 age <65) | Rate of complete response was higher in patients aged ≥65 compared to age <65. There was no difference in PFS, OS, and toxicities between patients aged <65 and ≥65. |
| Bethge et al.34 Retrospective cohort from the German Registry for Stem Cell Transplantation | Axi-cel Tisa-cel | 173 axi-cel 183 tisa-cel | 13% eligible for ZUMA-1 89% eligible for JULIET | Axi-cel: ORR 74% (CR 42%) Tisa-cel: ORR 53% (CR 32%) | Axi-cel: 1 y PFS 35%, OS 55% Tisa-cel: 1 y PFS 24%, OS 53% | Not assessed | Axi-cel: 81% (10% grade 3+) Tisa-cel: 65% (13% grade 3+) | Axi-cel: 44% (16% grade 3+) Tisa-cel: 22% (7% grade 3+) | Axi-cel: 2 y 10.4% Tisa-cel: 2 y 3.5% | Real-world ORR, CR, and OS were comparable to clinical trials, but real-world PFS was worse. Higher rates of delayed infection-related NRM in real-world cohort. |
| Riedell et al.35 Retrospective cohort of 8 US centers | Axi-cel Tisa-cel | 168 axi-cel 92 tisa-cel | 61% axi-cel ineligible for ZUMA-1 43% tisa-cel ineligible for JULIET | Axi-cel: ORR 52% (CR 44%) Tisa-cel: ORR 41% (CR 35%) | Axi-cel: 1 y PFS 42%, OS 62% Tisa-cel: 1 y PFS 32%, OS 59% | Axi-cel: 1 y DOR 70% Tisa-cel: 1 y DOR 75% | Axi-cel: 85% (9% grade 3+) Tisa-cel: 39% (1% grade 3+) mDOR NR for either cohort | Axi-cel: 56% (38% grade 3+) Tisa-cel: 11% (1% grade 3+) | Axi-cel: 9%, 4 deaths from ICANS Tisa-cel: 7% | Real-world outcomes were slightly lower compared to ZUMA-1 and JULIET, although safety outcomes were comparable. Axi-cel and tisa-cel had comparable efficacy, although less CRS and ICANS with tisa-cel. |
| Pasquini et al.36 Postauthorization safety study through the CIBMTR registry | Tisa-cel | 155 | Not assessed | ORR 62% (CR 40%) | 1 y: EFS 52% OS 77% | 1 y DOR 61% | 45% (4.5% grade 3+) | 18% (5.1% grade 3+) | 1.2% | Similar efficacy and improved safety compared to JULIET. |
| Kittai et al.37 Retrospective cohort from 4 centers | Axi-cel Tisa-cel | 94 axi-cel 36 tisa-cel | 57% of patients had high comorbidity based on CIRS score | ORR 68% (CR 42%) | mPFS 6.7 mo mOS NR 1 y OS 60% | Not assessed | 79% | 58% | 12.3%, 2 deaths due to CRS, 1 death due to ICANS | Worse ECOG PS and comorbidities by CIRS score were associated with worse survival. Presence of more comorbidities was not associated with worse CRS or ICANS. |
| Cook et al.38 Meta-analysis of prospective and retrospective studies of CAR-T in primary and secondary CNS lymphoma | Multiple CAR-T products | 30 patients with primary CNS lymphoma (PCNSL) 98 patients with secondary CNS lymphoma (SCNSL) | Not assessed | PCNSL: ORR 64% (CR 56%) SCNSL: ORR 57% (CR 47%) | PCNSL: 6-mo PFS 37% SCNSL: 6-mo PFS 37% | PCNSL: mDOR 9 mo SCNSL: mDOR 4.6 mo | PCNSL: 70% (13% grade 3+) SCNSL: 72% (11% grade 3+) | PCNSL: 53% (18% grade 3+) SCNSL: 48% (26% grade 3+) | Not assessed | Incidence of CRS and ICANS in real-world cohort of PCNSL and SCNSL is comparable to clinical trials. Approximately one-third of patients with PCNSL and SCNSL had 6-mo complete responses, which could be durable with longer follow-up. |
| Lin et al.50 Single-center retrospective cohort of older adults who did and did not receive CAR-T | Axi-cel and tisa-cel | 24 older adults received CAR-T 18 older adults did not receive CAR-T 25 younger adults (age <65) received CAR-T | Not assessed | 100-d CR 51% (for 49 patients who received CAR-T) | 6-mo PFS 48%, OS 71% (for 49 patients who received CAR-T) Older adults who received CAR-T had a significantly lower risk of death (HR 0.31) compared to older adults who did not receive CAR-T No difference in PFS or OS between older or younger adults who received CAR-T by chronological age, functional limitations, cognitive impairment, or comorbidity burden | Not assessed | 83% (8% grade 2±) | 54% (25% grade 2±) | 2 deaths in older adults after CAR-T | Older adults who receive CAR-T have better postrelapse OS compared to patients who instead receive chemotherapy and/or supportive care. PFS, OS, and rates of CRS and ICANS are not different between older and younger adults who receive CAR-T. |
AE, adverse event; CIBMTR, Center for International Blood and Marrow Transplant Research; ECOG PS, Eastern Cooperative Oncology Group Performance Status; NRM, nonrelapse mortality.
When considering BsAb vs CAR-T therapy in the third-line setting, cross-trial comparisons (Table 1) suggest lower response rates and PFS with BsAb therapies with shorter follow-up. In particular, patients with refractory disease have lower CR rates to BsAb therapies as compared to CAR-T: for glofitamab, CR rates are 34% with refractory as compared to 70% with nonrefractory disease,8 and for epcoritamab, CR rates are 30% with refractory vs 53% with nonrefractory disease.11 The lack of a costimulatory domain on current CD20xCD3 BsAb constructs may account for this observation, as cytotoxicity of BsAb therapy is dependent on innate immunity, whereas CAR-T constructs contain 4-1BB or CD28 costimulatory domains, which promote T-cell proliferation and persistence. From a logistic perspective, although CAR-T therapy requires significant upfront resources surrounding apheresis, CAR production, and intensive monitoring and toxicity management, current BsAb therapy strategies require ongoing dosing every several weeks for up to 12 cycles (glofitamab) or indefinitely until progression (epcoritamab), which may incur additional time and resource costs for patients as well as centers. Although CAR-T is considered cost-effective compared to other chemoimmunotherapy regimens and transplantation,39 cost-effectiveness studies between CAR-T and BsAb in the third-line setting are warranted. An additional advantage to sequencing CAR-T first is the demonstrated efficacy of BsAb therapies in patients with previous CAR-T exposure, whereas limited data have demonstrated efficacy of the converse strategy, with 1-year PFS and OS of 37% and 54%, respectively, in a registry cohort of 28 patients who received CAR-T after BsAb therapies.51 Further, there is theoretical concern about T-cell exhaustion post-BsAb therapy, which could limit the ability to apherese and manufacture an efficacious CAR T-cell product.40
CAR-T eligibility
Given the curative potential of CAR-T among diverse patient cohorts, there is not a uniform definition for CAR-T eligibility, specifically for patients who may be considered too frail for CAR-T. Automatic exclusion of patients based on chronological age, presence of comorbidities, or cognitive and/or functional impairments is not advised as these characteristics do not consistently affect post–CAR-T outcomes.28,33,41,42 Further, transplant eligibility is not the same as CAR-T eligibility, as demonstrated in the PILOT trial of liso-cel in transplant-ineligible patients.20,43
Based on patient cohorts with inferior outcomes after CAR-T (Table 2), we suggest that the following patients be referred to a multidisciplinary clinic with involvement by geriatrics, physical medicine and rehabilitation, physical therapy, occupational therapy, social work, and nutrition for comprehensive frailty assessment: age >70 to 75,31 Eastern Cooperative Oncology Group (ECOG) Performance Status of 2 or greater,25,28,29,32,37 multiple comorbidities per the Cumulative Illness Rating Scale (CIRS)- derived “Severe4” comorbidity index validated in CAR-T (CIRS score of 3 or higher in any of the respiratory, upper gastrointestinal, renal, or hepatic organ systems),37,44 significant weight loss and/or poor nutrition, and secondary CNS involvement and/or baseline cognitive impairment.41
Although these characteristics have not yet been validated for determining CAR-T patient eligibility in routine clinical care, extrapolated evidence from the transplantation literature suggests that comprehensive frailty assessments in a dedicated multidisciplinary clinic may improve outcomes.45,46 Planning for functional optimization of at-risk patients before (“prehab”) and after (“rehab”) CAR-T, as well as discussion of bridging therapies and prophylactic steroids and/or anticytokine therapies prior to CAR-T infusion, should also occur.41
CLINICAL CASE (continued)
After discussion with the patient, the decision was made to proceed with liso-cel CAR-T therapy, which was complicated by grade 2 CRS and grade 2 neurotoxicity, both of which completely resolved. One year after CAR-T therapy, he developed relapsed DLBCL in a single cervical lymph node, which was treated with radiation therapy resulting in a CR. Six months later, he developed progressive DLBCL.
Case for bispecific antibody therapy first
Although CAR-T has the potential for cure in LBCL, two-thirds of patients will eventually relapse after CAR-T, and outcomes for these patients are dismal, with a median OS of only 5.2 months.47 There are not currently any data to inform which patients may preferentially benefit from BsAb first rather than CAR-T therapy to maximize survival. For the aforementioned patients who are known to have inferior survival after CAR-T, the potential benefits of BsAb therapies over CAR-T (Table 3) should be discussed, particularly the availability of off-the-shelf BsAb products and the generally lower rates of high-grade toxicities. These benefits should be placed in context of the unknown long-term curative potential of BsAb therapies, as well as the known activity of BsAb therapies in the post–CAR-T setting.
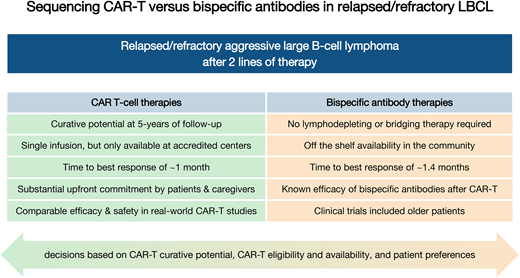
Pros and cons of CAR-T vs bispecific antibody therapies in third-line LBCL
| Characteristic . | CAR-T therapy . | Bispecific antibody therapy . |
|---|---|---|
| Curative potential | Confirmed at 5 years of follow-up | Data not yet mature; complete responses appear to be durable with short follow-up |
| Administration | Single infusion | Repeated infusions required |
| Off-the-shelf availability | Not currently | Yes |
| Time to treatment initiation | Several weeks required for product manufacturing | Treatment can be started immediately |
| Bridging therapy | Optional, but may be needed in patients with aggressive disease | Not needed |
| Geographic availability | Only at specialized, accredited centers | Community settings |
| Upfront commitment | Substantial: hospitalization often required for administration | Less than CAR-T: initial hospitalization required for dose escalation, although emerging data regarding safety of outpatient initiation |
| Caregiver support required | Substantial | Less than CAR-T |
| Time to best response | ∼1 month | ∼1.4 months |
| T-cell requirements | Dependent on T-cell health (manufacturing failures, bendamustine exposure) | No costimulatory domain, so relies on innate immunity, which may be impaired by T-cell exhaustion |
| Activity in CNS involvement | CAR-T has demonstrated activity and comparable safety in primary and secondary CNS lymphoma | Not yet studied |
| Real-world data | Comparable efficacy and safety in real-world cohorts as compared to clinical trial cohorts | No real-world data regarding safety or efficacy |
| Patient-reported outcomes | Clinically meaningful longitudinal improvements in quality of life in the second- and third-line settings | Data not yet published |
| Cost-effectiveness | Cost effective in the second and third lines | Not yet studied |
| Existing sequencing data | No data for CAR-T after BsAb therapy | Known efficacy and safety of BsAb therapy after CAR-T |
| Characteristic . | CAR-T therapy . | Bispecific antibody therapy . |
|---|---|---|
| Curative potential | Confirmed at 5 years of follow-up | Data not yet mature; complete responses appear to be durable with short follow-up |
| Administration | Single infusion | Repeated infusions required |
| Off-the-shelf availability | Not currently | Yes |
| Time to treatment initiation | Several weeks required for product manufacturing | Treatment can be started immediately |
| Bridging therapy | Optional, but may be needed in patients with aggressive disease | Not needed |
| Geographic availability | Only at specialized, accredited centers | Community settings |
| Upfront commitment | Substantial: hospitalization often required for administration | Less than CAR-T: initial hospitalization required for dose escalation, although emerging data regarding safety of outpatient initiation |
| Caregiver support required | Substantial | Less than CAR-T |
| Time to best response | ∼1 month | ∼1.4 months |
| T-cell requirements | Dependent on T-cell health (manufacturing failures, bendamustine exposure) | No costimulatory domain, so relies on innate immunity, which may be impaired by T-cell exhaustion |
| Activity in CNS involvement | CAR-T has demonstrated activity and comparable safety in primary and secondary CNS lymphoma | Not yet studied |
| Real-world data | Comparable efficacy and safety in real-world cohorts as compared to clinical trial cohorts | No real-world data regarding safety or efficacy |
| Patient-reported outcomes | Clinically meaningful longitudinal improvements in quality of life in the second- and third-line settings | Data not yet published |
| Cost-effectiveness | Cost effective in the second and third lines | Not yet studied |
| Existing sequencing data | No data for CAR-T after BsAb therapy | Known efficacy and safety of BsAb therapy after CAR-T |
It is critically important to assess patient preferences when deciding on sequencing of BsAb vs CAR-T therapies, as there are significant logistic disadvantages to CAR-T administration that may be burdensome to patients. CAR-T therapies are generally only available at large specialized cancer centers, which may require patients to travel great distances for consultation and apheresis, followed by prolonged stays away from home for CAR-T infusion and postinfusion monitoring.48 Further, although there are some centers that administer CAR-T therapies in the outpatient setting, most centers pursue inpatient administration for several weeks, which may be undesirable for patients and may lead to progressive frailty in older adults. BsAb therapies, on the other hand, will likely be available in the community setting and require much shorter inpatient stays for initial dose escalation, with formal studies ongoing to assess the safety of outpatient administration with premedication.8 A significant advantage of BsAb therapies is their off-the-shelf availability and capability for immediate treatment initiation, without requirement for apheresis and manufacturing, which may take several weeks for CAR-T therapies. This is particularly important for patients with florid and rapidly progressive LBCL, as interruptions in bridging therapies surrounding necessary washout periods before CAR-T apheresis and lymphodepleting chemotherapy may lead to significant disease progression during the manufacturing period and worse post–CAR-T outcomes and toxicities. In this vein, although CAR-T therapies have a time to best response of approximately 1 month, the time to best response of 1.4 months of glofitamab and epcoritamab may be preferable for patients with aggressive disease who may not be able to tolerate the additional several weeks of CAR-T manufacturing time. Finally, BsAb clinical trials in R/R LBCL included significant proportions of older patients (19% were age ≥75 years for epcoritamab and 54% were age ≥65 years for glofitamab),8,11 demonstrating efficacy and safety in this patient cohort. However, it is important to note that a subgroup analysis of patients aged ≥65 years in ZUMA-1 did not find any age-related differences in the efficacy or safety of axi-cel,49 which has been confirmed in real-world cohorts (Table 2). These data underscore the importance of a nuanced discussion with patients and their caregivers regarding the risks and benefits of CAR-T vs BsAb therapies.
CLINICAL CASE (continued)
The patient elected to enroll on a clinical trial of BsAb therapy mosunetuzumab in combination with polatuzumab vedotin. He received 8 cycles of combination therapy on trial and achieved a complete metabolic response, with his treatment course complicated by grade 1 CRS during the first 2 cycles and grade 3 neutropenia after cycle 5. His serial positron emission tomography scans at 12, 18, and 24 months after study enrollment continued to demonstrate ongoing remissions.
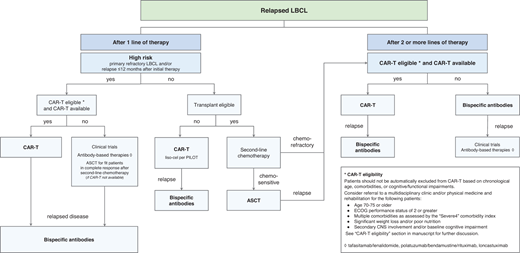
Our sequencing approach for relapsed/refractory LBCL and conclusions
We propose a treatment algorithm for R/R LBCL that prioritizes CAR-T administration in all eligible patients in the second- and third-line settings given the curative potential and manageable toxicities of CAR-T in real-world cohorts (Figure 1).
Our approach to sequencing CAR T-cell and bispecific antibody therapies in relapsed large B-cell lymphomas.
Our approach to sequencing CAR T-cell and bispecific antibody therapies in relapsed large B-cell lymphomas.
However, discussions with patients regarding their treatment preferences will be paramount as more long-term data on BsAb efficacy emerge in the literature. We recognize the limitations of cross-trial comparisons between CAR-T and BsAb therapies; prospective trials that compare the 2 strategies will be critical to treatment decision-making, particularly with risk stratification schema to study which patient cohorts may most benefit from CAR-T or BsAb therapies as the first in sequence. Ongoing trials studying specific CAR-T and BsAb sequencing strategies, such as SWOG 2114 and NCT04889716, which investigate various BsAb therapies as consolidation after commercial CAR-T, will also define optimal sequencing paradigms.52 Finally, efforts to increase the accessibility and safety of CAR-T delivery in community settings that are closer in proximity to patients and their caregivers will be vital in improving patient-centered outcomes.
Conflict-of-interest disclosure
Ajay Major: no competing financial interests to declare.
Manali Kamdar declares research support from Novartis; consultancy from AbbVie, AstraZeneca, Celgene/Bristol-Myers Squibb, Adaptive Biotechnologies, ADC Therapeutics, Beigene, Genentech, Impact Bio, Syncopation, and Caribou Biosciences; speaker's bureau from SeaGen; and data monitoring committee from Celgene and Genentech.
Off-label drug use
Ajay Major: Nothing to disclose.
Manali Kamdar: Nothing to disclose.