Abstract
Hematologic malignancies often present acutely with a constellation of infectious complications, pancytopenia, tumor lysis, and renal dysfunction. Acute leukemias and aggressive lymphomas often require hospitalization for rapid diagnostic evaluation, urgent management of complicating presentations, and timely management of intensive systemic therapies. There is an emerging paradigm whereby complex cancer care can be safely and effectively provided in the community, where the majority of cancer is treated. A substantive and effective network between local oncologists and their academic counterparts will enhance care for the patient, advance research, and help bring complicated therapies to local centers, thereby improving access. Here we present several cases that highlight a collaborative approach to complicated hematologic malignancies in the community.
Learning Objectives
Identify aggressive hematologic malignancies and initiate appropriate diagnostic and supportive measures rapidly
Understand the timing of involving an academic center or disease expert
Explore the role and use of sophisticated therapies along with toxicities in the community
Introduction
Acute leukemias (acute myeloid leukemia/acute lymphoblastic leukemia) and non-Hodgkin lymphoma (NHL) represent about 1.3% and 4.1%, respectively, of all new cancer cases annually, with the aggressive NHL subgroup approximation likely to be in the 2% to 3% range.1,2 The combination of rarity, the need for intensive and urgent therapies, protracted transfusion support, and significant complications have traditionally left these challenges to be managed largely in the academic arena with disease experts.
While the historical precedent was tertiary center referral if options were locally unavailable, geographic and socioeconomic considerations limit this option for most patients. Fortunately, runaway advances in immune engager therapy and the expansive infrastructure of community- based clinical trials that has developed over the past 2 decades have helped bridge this gap considerably. We present cases that crystalize the democratized access to sophisticated therapy in patients with hematologic malignancies in the community.
CLINICAL CASE 1
An 18-year-old woman with heavy menses/gingival bleeding presents with the following: white blood cell (WBC) count, 28; hemoglobin, 10; platelets, 15; coagulopathy; and a peripheral smear showing 75% blasts. She was empirically initiated on all trans-retinoic acid (ATRA) for possible acute promyelocytic leukemia (APL) vs M4 acute myeloid leukemia. She was aggressively transfused to maintain platelets >50 000, fibrinogen >150, and fresh frozen plasma to normalize prothrombin time/partial thromboplastin time. Prophylactic prednisone (0.5 mg/kg/d) was instituted with ATRA to preemptively address the possibility of high-risk APL presenting with a WBC >10.
APL has historically had the highest incidence of early mortality rates mostly due to hemorrhagic complications leading to death. Early intervention with ATRA, along with transfusion support to remediate coagulopathies, has yielded cure rates of >80%. In conjunction with empiric therapy, minimizing invasive procedures decreases complications related to disseminated intravascular coagulation (DIC). ATRA should be started before confirmatory molecular studies if there is a high index of suspicion based on presentation, peripheral smear, and coagulopathy. Delays in starting ATRA are associated with potentially fatal hemorrhagic complications while initiating ATRA in a non-APL diagnosis has little associated toxicity and can readily be discontinued once a diagnosis has been formally made. While it is not unreasonable to reach out to an academic center for a potential transfer of care, the diagnostic and supportive measures instituted locally within the first 24 to 48 hours can be life-saving and need to be recognized by all.
CLINICAL CASE 1 (continued)
Flow cytometry on the peripheral blood/bone marrow aspiration revealed HLA-DR– and CD33+ with fluorescence in situ hybridization ultimately showing t(15;17) 48 hours later consistent with APL.
The patient developed pulmonary decompensation with ATRA syndrome, requiring intubation despite initiating high-dose dexamethasone. ATRA was held and arsenic trioxide (ATO) and idarubicin were initiated since differentiation syndrome rates are similar with ATRA/ATO or ATRA/chemotherapy, despite arsenic having a risk of DS.3 With aggressive management of the DIC, tumor lysis syndrome prophylaxis, and differentiation syndrome, oxygenation improved, permitting the reintroduction of ATRA. The remainder of her course was notable for an upper-extremity deep venous thrombosis managed with serial Dopplers in place of anticoagulation due to thrombocytopenia and ongoing DIC, along with an asymptomatic 4-mm central nervous system (CNS) bleed.
In high-risk APL, it is reasonable to use an ATRA/ATO backbone, which has established data in the low and intermediate subgroups but remains an outstanding question for use in all risk groups. The addition of gemtuzumab ozogamicin or idarubicin to ATRA/ATO has been shown to yield overall survival >85% in high-risk patients.4-6 In the APL 15 trial, Wang et al7 investigated ATRA/ATO with and without chemotherapy for all risk groups. The high-risk group represented about one-third of the patients, and the 2-year disease-free survival was 94% and 87% for the ATRA/ATO and ATRA/ATO/chemotherapy, respectively. This study was designed to be a noninferiority study, which opens the way for further investigation before holding all gemtuzumab ozogamicin or idarubicin.
CLINICAL CASE 1 (continued)
The patient was polymerase chain reaction negative about 36 days from the start of induction and facilitated the transition to outpatient ATO/ATRA consolidation along with continued CNS prophylaxis.
ATO and ATRA consolidation was initiated with ATO 0.15 mg/kg 5 d/wk for 1 to 4 weeks and then repeated every 8 weeks × 4 cycles with ATRA 45 mg/m2 for 7 days on and 7 days off for 28 weeks. Our group has a dedicated team approach to continue consolidation with a nurse navigator, advanced practice provider (APP), and a malignant hematology physician that leads the care team (see Table 1).8 We set up thrice-weekly APP visits with electrocardiograms, as well as supportive labs/infusions on site to replete electrolytes in the setting of arsenic. Our supportive ancillary team with nutrition, social work, and palliative care buttressed the clinical management. Our clinic is open 7 days a week to effectively manage these complicated patients and their needs as well as 24-hour/7-day availability of our hematologic malignancy team to any of the surrounding clinics and smaller hospitals to help manage or transfer patients to a central site of care.
Infrastructure required to support delivery of supportive care after intensive chemotherapy in the outpatient setting
| Inpatient management | • Nursing education on treatment, roadmap, expected complications • CVC education and training • Clear written discharge instructions with information for nonurgent and emergent situations • Clear communication with outpatient team |
| Outpatient management | • SOP for CVC care, antimicrobial prophylaxis, transfusion thresholds, management of neutropenic fever • 24-hour phone access to experienced provider in AML for emergencies • Regular care team available for 3 times per week visits and nonscheduled evaluations of symptoms • Infusion center with extended daily and weekend/holiday hours for frequent monitoring and transfusion • Blood bank with large transfusion capability and rapid delivery of blood products to clinic setting • Ability to rapidly evaluate and initiate treatment of neutropenic fever in clinic (eg, antimicrobial cocktail available for rapid administration before hospital transfer) • Multidisciplinary expertise (infectious disease, pulmonary) in management of AML and therapy complications • Ancillary support staff with expertise in AML management; nursing, social worker, pharmacists, physical therapists, nutritionists |
| Inpatient management | • Nursing education on treatment, roadmap, expected complications • CVC education and training • Clear written discharge instructions with information for nonurgent and emergent situations • Clear communication with outpatient team |
| Outpatient management | • SOP for CVC care, antimicrobial prophylaxis, transfusion thresholds, management of neutropenic fever • 24-hour phone access to experienced provider in AML for emergencies • Regular care team available for 3 times per week visits and nonscheduled evaluations of symptoms • Infusion center with extended daily and weekend/holiday hours for frequent monitoring and transfusion • Blood bank with large transfusion capability and rapid delivery of blood products to clinic setting • Ability to rapidly evaluate and initiate treatment of neutropenic fever in clinic (eg, antimicrobial cocktail available for rapid administration before hospital transfer) • Multidisciplinary expertise (infectious disease, pulmonary) in management of AML and therapy complications • Ancillary support staff with expertise in AML management; nursing, social worker, pharmacists, physical therapists, nutritionists |
AML, acute myeloid leukemia; CVC, central venous catheter; SOP, standard operating policy.
Ref. Halpern and Walter.8
The role of maintenance therapy in the era of ATRA/ATO remains controversial if molecular remission is achieved by the end of consolidation.9 The high-risk population who has had an ATRA/ATO backbone as part of the induction warrants further investigation with regards to the benefits vs toxicity of a maintenance regimen and a corollary of the optimal drugs, whether it be ATRA alone or in combination with ATO.7
While arsenic does have CNS penetration, there remains a small risk of CNS relapse in patients with a CNS bleed or presenting with a high white count. Many of the large pinnacle APL trials did not offer CNS prophylaxis despite a number of them including high-risk patients, and despite that, very low rates of CNS relapse were noted.10-12 Since the patient presented with a high WBC and a small CNS bleed, a comprehensive discussion was had with the patient and her family regarding the current data. Due to her age and the negative factors noted, CNS prophylaxis was delivered via the intrathecal route once her coagulopathy and circulating blasts had cleared. She remains in complete remission 3 years out.
CLINICAL CASE 2
A 19-year-old man presented with stage IV diffuse large B-cell lymphoma (DLBCL) with a T-cell–rich component, a 30-cm spleen, a 20-cm abdominal mass, significant pancytopenia, and ascites. Fluorescence in situ hybridization testing revealed rearrangements of C-MYC but failed to show abnormalities in BCL-2 and BCL-6. His bone marrow biopsy specimen was negative. National Institutes of Health pathology review confirmed the initial diagnosis.
Given the presentation and C-MYC rearrangement, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (R-EPOCH) was chosen for induction therapy with the hope of intensifying doses based on the dose-adjusted algorithm, although cytoxan, doxorubicin, vincristine, prednisone, rituximab (R-CHOP) would be a completely viable and comparable option.13,14 From a practical standpoint, this critically ill young man needed his first cycle of therapy in the hospital setting due to tumor lysis syndrome, extensive cytopenias, and borderline performance status. This case predated the approval of polatuzamab-cyclophosphamide, doxorubicin, prednisone (CHP), which could also have been a first-line option,15 but polatuzamab may not be readily available in all hospital settings.
After 3 cycles of dose-adjusted R-EPOCH, he had an improvement in his hematologic parameters, spleen size, and tumor masses, but his disease progressed within weeks of completing his sixth R-EPOCH. Transfusion-dependent cytopenias precluded him from participating in clinical trials, and he underwent splenic external beam radiotherapy to improve his counts followed by second-line therapy with rituximab, ifosfamide, carboplatin, etoposide (R-ICE). Colleagues at an academic center were consulted for stem cell evaluation vs chimeric antigen receptor T cells (CAR-T) depending on response.
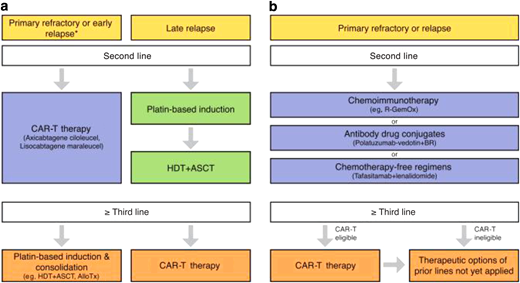
An early referral to an academic center at first relapse allows for greater coordination of care since the ultimate path would lead to CAR-T or stem cell transplant (see Figure 1).16 Having strong relationships with academic centers and disease experts can help facilitate the rapid evaluation of patients in need of advanced therapies, especially since primary refractory DLBCL has broad resistance to cytotoxic therapy.
Therapeutic algorithm for patients with relapsed/refractory DLBCL. (a) For transplant-eligible patients, depending on the time point of relapse, either an anti-CD19 CAR-T therapy (using axicabtagene ciloleucel or lisocabtagene maraleucel) or platin-based induction followed by high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) represents the standard approach (*within 12 months after completion of first-line therapy). (b) For transplant-ineligible patients, chemoimmunotherapy, antibody drug conjugates, and chemotherapy-free regimens represent potential therapeutic options in second line. Third-line therapy using anti-CD19–directed CAR-T represents a potentially curative option for eligible patients.
Therapeutic algorithm for patients with relapsed/refractory DLBCL. (a) For transplant-eligible patients, depending on the time point of relapse, either an anti-CD19 CAR-T therapy (using axicabtagene ciloleucel or lisocabtagene maraleucel) or platin-based induction followed by high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) represents the standard approach (*within 12 months after completion of first-line therapy). (b) For transplant-ineligible patients, chemoimmunotherapy, antibody drug conjugates, and chemotherapy-free regimens represent potential therapeutic options in second line. Third-line therapy using anti-CD19–directed CAR-T represents a potentially curative option for eligible patients.
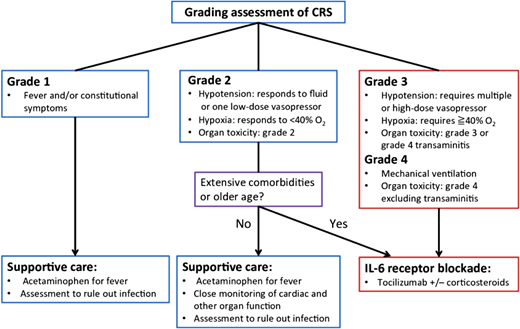
CAR-T has revolutionized treatment for patients with relapsed/refractory DLBCL who cannot attain a complete remission to move to allogeneic hematopoietic cell transplantation (AHCT) or have relapsed after AHCT. While response rates are high, durable remission is achieved in approximately 30% to 40% of patients. Axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel are the 3 anti-CD19 CAR-T therapies that are approved by the US Food and Drug Administration for relapsed DLBCL after ≥2 prior lines of systemic therapy or for primary refractory disease. Significant toxicities such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) can impact up to 20% and 30% of patients, respectively (see Figure 2).17-20 Accessibility and cost remain barriers to broader utilization as well.
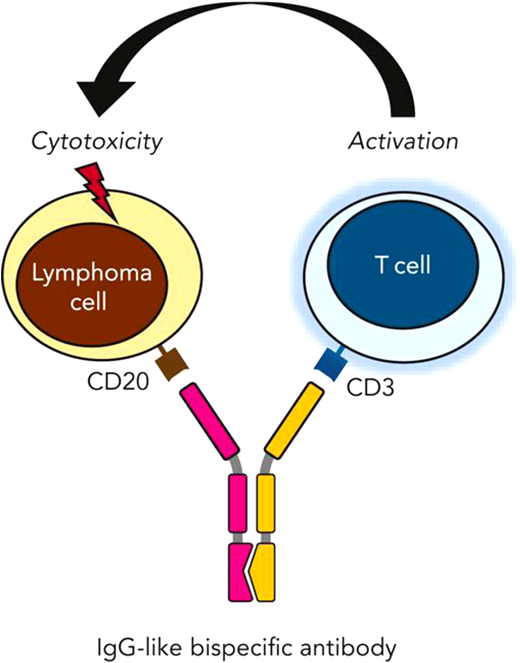
Possessing a Fab specific to an epitope on CD20 of malignant lymphoma cells along with a separate Fab segment for CD3 binding and subsequent T-cell activation, bispecific antibodies facilitate the creation of an immune synapse, resulting in T-cell–mediated cytotoxicity. Falchi et al.26
Possessing a Fab specific to an epitope on CD20 of malignant lymphoma cells along with a separate Fab segment for CD3 binding and subsequent T-cell activation, bispecific antibodies facilitate the creation of an immune synapse, resulting in T-cell–mediated cytotoxicity. Falchi et al.26
Phase 3 trials are exploring the benefit of second-line CAR-T over standard of care (SOC) followed by AHCT.21,22 Westin et al22 show in the ZUMA 7 trial an overall survival benefit with axicabtagene ciloleucel in the second-line setting compared to SOC. However, Bishop et al21 show in the BELINDA trial that tisagenlecleucel did not show a higher response rate or event-free survival in the AHCT group. Comparison is hindered by the differing number of salvage regimens, bridging therapy, organ-compromising disease, and percentage of patients receiving CAR-T. The PILOT study also offers an evolving CAR-T landscape for transplant-ineligible patients in the second line as well.23 Our patient's cytopenias precluded pheresis, and rapid disease progression necessitated cytotoxic salvage over moving to CAR-T at this point.
CLINICAL CASE 2 (continued)
In this clinical case, 2 cycles of R-ICE yielded a good partial response but residual hypermetabolic disease on positron emission tomography. However, count improvement allowed T-cell collection for CAR-T and ultimately administration. Unfortunately, his disease relapsed within 30 days. Repeat biopsy showed the absence of CD19, the presence of CD20, and no actionable mutations on next-generation sequencing.
Progression after CAR-T is associated with dismal outcomes,24 and the loss of surface CD-19 expression may render treatments approved by the Food and Drug Administration, such as tafasitamab-based25 therapy or loncastuximab tesirine,25 less effective, emphasizing the need for biopsy-informed therapy. For this reason, polatuzumab-bendamustine, rituximab was given locally. The local team and colleagues at academic centers remained engaged throughout the entirety of his clinical course.
He enrolled in a protocol at a quaternary academic center with an epicortimab/gemcitabine/oxaliplatin trial in place of an epicortimab monotherapy trial locally. He continued to be seen locally to manage complications and transfusions and remain with family.
Durable remissions can be challenging after CAR-T therapy, but bispecific monoclonal antibodies (BsAbs) are offering encouraging results (see Figure 3).26 With antibodies targeted at the CD20 on DLBCL and the CD3 on T cells, glofitamab has shown complete response in about 39% of heavily treated patients for a median of 12.6 months. However, much like CAR-T, these BsAb therapies have significant toxicities, including CRS and ICANS, which need to be carefully considered and managed.27
CRS is a known complication of T-cell redirecting therapy, observed in patients receiving CAR-T and/or bispecific antibodies, with clinical manifestations a result of an exuberant immune activation and concomitant proinflammatory cytokine production. Makita et al.20 Grading is predicated on progressive symptoms from the hyperinflammatory state ranging from fevers to the development of hypoxia and vasodilatory mediated hypotension. Stepwise but rapid escalation of therapy is needed according to the severity of symptoms and is critical to avoid adverse clinical outcomes.
CRS is a known complication of T-cell redirecting therapy, observed in patients receiving CAR-T and/or bispecific antibodies, with clinical manifestations a result of an exuberant immune activation and concomitant proinflammatory cytokine production. Makita et al.20 Grading is predicated on progressive symptoms from the hyperinflammatory state ranging from fevers to the development of hypoxia and vasodilatory mediated hypotension. Stepwise but rapid escalation of therapy is needed according to the severity of symptoms and is critical to avoid adverse clinical outcomes.
CLINICAL CASE 2 (continued)
Unfortunately, his disease proved to be resistant to epcortimab and a further salvage with tafasitamab/lenalidomide as a bridge to a trial with allogeneic CAR-T cell therapy. He succumbed to his progressive disease with hepatic failure after an 18-month struggle.
While the outcome in this young adult with primary refractory NHL was quite devastating, his parents and family took solace in the fact that he received novel options and were deeply grateful for our guidance and quaternary care collaboration toward a common goal.
CLINICAL CASE 3
An 84-year-old man with excellent health and an Eastern Cooperative Oncology Group Performance Status of 0 developed mantle cell lymphoma 6 years before presentation to our clinic. He achieved a durable response to rituximab bendamustine followed by rituximab maintenance. Approximately 2 years after completion of maintenance, he developed rapidly progressive abdominal adenopathy with compression of the renal vasculature, resulting in acute kidney injury. Successive trials of acalabrutinib and rituximab lenalidomide were met with an attenuated response, a lymphoma mass effect upon the inferior vena cava resulting in volume retention, and electrolyte derangements.
Relapsed mantle cell lymphoma has been a notoriously challenging disease, with the historical literature reporting 20% to 30% response rates on various combinations of salvage chemoimmunotherapy, discouragingly brief progression-free survival intervals,28 and the absence of a coalescent approach. Consequently, the efficacy of Bruton tyrosine kinase inhibitors (BTKis) was particularly welcomed. First reported by Wang and colleagues,29 ibrutinib resulted in unprecedented response rates of 68% and a progression-free survival of 17.5 months. Acalabrutinib30 and zanubrutinib31 have similarly improved upon this benchmark and are regularly used by community oncologists, familiar with this class of agents after almost a decade of experience with chronic lymphocytic leukemia. Nonetheless, they are not curative, and at the inexorable progression, there is a dearth of choices. While cellular therapy with brexucaptagene autocel has regulatory approval, the geographic logistics precluded our ability to realistically consider this as an option.32
Fortunately, our center was participating in a clinical trial evaluating outpatient administration of a BsAb therapy. The study is pivotally tasked with assessing both efficacy and safety, with emphasis on community-based outpatient management of CRS and ICANS. The patient received the investigational agent on a weekly step-up dosing regimen before commencing the maintenance phase. His course was complicated by grade 1 CRS, managed entirely as an outpatient with supportive care medications, close monitoring with daily phone calls and home vital signs, and immediate office evaluation for concerning symptoms. After the first 3 weeks of therapy, he had complete resolution of his significant lower extremity edema, orthopnea, and acute kidney injury. Surveillance imaging corroborated the clinical improvement with a complete metabolic response on functional imaging, which remains ongoing. It is not difficult to envision a scenario where this patient would have been recommended entirely supportive measures with a transition to end-of-life care in the recent past. The availability of an investigational bispecific antibody at an experienced nonacademic research center, however, was able to meaningfully extend a life of excellent quality in a previously dire scenario.
Discussion
Hematologic malignancies can present in a clinical crisis with a high disease burden, transfusion needs, complicated infections, and rapid proliferation all requiring a rapid activation of medical, diagnostic, and therapeutic interventions. Traditionally, this has been the domain of disease experts in academic centers. However, greater than 80% of cancer care is provided in the community setting. With the advent of complex, targeted regimens, older and even medically unfit patients may have more options. However, these are the very patients who want to manage therapy in their communities where support is the strongest and quality of life is optimized.33
Oncology has had a seismic shift toward personalized care with a better understanding of the molecular drivers of the disease informing treatment choices. In this evolving environment, even within community oncology practices, there is a subspecialization that aligns with therapeutic options similar to academic centers.34 Outcome data for patients treated at academic centers vs community centers along with clinical trial engagement are confounded by socioeconomic, racial, and financial biases. These same barriers also impact the access of patients to clinical trial enrollment and are now a focus of regulatory authorities and trial sponsors (https://www.fda.gov/consumers/minority-health-and-health-equity/clinical-trial-diversity).
Community-hybrid practices combine medical oncologists, radiation and surgical oncologists, palliative care MD/APPs, genetic counselors, and ancillary supportive care such as nutritionists and social workers to provide comprehensive care locally. With the increase in subspecialization, the number of clinical trials and participation in these trials also rise.35 Our common goal is to provide patients with excellent cancer care comprehensively with access to clinical trials closer to home, which may lower some barriers to enrollment. Breakthrough therapies may originate in the academic setting, but a closely coordinated clinical and educational paradigm between the community and academic oncologists can help operationalize new therapies quickly and safely locally as well as define options for the academic setting.
The simplest method to achieve this is through direct communication, strong ties, and transparency.
The cost of cancer care is rising exponentially with cellular therapies, targeted drugs, and management of associated therapies in hematologic malignancies, but also in all aspects of oncology. In the era of value-based oncology care, treatments in community-based clinics are more cost-efficient than the same care offered in hospital-based clinics.36 As the cost of our complicated therapeutics increases, further investigation is certainly warranted for the operationalization of novel therapies quickly and effectively into the community for equity and impact.
Jillella et al37 provide an effective, collaborative model of early education with their community practices on the identification of APL and access to experts, along with defined treatment algorithms and a comanagement strategy that allows community oncologists to maintain early mortality rates similar to academic institutions. Engagement with community oncologists and their academic peers more intricately can lead to gains toward a common goal with the possibility of lowering costs and increasing clinical trial enrollment locally while ultimately benefiting patients.
Conflict-of-interest disclosure
Dipti Patel-Donnelly: ABIM medical oncology Longitudinal Knowledge Assessment Committee.
Mitul Gandhi: Advisory boards: Janssen Oncology 11/2021, Sanofi 12/2022, Janssen 5/2023.
Off-label drug use
Dipti Patel-Donnelly: none.
Mitul Gandhi: none.