Abstract
The care of the multiple myeloma (MM) patient is complex, with most patients requiring multiple lines of therapy over a span of many years to decades. Since the days when autologous stem cell transplantation became the standard of care for a large subset of patients, it was imperative that community practices and specialized academic centers work together to optimize the initial care of patients. Now, with the unprecedented number of treatment options and the introduction of chimeric antigen receptor T-cell therapies and bispecific T-cell engagers, that collaboration has become even more important and stretches from the upfront treatment to the relapsed and refractory disease setting. I will discuss the unique safety profile and logistical aspects that pose challenges and opportunities for the safe and successful delivery of these therapies. Close interaction, communication, and established partnerships between the primary oncologist, the myeloma specialist, and the transplant or immune effector cell provider will be required to provide the optimal care longitudinally for each patient. This multidisciplinary approach to treating MM can serve as a paradigm for blending community and academic care.
Learning Objectives
Identify best practices for safe and successful delivery of therapies for multiple myeloma
Identify opportunities to anticipate and address acute phase and late phase toxicity
Prepare and plan for a clinical model that promotes a good rapport between academic and community practices
CLINICAL CASE
A 66-year-old man is diagnosed with revised international staging system II IgGk multiple myeloma with standard risk cytogenetics after presenting with back and left hip pain. He undergoes induction with bortezomib, lenalidomide, and dexamethasone and achieves a very good partial response (VGPR) after 4 cycles. He is referred to the local transplant center, where he undergoes autologous stem cell transplantation (ASCT). He does well and is maintained on single-agent lenalidomide. Three years later, he has progression indicated by rising paraprotein and begins salvage therapy with daratumumab, carfilzomib, and dexamethasone. He achieves VGPR, but 18 months later, he has elevated calcium and rising paraprotein. He then moves on to elotuzumab, pomalidomide, and dexamethasone. He achieves partial response (PR) but, 12 months later, has progression indicated by rising paraprotein. You begin fourth-line selinexor and dexamethasone. He has a minor response and has progression 4 months later. He is felt to be an excellent candidate for bispecific or chimeric antigen receptor (CAR) T-cell therapy. Are you and your institution ready to proceed with this type of therapy? Will you need to refer to an academic/ transplant center? What are the next steps in shepherding this patient safely and successfully through this process?
Introduction
Multiple myeloma (MM) is the second-most common hematologic malignancy and is characterized by clonal expansion of malignant plasma cells.1 Although largely incurable, the incorporation of ASCT, proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and anti-CD38 monoclonal antibodies have resulted in continued improvement in overall survival.2 Unfortunately, despite these advances, the median survival of patients who progress after a PI, IMiD, and anti-CD38 antibody is quite poor.3 B-cell maturation antigen targeting treatments have emerged as effective antimyeloma therapies in triple class refractory (TCR) MM patients. Two CAR T-cell therapies, idecabtagene vicleucel (ide-cel)4 and ciltacabtagene autoleucel (cilta-cel),5 and one bispecific T-cell engager, teclistamab,6 are now approved by the US Food and Drug Administration (FDA) for patients in this setting. Several others are being investigated in clinical trials.
ASCT and use of CAR T-cells, and bispecific T-cell engagers may require referral from the community to an academic or transplant center. Although these therapies are not unique to MM, the inability for any of these therapies to be curative on their own will likely mean incorporation of more than 1 of these types of therapies along the treatment course for a patient. Thus, to provide optimal care for a myeloma patient, a good strategy between the community practitioner and the specialist will be of utmost importance at several times along the care of an MM patient. These unique nuances may pose both a challenge and opportunity for delivery and access of care, but MM treatment is poised to serve as a paradigm for the treatment other diseases.
Autologous stem cell transplantation
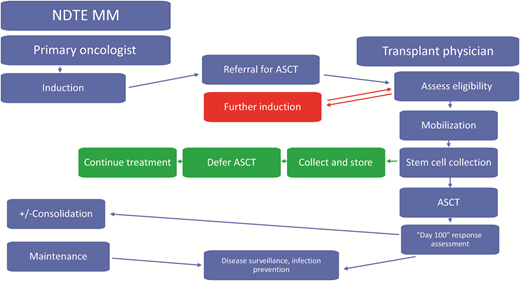
The initial treatment of a transplant-eligible patient with myeloma currently is likely to require induction chemotherapy followed by ASCT consolidation and finally maintenance therapy.7,8 This initial line of therapy already requires and establishes the relationship between the treating oncologist and a transplant physician who often is located in a separate practice at an academic center or specialized transplant center. Depending on when the initial referral is made, establishing close communication is key and includes discussion about the specific induction regimen used, the number of cycles needed, and coordination of transfer for leukapheresis and stem cell collection (Figure 1). Sometimes further or alternate induction is needed prior to ASCT, and this is usually delivered at the primary oncologist practice in close coordination with the transplant center. Following ASCT, the close contact continues and includes choice of consolidation and maintenance therapy and posttransplant interventions, such as prophylactic medications and revaccination. On occasion, the decision may be made to collect stem cells to store for future use and not proceed with the transplant at that time. In that scenario, the patient will need to continue further induction and maintenance per the nontransplant standard of care. This paradigm is well established and highlights the close communication between the treating oncologist and the transplant physician for the optimal delivery of care for this first line of therapy already in place.
Management of a newly diagnosed transplant-eligible (NDTE) patient with multiple myeloma (MM): key roles of the primary oncologist and transplant physician. ASCT, autologous stem cell transplantation.
Management of a newly diagnosed transplant-eligible (NDTE) patient with multiple myeloma (MM): key roles of the primary oncologist and transplant physician. ASCT, autologous stem cell transplantation.
Autologous CAR T-cell therapy
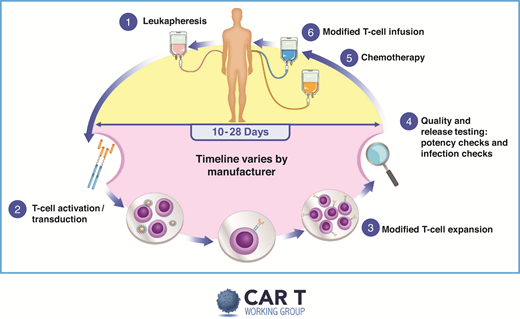
Without a doubt, the resource-heavy processing and delivery of autologous CAR T products provide unique challenges. A pictorial representation of the basic autologous CAR T process is shown in Figure 2. The initial process best mirrored what had already been established by transplant programs. But, even then, the processes in place required adjustments, and specific immune effector cell programs/paradigms were created. Figure 3 depicts some of the practical and logistic differences between the standard autologous hematopoietic cell transplantation process and the autologous CAR T process. The current indication for use of CAR T-cells in myeloma is in the relapsed and refractory space. Thus, patients often are being evaluated in the setting of relapsing or high burden disease that may make it difficult for the patient to withstand the inherent treatment delays. Furthermore, having undergone multiple lines of therapy over the course of many years, the patient may have developed residual cytopenias and organ dysfunction that may preclude eligibility.
Overview of CAR T therapy. Reproduced with permission from the slide library of the chimeric antigen receptor (CAR) T Working Group (v2 9.4.2019).
Overview of CAR T therapy. Reproduced with permission from the slide library of the chimeric antigen receptor (CAR) T Working Group (v2 9.4.2019).
AHCT vs CAR T therapy: similarities and differences. AHCT, autologous hematopoietic cell transplantation; CAR, chimeric antigen receptor; IT, information technology; PBMC, peripheral blood mononuclear cells.
AHCT vs CAR T therapy: similarities and differences. AHCT, autologous hematopoietic cell transplantation; CAR, chimeric antigen receptor; IT, information technology; PBMC, peripheral blood mononuclear cells.
Table 1 depicts some considerations to determine if a patient is appropriate for CAR T therapy. Close attention should be paid to the immediate therapy given prior to planned T-cell collection in order to maximize a functional and efficacious CAR T product.9-12 After T cells are secured for CAR T manufacturing, there will likely be a 4- to 6-week window of time before CAR T-cells are infused. Many patients will need to undergo bridging therapy to control or debulk their disease to both minimize toxicity and maximize efficacy of therapy. Figure 4 outlines some consideration for selection of the most appropriate bridging therapy, which may be different for each patient. These various time points require very specific decisions and treatments delivered by both the primary oncologist and the immune effector cell provider, which again highlights the importance of close communication and collaboration.
Bridging therapy considerations for CAR T therapy. BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; CD, clusters of differentiation; DCEP, dexamethasone, cyclophosphamide, etoposide, and cisplatin; PI, proteasome inhibitor; IMID, immunomodulatory drugs; VDPACE, bortezomib, dexamethasone, cisplatin, adriamycin, cyclophosphamide, and etoposide.
Bridging therapy considerations for CAR T therapy. BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; CD, clusters of differentiation; DCEP, dexamethasone, cyclophosphamide, etoposide, and cisplatin; PI, proteasome inhibitor; IMID, immunomodulatory drugs; VDPACE, bortezomib, dexamethasone, cisplatin, adriamycin, cyclophosphamide, and etoposide.
Key considerations to determine if CAR T-cell therapy is appropriate for a patient with multiple myeloma
| Consideration . | Comments . |
|---|---|
| Indication | • Does the patient meet the label indication or clinical trial eligibility? |
| Kinetics of disease progression | • Is the patent able to come off all therapy, including a 2-week washout, prior to leukapheresis? • Does the patient need alternative therapy prior to CAR T-cell therapy consideration? |
| Immediate therapy prior to leukapheresis | • How would this affect the ability to successfully manufacture CAR T-cells (ie, obtain sufficient numbers of T-cells and expand)? • Recent alkylators, prior BCMA therapies may impact effectiveness of CAR T-cells. |
| Need for bridging | • Does patient need bridging therapy while waiting for CAR T-cell manufacturing? |
| Contraindicated medications | • Immunosuppressants, anticoagulants. • Can these be safely stopped prior to collection and acute treatment phase? |
| No active infection | • Higher risk of complications if patient experiences CRS. |
| Adequate organ function (renal, cardiac, pulmonary, BM) | • Is the patient healthy enough to receive LD chemotherapy? • Does the patient have organ function reserve to tolerate toxicities of CR T-cell therapy, namely CRS and ICANS? |
| Consideration . | Comments . |
|---|---|
| Indication | • Does the patient meet the label indication or clinical trial eligibility? |
| Kinetics of disease progression | • Is the patent able to come off all therapy, including a 2-week washout, prior to leukapheresis? • Does the patient need alternative therapy prior to CAR T-cell therapy consideration? |
| Immediate therapy prior to leukapheresis | • How would this affect the ability to successfully manufacture CAR T-cells (ie, obtain sufficient numbers of T-cells and expand)? • Recent alkylators, prior BCMA therapies may impact effectiveness of CAR T-cells. |
| Need for bridging | • Does patient need bridging therapy while waiting for CAR T-cell manufacturing? |
| Contraindicated medications | • Immunosuppressants, anticoagulants. • Can these be safely stopped prior to collection and acute treatment phase? |
| No active infection | • Higher risk of complications if patient experiences CRS. |
| Adequate organ function (renal, cardiac, pulmonary, BM) | • Is the patient healthy enough to receive LD chemotherapy? • Does the patient have organ function reserve to tolerate toxicities of CR T-cell therapy, namely CRS and ICANS? |
BM, bone marrow; BCMA, B-cell maturation antigen; CART, chimeric antigen receptor T-cell; CR, complete response; CRS, cytokine release syndrome; ICANS, immune effector cell–associated neurotoxicity syndrome; LD, lymphodepletion.
Toxicity profile
The toxicity profile associated with cellular redirecting therapies have been extensively described elsewhere and are summarized in Table 2.13-16 In general, toxicity can be divided into an acute phase (30 days) and a late phase (beyond 30 days). In the acute phase, cytokine release syndrome, neurotoxicity, and cytopenias predominate. The rapidity and onset of these potential toxicities require expert, multidisciplinary vigilance, availability, and management. Currently, these toxicities are treated mostly in the inpatient setting, but they are slowly starting to be managed as in outpatient settings as well.
CAR T-associated toxicities
| Acute phase (Days 0-30) . | Late phase (After first 30 days) . |
|---|---|
| • CRS • ICANS • Cytopenias ○ MAS or HLH is a very rare and severe form ○ DIC • B-cell aplasia and hypogammaglobulinemia • Life threatening if not managed by expert multidisciplinary team • Tumor lysis is rare and likely varies by disease and disease burden | • Persistent cytopenias • B-cell aplasia and hypogammaglobulinemia ○ IVIG replacement? • T-cell deficiency ○ PJP and VZV prophylaxis, other? • Infection prophylaxis • Residual effects of acute toxicity • Delayed CRS and neurotoxicity is rare but can occur • Impairment to QOL—fatigue, memory issues, not yet well described |
| Acute phase (Days 0-30) . | Late phase (After first 30 days) . |
|---|---|
| • CRS • ICANS • Cytopenias ○ MAS or HLH is a very rare and severe form ○ DIC • B-cell aplasia and hypogammaglobulinemia • Life threatening if not managed by expert multidisciplinary team • Tumor lysis is rare and likely varies by disease and disease burden | • Persistent cytopenias • B-cell aplasia and hypogammaglobulinemia ○ IVIG replacement? • T-cell deficiency ○ PJP and VZV prophylaxis, other? • Infection prophylaxis • Residual effects of acute toxicity • Delayed CRS and neurotoxicity is rare but can occur • Impairment to QOL—fatigue, memory issues, not yet well described |
CRS, cytokine release syndrome; DIC, disseminated intravascular coagulopathy; HLH, hemophagocytic lymphohistiocytosis; ICANS, immune effector cell–associated neurotoxicity syndrome; IVIG, intravenous immunoglobulin; MAS, macrophage activation syndrome; PJP, pneumocystis jirovecii pneumonia; QOL, quality of life; VZV, varicella zoster virus.
For the most part, late-phase toxicity can be dominated by persistent or recurrent cytopenias and increased infection risk. Issues pertaining to infectious prophylaxis, revaccination, immunoglobulin deficiency, and need for replacement predominate. More and more, longer-term sequelae, such as fatigue, memory loss, and lapses in concentration, are starting to become better described.16-18 Although the optimal management of patients continues to evolve and be defined, there are clear processes and resources required for the safe delivery of these therapies.
Bispecific T-cell engagers
Unlike ASCT and autologous CAR T-cell therapy, which are usually done only once, the bispecific T-cell engagers require continuous therapy, much like other standard myeloma therapies. Furthermore, the off-the-shelf nature of these products significantly simplifies the overall process and allows for prompt initiation of therapy (Table 3) Thus, this therapy has the most potential for direct management and treatment by the community oncologist. However, although acute and late-phase toxicities are similar, both differ qualitatively and quantitatively from CAR T-cells and require careful attention to determine how to proceed next. In fact, the FDA recommends 48-hour hospitalization following each of the first 3 doses (first full dose and 2 step-up doses) of teclistamab. Some practices may continue the paradigm of referring patients to a specialized center for the entire treatment or at least the initial cycle, when the risks of cytokine release syndrome and neurotoxicity and the need for hospitalization may be high. Others practices will develop a spoke and wheel setup, with certain clinics but not others having the expertise to deliver the treatment. It should be noted that long-term data are not available. As studies mature, there is an emerging infection signal that does not abate but continues and may increase for as long as patient is on therapy. Thus, continuous surveillance and monitoring is imperative. The degree of collaboration between the primary oncologist and the academic center will depend on the treatment setting in place to deliver this therapy.
Bispecific antibodies vs CAR T-cell therapy
| . | Bispecific antibody . | CAR T-cell . |
|---|---|---|
| Approved product | Teclistimab | Ide-cel and Cilta-cel |
| Efficacy | +++ | ++++ |
| CRS | ++ | +++ |
| Neurotoxicity | + | ++ |
| Infections | +++ | +++ |
| How given | IC/SC every 1-4 weeks continuous | Only once |
| Availability | Off-the-shelf | Limited |
| Need for bridging | No | Often |
| Where given | Specialized center, Community possible | Specialized center |
| Unfit patient | Possible | Challenging |
| . | Bispecific antibody . | CAR T-cell . |
|---|---|---|
| Approved product | Teclistimab | Ide-cel and Cilta-cel |
| Efficacy | +++ | ++++ |
| CRS | ++ | +++ |
| Neurotoxicity | + | ++ |
| Infections | +++ | +++ |
| How given | IC/SC every 1-4 weeks continuous | Only once |
| Availability | Off-the-shelf | Limited |
| Need for bridging | No | Often |
| Where given | Specialized center, Community possible | Specialized center |
| Unfit patient | Possible | Challenging |
CAR, chimeric antigen receptor; CRS, cytokine release syndrome; IV, intravenous; SQ, subcutaneous.
The patient in this case was given the option of enrolling in a clinical trial or proceeding with FDA-approved therapy. The patient opted for teclistamab as a standard of care. In our large community practice, we currently follow the spoke and wheel model. The patient was able to receive therapy in a clinic only 20 minutes away from his home; although this was not his home clinic, he still did not have to travel 3 hours to get to the nearest academic center.
Conclusion
Since the adoption of ASCT as a standard of care for a subset of patients with MM, primary oncologists have established close relationships with myeloma specialists and/or transplant physicians in order to deliver safe and optimal care for their patients. Now, with the unprecedented number of treatment options and the introduction of CAR T-cell therapies and bispecific T-cell engagers, which have unique logistics and toxicities that span the entire treatment journey of a patient, the need for multidisciplinary collaboration has only strengthened. Close interaction, communication and established partnerships will be required to provide the optimal care longitudinally for any patient. This multidisciplinary approach to treating MM can serve as a paradigm for blending community and academic care.
Conflict-of-interest disclosure
Jesús G. Berdeja's company received research funding in his name from 2 Seventy Bio, Acetylon, BMS, CARsgen, Celgene, CRISPR Therapeutics, Fate Therapeutics, GSK, Incyte, Karyopharm, Novartis, Sanofi, and Teva. His company received consultation funding in his name from BMS, Celgene, CRISPR Therapeutics, Janssen, Kite Pharma, Legend Biotech, Roche, and Takeda.
Off-label drug use
Jesús G. Berdeja: Nothing to disclose.