Abstract
Several recent advances have affected the treatment landscape of diffuse large B-cell lymphoma. Chimeric antigen receptor (CAR) T-cell therapy has transformed the management of chemorefractory disease. Two randomized studies in early relapse disease have expanded the label to provide access to CAR T-cell therapy as early as second line for some patients. Despite the durable remissions that have been achieved, many patients will experience relapse. There is a growing population of patients previously treated with CAR T-cell therapy facing dismal outcomes. We review the prospective studies that inform treatment options in later lines and highlight the limited data examining outcomes with novel therapies after CAR T-cell failure. The treatment landscape is anticipated to continue to evolve with the emergence of bispecific antibodies that appear to be a promising approach, particularly after CAR T-cell therapy, although little is known about overlapping mechanisms of resistance. Enrichment for patients who have received prior CAR T-cell therapy on prospective trials is a critical unmet need to inform the preferred management for these high-risk patients.
Learning Objectives
Evaluate the available evidence that may inform treatment strategies for patients after chimeric antigen receptor (CAR) T-cell therapy, including emerging therapies
Understand potential mechanisms of resistance of CAR T-cell therapy that may inform subsequent treatment
CLINICAL CASE
A 67-year-old woman presents with advanced stage diffuse large B-cell lymphoma (DLBCL), nongerminal center immunophenotype with double expression of MYC and BCL2 and no MYC gene rearrangement. She had additional high-risk features, including extranodal involvement (bone and soft tissue) and elevated lactate dehydrogenase, resulting in an International Prognostic Index of 4. She completed 6 cycles of rituximab (R), cyclophosphamide, doxorubicin, vincristine, and prednisone, achieving a complete response (CR) at the end of induction imaging. However, 6 months later, she presents with recurrent bone pain and imaging confirms recurrent lymphoma involving multiple bone lesions, soft tissue, muscle, and adenopathy above and below the diaphragm. Largest mass is 6 cm in diameter. Biopsy confirms recurrent DLBCL. With early-relapse DLBCL, she is evaluated for chimeric antigen receptor (CAR) T-cell therapy and is deemed to be a good candidate. She receives bridging therapy with R and polatuzumab. Positron emission tomography/computed tomography postbridging reveals progressive lymphoma in extranodal sites, including bone and soft tissue. She proceeds with standard-of-care lisocabtagene maraleucel (liso-cel), tolerating it well with no ongoing toxicity at day 30. Imaging reveals she has achieved a CR. She remains in CR for 6 months; however, she returns for follow-up with concerns about recurrent lymphoma.
CAR T-cell therapy has transformed the management of chemorefractory DLBCL based on the pivotal, single-arm phase 2 trials leading to approval of 3 anti-CD19 autologous CAR T-cell therapies for the management of relapsed or refractory DLBCL.1-3 Objective response rates across these studies were very promising, ranging from 52% to 82%, with approximately 40% maintaining a CR beyond 12 months. In addition to providing novel cellular therapy for patients otherwise facing dismal outcomes, these studies provided the rationale for the randomized trials conducted in second line examining CAR T-cell therapy vs standard of care for early-relapse DLBCL. ZUMA-7, TRANSFORM, and BELINDA each explored whether an anti-CD19 CAR T-cell therapy would be superior to platinum-based salvage chemotherapy followed by high-dose therapy and autologous stem cell transplant among chemosensitive patients deemed appropriate candidates for intensive therapy.4-6 Both ZUMA-7 and TRANSFORM met their primary end points of significant improvement in event-free survival, resulting in approval for axicabtagene ciloleucel (axi-cel) and liso-cel as early as second line for patients with primary refractory or early-relapse DLBCL.
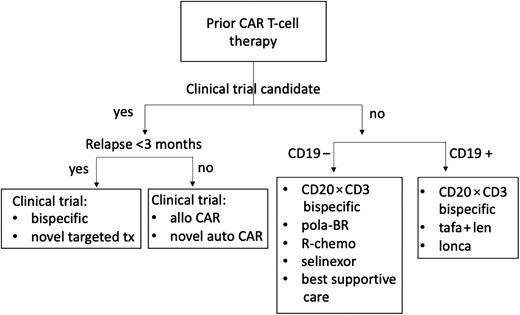
Despite the enthusiasm surrounding CAR T-cell therapy in early-relapse DLBCL or for those who have had at least 2 prior lines of therapy, at least 50% of patients will fail to achieve a durable remission. As CAR T-cell therapy moves into earlier lines of treatment, there is an increasing population in need of effective management options after CAR T-cell therapy (Figure 1). There are many challenges these patients will face. The acute toxicity of cellular therapy, including cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, if severe, can result in debilitation. In addition, real-world analyses report patients treated with standard-of-care CAR T-cell therapy often would not meet the stringent eligibility criteria of prospective trials.7-9 Late toxicity, including prolonged cytopenia (observed in 20%-40% of patients), and B-cell aplasia increase the risk for opportunistic infections and necessitate the need for prophylactic antimicrobials, growth factors, and/or transfusion support. This undoubtedly affects eligibility for participation in clinical trials. However, pursuit of clinical trials for these patients should be of highest priority. Many patients exhibit high-risk disease from the onset, and progressing after CAR T-cell therapy has been associated with very poor outcomes.10,11 Not surprisingly, failing to achieve a response to CAR T-cell therapy or progressing within 90 days is associated with the most unfavorable outcomes.
Treatment algorithm, after CAR T-cell therapy management. allo, allogeneic; BR, bendamustine-rituximab; pola, polatuzumab; R-chemo, rituximab plus chemotherapy; tafa + len, tafasitamab plus lenalidomide; tx, treatment.
Treatment algorithm, after CAR T-cell therapy management. allo, allogeneic; BR, bendamustine-rituximab; pola, polatuzumab; R-chemo, rituximab plus chemotherapy; tafa + len, tafasitamab plus lenalidomide; tx, treatment.
Little is currently known about mechanisms of resistance to CAR T-cell therapy, and few prospective studies provide a hint at strategies to successfully overcome them. Our current understanding of resistance can be summarized as antigen loss, diminished T-cell fitness, and/or a protumor microenvironment. Among patients with lymphoma treated with CD19-directed CAR T-cell therapy, antigen loss in the form of CD19 mutations or decreased CD19 membrane expression is relatively uncommon.11,12 As the treatment landscape continues to evolve with several CD19-directed therapies emerging, it is advisable to pursue a biopsy if feasible in the post-CD19 CAR T-cell setting to determine the immunophenotype and potentially guide therapy.
It is unclear whether it is necessary to demonstrate maintained expression of CD19 to achieve efficacy from 2 approved CD19- directed therapies in relapsed DLBCL, tafasitamab, an Fc-modified, humanized, anti-CD19 monoclonal antibody, or loncastuximab (lonca) tesirine, an anti-CD19 antibody conjugated to a pyrrolobenzodiazepine dimer. The L-MIND study enrolled patients with relapsed DLBCL who had 1 to 3 prior lines of therapy and were not candidates for high-dose therapy and autologous stem cell transplant, leading to approval of tafasitamab in combination with lenalidomide for relapsed DLBCL (Table 1).13 This study did not include patients previously treated with CAR T-cell therapy. Limited data suggest tafasitamab is not associated with antigen loss or limitation of CAR-T effector function, which may lessen concerns about sequencing tafasitamab plus lenalidomide prior to CD19-directed CAR-T cell therapy,13,14 but little is known about the efficacy of this regimen after CAR T-cell therapy. A multicenter, retrospective analysis of standard-of-care tafasitamab plus lenalidomide included 17 patients who had received prior CAR T-cell therapy.15 Outcomes among these patients were similar to the larger cohort, with a median time of 14.3 months from CAR T-cell therapy to the start of tafasitamab plus lenalidomide. Response rates were slightly higher among patients with relapsed disease after CAR T-cell therapy as opposed to those with refractory disease (objective response rate [ORR] 36% vs 17%), suggesting a higher chance of success among those who respond to CAR T-cell therapy but then relapse.
Targeted therapies approved by the US Food and Drug Administration in R/R DLBCL, pivotal trials
| Characteristic . | Tafasitamab + lenalidomide . | Loncastuximab tesirine . | Rituximab-bendamustine-polatuzumab vedotin . | Selinexor . |
|---|---|---|---|---|
| Trial name | L-MIND13 | LOTIS-216 | R-Benda-Pola17 | SADAL19 |
| Sample size | 80 | 145 | 80 | 267 |
| ORR, % | 60 | 48 | 63 | 28 |
| Follow-up, mo | 17.3 | 7.3 | 27 | 14.7 |
| PFS, mo | 12.1 | 4.9 | 9.2 | 2.6 |
| OS, mo | Not reached | 9.9 | 12.4 | 9.1 |
| DOR, mo | 21.7 | 10.3 | 10.9 | 9.3 |
| ORR post-CART, % | 36 (n = 42)15 | 46 (n = 13)28 | 44 (n = 57)29 | — |
| PFS after CAR T-cell, mo | 1.4 | 1.4 | 2.5 | — |
| Characteristic . | Tafasitamab + lenalidomide . | Loncastuximab tesirine . | Rituximab-bendamustine-polatuzumab vedotin . | Selinexor . |
|---|---|---|---|---|
| Trial name | L-MIND13 | LOTIS-216 | R-Benda-Pola17 | SADAL19 |
| Sample size | 80 | 145 | 80 | 267 |
| ORR, % | 60 | 48 | 63 | 28 |
| Follow-up, mo | 17.3 | 7.3 | 27 | 14.7 |
| PFS, mo | 12.1 | 4.9 | 9.2 | 2.6 |
| OS, mo | Not reached | 9.9 | 12.4 | 9.1 |
| DOR, mo | 21.7 | 10.3 | 10.9 | 9.3 |
| ORR post-CART, % | 36 (n = 42)15 | 46 (n = 13)28 | 44 (n = 57)29 | — |
| PFS after CAR T-cell, mo | 1.4 | 1.4 | 2.5 | — |
Benda, bendamustine; DOR, duration of response; Pola, polatuzumab vedotin; R/R, relapsed/refractory.
Lonca is approved for relapsed DLBCL after 2 lines of therapy based on the LOTIS-2 trial.16 This heavily pretreated and refractory population achieved an ORR of 48%, including similar responses among 13 (9%) patients who had prior anti-CD19 CAR T-cell therapy (Table 1). Patients were required to have a biopsy demonstrating CD19 expression to be eligible for this prospective trial. One potential advantage of lonca is that it is an intravenous formulation readily available for refractory patients in need of urgent therapy. We might consider this first among those patients with refractory disease.
Among patients with loss of CD19 or prescriber preference for an alternate target, polatuzumab vedotin is an antibody drug conjugate targeting CD79b approved for relapsed DLBCL in combination with R-bendamustine based on a randomized phase 2 study in transplant-ineligible patients. The polatuzumab- containing arm resulted in a significant improvement in progression-free survival (PFS, 9.2 vs 3.7 months) and overall survival (OS, 12.4 vs 4.7 months) (Table 1).17 The efficacy of the control arm (R-bendamustine) was limited, leading many to question the role of bendamustine in relapsed DLBCL and omission of bendamustine in some retrospective series reporting outcomes with polatuzumab-based approaches after CAR T-cell therapy.11,18 Responses ranged from 44% to 48% with polatuzumab-based approaches, but responses were not durable in either series.
Selinexor, a selective inhibitor of the nuclear export protein XPO1, is an oral option approved for patients with relapsed or refractory DLBCL who have received at least 2 lines of therapy. The SADAL study was a single-arm phase 2 trial demonstrating an ORR of 28% (12% CR) with single-agent selinexor.19 With the modest efficacy and common grade 3 to 4 cytopenias, selinexor should be reserved for those without an acceptable alternative option.
Targeting CD20 has been an established approach for B-cell lymphomas for the past 2 decades. Chemotherapy in combination with CD20 monoclonal antibodies after CAR T-cell therapy has not resulted in favorable outcomes.10,11 This may be due to the prescription of CAR T-cell therapy for patients with chemorefractory disease; therefore, expecting to overcome early signs of chemoresistance is unlikely with retreatment. Can we enhance CD20 targeting and omit chemotherapy after CAR T-cell therapy? An emerging class of bispecific antibodies that target tumor antigen and recruit T cells may provide a new and effective option for the post–CAR T-cell space (Table 2).
Emerging bispecific antibodies
| Characteristic . | Epcoritamab21 . | Glofitamab20 . | Mosunetuzumab23 . | Odronextamab22 . |
|---|---|---|---|---|
| Overall cohort | ||||
| Sample size | 157 | 155 | 129 | 49* |
| ORR, % | 63 | 52 | 34.9 | 39* |
| CR, % | 39 | 39 | 19.4 | 24* |
| DOR, mo | 12 | 18.4 | 7.6 | 4.4* |
| PFS, mo | 4.4 | 4.9 | 1.4 | 11.5 |
| Follow-up, mo | 10.7 | 12.6 | 11.9 | 4.2 |
| Post–CAR T-cell cohort | ||||
| Sample size | 61 | 52 | 19 | 33 |
| ORR, % | 54 | Not reported | 37 | 33 |
| CR, % | 34 | 35 | 26 | 24 |
| DOR (mos) | 9.7 | Not reported | Not reported | Not reached |
| Characteristic . | Epcoritamab21 . | Glofitamab20 . | Mosunetuzumab23 . | Odronextamab22 . |
|---|---|---|---|---|
| Overall cohort | ||||
| Sample size | 157 | 155 | 129 | 49* |
| ORR, % | 63 | 52 | 34.9 | 39* |
| CR, % | 39 | 39 | 19.4 | 24* |
| DOR, mo | 12 | 18.4 | 7.6 | 4.4* |
| PFS, mo | 4.4 | 4.9 | 1.4 | 11.5 |
| Follow-up, mo | 10.7 | 12.6 | 11.9 | 4.2 |
| Post–CAR T-cell cohort | ||||
| Sample size | 61 | 52 | 19 | 33 |
| ORR, % | 54 | Not reported | 37 | 33 |
| CR, % | 34 | 35 | 26 | 24 |
| DOR (mos) | 9.7 | Not reported | Not reported | Not reached |
Responses reported are for patients with DLBCL without prior CAR T-cell therapy.
Glofitamab is a 2:1 CD20 × CD3 bispecific monoclonal antibody with promising efficacy in a phase 1/2 study of patients with relapsed or refractory DLBCL after at least 2 prior lines of therapy.20 A third of the study population (51 patients) had prior CAR T-cell therapy, including 30% who were refractory to CAR T. The CR rates were similar for this subgroup, 35% vs 39% compared with the overall study population. There were also no reports of increased toxicity among patients receiving a bispecific antibody after CAR T-cell therapy. Epcoritamab, a subcutaneous CD20 × CD3 bispecific antibody, was also explored in a phase 1/2 study of patients with relapsed or refractory DLBCL after at least 2 prior lines of therapy.21 Nearly 40% (61 patients) of this study population included patients with prior CAR T-cell therapy, with 46 (75%) having progressed within 6 months of CAR T-cell therapy. The ORR among patients who received prior CAR T-cell therapy was 54%, 34% achieved a CR, and the median duration of response was 9.7 months. The CR rate was lower among those refractory to prior CAR T-cell therapy, 28% but still meaningful.
Odronextamab, a fully human IgG4-based CD20 × CD3 bispecific, included 33 patients with relapsed/refractory DLBCL and prior CAR T-cell therapy in a phase 1/1b study.22 Among these patients, ORR was 33%; 24% achieved a CR, with an estimated 4.4-month duration of response; and duration of CR was not reached. Mosunetuzumab, a CD20 × CD3 bispecific antibody currently approved for the treatment of relapsed follicular lymphoma following at least 2 lines of therapy, included 19 (n = 15 DLBCL) patients who had received prior CAR T-cell therapy in the dose escalation study.23 The responses observed were comparable to the those observed with aggressive lymphoma who had not undergone CAR T-cell therapy. As a class, the CD20-bispecific antibodies appear very promising for patients who have progressed following CAR T-cell therapy. The favorable toxicity profile lends itself to combination approaches that are under investigation (Table 3). Little is known about overlapping mechanisms of resistance such as T-cell exhaustion. Effort should be made to enroll patients on ongoing prospective trials to gain more experience.
Prospective trials recruiting after CAR T-cell therapy
| Trial . | Therapy . | Clinical trial info . |
|---|---|---|
| SWOG | Mosunetuzumab ± polatuzumab | NCT05633615 |
| ALPHA2 | ALLO-501A CD19 allo CAR | NCT04416984 |
| PBCAR0191 | CD19 allo CAR | NCT03666000 |
| ANTLER CB-010 | CRISPR edited CD19 allo CAR | NCT 04637763 |
| TAK-007 | CD19 allo natural killer CAR | NCT05020015 |
| Phase 1/2 CD20 CAR T | CD20-specific CAR T cell | NCT03277729 |
| Phase 1/2 CD19/20 CAR T | CD19/CD20 CAR T cell | NCT04186520 |
| KITE-363, phase 1 | CD19/CD20 CAR T cell | NCT04989803 |
| JV-213, phase 1, | CD79b CAR T cell | NCT05773040 |
| Phase 2/3, NKTR-255 vs placebo following CD19 CAR T-cell therapy | NKTR-255 (IL-15 receptor agonist) | NCT05664217 |
| Trial . | Therapy . | Clinical trial info . |
|---|---|---|
| SWOG | Mosunetuzumab ± polatuzumab | NCT05633615 |
| ALPHA2 | ALLO-501A CD19 allo CAR | NCT04416984 |
| PBCAR0191 | CD19 allo CAR | NCT03666000 |
| ANTLER CB-010 | CRISPR edited CD19 allo CAR | NCT 04637763 |
| TAK-007 | CD19 allo natural killer CAR | NCT05020015 |
| Phase 1/2 CD20 CAR T | CD20-specific CAR T cell | NCT03277729 |
| Phase 1/2 CD19/20 CAR T | CD19/CD20 CAR T cell | NCT04186520 |
| KITE-363, phase 1 | CD19/CD20 CAR T cell | NCT04989803 |
| JV-213, phase 1, | CD79b CAR T cell | NCT05773040 |
| Phase 2/3, NKTR-255 vs placebo following CD19 CAR T-cell therapy | NKTR-255 (IL-15 receptor agonist) | NCT05664217 |
Several trials are under way exploring the safety and preliminary activity of allogeneic CAR T-cell therapy, using T cells from healthy donors to overcome limitations of T-cell fitness and the delay necessary to manufacture from an autologous product (Table 3). Many of these trials are recruiting patients who have had prior autologous CAR T-cell therapy given the nature of the experimental treatment. ALLO-501A is an allogeneic anti-CD19 CAR T-cell product with disrupted T-cell receptor (TCR) α gene (reduce graft-versus-host disease risk), and the edited CD52 gene may permit use of ALLO-647 (a humanized anti-CD52 monoclonal antibody) to selectively deplete host T cells to enhance lymphocyte depletion.24 The ongoing ALPHA2 study allows prior CD19 autologous CAR T-cell therapy if tumors retain CD19 expression. Several other phase 1 studies are actively recruiting, using allogeneic CARs with novel gene editing to knock out the native TCR, eliminate immune checkpoints, or enhance avoiding immune detection, including enhanced lymphocyte-depleting therapy (Table 3). Other sources such as natural killer cells or dual-targeting CARs are also under exploration. A number of studies are targeting the postautologous CAR T-cell failures given the unmet clinical need. Prioritizing trial enrollment is critical.
Radiation can also be an effective treatment option for patients with relapsed disease after CAR T-cell therapy that is localized. In the large multicenter French registry DESCAR-T study, patients with localized disease (n = 12) who received radiation therapy had an ORR of 35.7% with a CR of 14.3%, median PFS of 3.7 months, and a median OS of 9.6 months.25 Another series of 14 patients with localized relapse after CAR T-cell therapy demonstrated sustained benefit with site-specific radiation, including in high-risk patients with double-hit lymphoma and patients refractory to chemotherapy, with a median response rate of 43% and median OS of 10 months. While radiation alone may not achieve durable remissions, radiation therapy can also be integrated with other novel agents or transplantation to achieve long-lasting remissions.26
Allogeneic stem cell transplant (allo-HCT) remains a potential strategy to address post–CAR T-cell relapses in fit patients with suitable donors. A recently published large Center for International Blood & Marrow Transplant Research (CIBMTR) multicenter study evaluating efficacy and toxicities of allo-HCT following CD19 CAR T-cell failure demonstrated 1-year OS, PFS, and graft-versus-host disease-free relapse-free survival rates of 59%, 45%, and 39%, respectively. One-year nonrelapse mortality and progression/relapse were 22% and 33%, respectively. These data suggest that allo-HCT can provide durable disease control in a proportion of transplant-eligible, fit patients.27
CLINICAL CASE (continued)
Given our patient had a 6-month response following CAR T-cell therapy, she was deemed an excellent trial candidate given full count recovery and no evidence of significant comorbidities. She enrolled on a novel phase 1 trial with a lenalidomide-based approach. Unfortunately, she experienced disease progression. She then went on to receive standard-of-care lonca and achieved a CR that lasted 9 months. With recent progression, we are exploring clinical trial options or a CD20- bispecific antibody.
Conclusions
CAR T-cell therapy has transformed our approach to chemorefractory DLBCL. However, many of these patients are expected to experience disease relapse, and with CAR T-cell therapy moving into earlier lines of treatment, there is a great unmet need for understanding the optimal management of patients in the post–CAR T-cell space. These patients can have persistent toxicity in the form of prolonged cytopenias, comorbidities, and risk of infection. However, prioritizing pursuit of novel therapeutic trials is critical given the promising therapies currently under investigation and the gap in knowledge about how to best serve these patients. In our practice, we will pursue a clinical trial as our preferred approach for patients experiencing disease progression after CAR T-cell therapy. However, for patients where a clinical trial is not feasible, we consider patient- and disease-specific characteristics to navigate the available standard-of-care options. Like many in the field, we are optimistic about the CD20 × CD3 bispecific antibodies that will be our preferred option after CAR T-cell therapy, but many questions remain, including the potential for overlapping mechanisms of failure such as T-cell exhaustion and potential risk for cytopenias or infection. We must continue to explore real-world evidence as these novel therapies enter the treatment landscape to inform treatment selection as the treatment landscape continues to evolve.
Conflict-of-interest disclosure
Loretta J. Nastoupil has received honorarium for participation in advisory boards/consulting from Abbvie, ADC Therapeutics, Atara Biotherapeutics, BMS, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Janssen, Incyte, Merck, Novartis, Regeneron, and Takeda; research support from BMS, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/ Roche, Genmab, Gilead/Kite, IGM Biosciences, Janssen, Novartis, and Takeda.
Swetha Kambhampati Thiruvengadam: no competing financial interests to declare.
Off-label drug use
Loretta J. Nastoupil: There is nothing to disclose.
Swetha Kambhampati Thiruvengadam: There is nothing to disclose.