Abstract
Patients with advanced chronic liver disease (CLD) often need procedures to both treat and prevent complications of portal hypertension such as ascites or gastrointestinal bleeding. Abnormal results for hemostatic tests, such as prolonged prothrombin time, international normalized ratio, and/or thrombocytopenia, are commonly encountered, raising concerns about increased bleeding risk and leading to transfusion to attempt to correct prior to interventions. However hemostatic markers are poor predictors of bleeding risk in CLD, and routine correction, particularly with fresh frozen plasma and routine platelet transfusions, should be avoided. This narrative review discusses the hemostatic management of patients with CLD using 2 case descriptions.
Learning Objectives
To evaluate periprocedural bleeding risk in patients with liver disease
To understand the role and limitations of prophylactic hemostatic interventions in the periprocedural setting
CLINICAL CASE 1
A 38-year-old woman attends a routine hepatology follow- up for known nonalcoholic steatohepatitis with unexplained jaundice. This has developed over the last few weeks and is associated with significant fatigue. Her blood tests reveal bilirubin 182 µmol/L, albumin 32 g/L, sodium 141 mmol/L, creatinine 89 µmol/L, hemoglobin 110 g/L, platelets 57 × 109/L, prothrombin time (PT) 24 s (international normalized ratio [INR] 2.4), activated partial thromboplastin time (APTT) 70 s, and Clauss fibrinogen 0.8 g/dL. Imaging confirms fatty liver disease but without definite features of cirrhosis. There is no evidence of portal hypertension, with spleen measuring 9.8 cm. A diagnostic liver biopsy is planned, and there is concern regarding the bleeding risk in view of her abnormal hemostatic test results.
Procedures are frequently required in patients with liver disease, and concern for bleeding arises both from the perception that chronic liver disease (CLD) constitutes a bleeding diathesis and that hemostatic parameters are commonly abnormal in patients with CLD. Reduced hepatic synthesis of hemostatic proteins along with portal hypertension and consequent hypersplenism commonly leads to prolonged PT, APTT, and thrombocytopenia in patients with CLD. While patients with CLD have a higher baseline risk of bleeding, this risk is largely mediated by portal hypertension. For example, in the PROLIVER study, of 280 patients (53% Child Pugh A) with CLD, 3.6% developed major bleeding per year, with 1.9% per year experiencing clinically relevant nonmajor bleeding.1 Of note, 91% of major bleeds were secondary to portal hypertension. Patients with acute decompensation or acute-on-chronic liver failure (ACLF) are reported to have higher rates of bleeding, and this remains predominantly mediated by portal hypertension. Drolz et al retrospectively reviewed bleeding in 211 patients with cirrhosis admitted to critical care and reported 17% had bleeding events.2 Bleeding was most commonly secondary to varices (5.9%), with 10 patients experiencing postprocedural/postoperative bleeding. Ow et al. reported 13% later bleeds (>48 h post admission) in a retrospective cohort of 623 consecutive patients with CLD admitted to critical care.3 Only 10 bleeds were related to procedures (ie, only 1.6% developed major bleeding related to procedures). This is of note because critically ill patients are frequently subject to multiple procedures over the course of their admission.
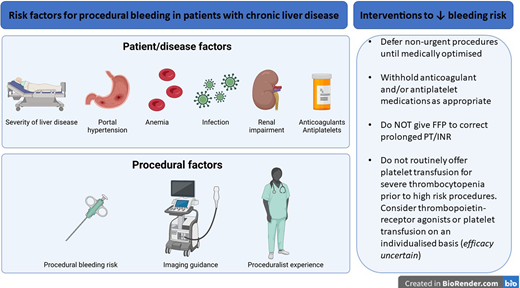
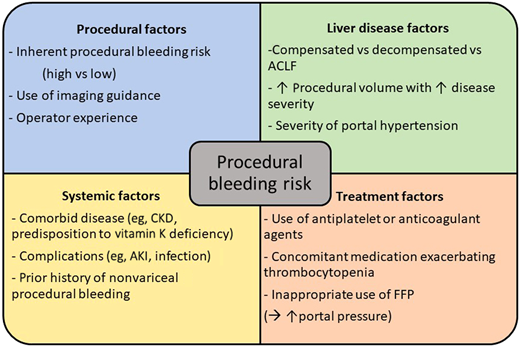
There is significant agreement in the recommended approach to periprocedural hemostatic management of patients with liver disease among recently published guidelines.4-8 All guidelines advocate considering the procedural bleeding risk and patient-specific bleeding risk factors. There is also agreement on avoiding fresh frozen plasma (FFP) and other blood components to correct prolonged PT/INR. The need for correction of thrombocytopenia and/or hypofibrinogenemia is less certain. The rationale for guidance recommendations is further presented in this manuscript. Factors contributing to procedural bleeding risk are summarized in Figure 1.
Factors which modulate bleeding risk in patients with chronic liver disease in the periprocedural setting. ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; CKD, chronic kidney disease; FFP, fresh frozen plasma.
Factors which modulate bleeding risk in patients with chronic liver disease in the periprocedural setting. ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; CKD, chronic kidney disease; FFP, fresh frozen plasma.
Procedural bleeding risk
Estimation of procedural bleeding risk for commonly performed procedures in patients with liver disease is largely derived from observational cohorts with variable use of prohemostatic therapy. Recent guidance categorizes procedures as low bleeding risk, for which the major bleeding rate is <1.5%, or high bleeding risk, for which the rate exceeds 1.5% or there is potential for uncontrolled bleeding or severe consequences (eg, organ failure or death).4-6 There is a need for data from large observational studies without empiric blood component support to accurately evaluate procedural bleeding risk; one such study is the PROC-BLeeD, the results of which are awaited (www.clinicaltrials.gov/ct2/show/NCT04076605). The suggested classification of bleeding risk for commonly performed procedures proposed by International Society of Thrombosis and Haemostasis is presented in Table 1. There is some variation in risk stratification between guidance documents; for example, the American Association for the Study of Liver Disease considers both transjugular and percutaneous approaches to liver biopsy to be high bleeding risk procedures, while the European Association of Liver Disease (EASL) considers both approaches to be low bleeding risk procedures, despite comparable definitions for bleeding risk. A recent observational cohort of 302 patients requiring diagnostic liver biopsy reported a low rate of major bleeding (0.6%), with no deaths and only 2 patients requiring vascular embolization to control bleeding.9 This study supports the EASL classification of both transjugular and percutaneous liver biopsy as low bleeding risk procedures.
Procedural bleeding risk classification of commonly performed procedures in patients with advanced chronic liver disease from the International Society of Thrombosis and Haemostasis Scientific Subcommittee
| . | Low bleeding risk . | High bleeding risk* . |
|---|---|---|
| Endoscopic | Diagnostic procedures Endoscopic variceal ligation Transesophageal echocardiogram | Bronchoscopy with biopsy Colonoscopy with polypectomy Endoscopic retrograde cholangiopancreatography with sphincterotomy |
| Percutaneous | Paracentesis Thoracocentesis | Percutaneous liver biopsy Tunneled ascitic/pleural drain placement Cranial/spinal surgery# |
| Vascular | Peripheral/central venous catheterization Transjugular liver biopsy | Transjugular intrahepatic portosystemic shunt Transcatheter arterial chemoembolization |
| Other | Dental procedures including extractions Skin biopsy | Intraocular procedures# |
| . | Low bleeding risk . | High bleeding risk* . |
|---|---|---|
| Endoscopic | Diagnostic procedures Endoscopic variceal ligation Transesophageal echocardiogram | Bronchoscopy with biopsy Colonoscopy with polypectomy Endoscopic retrograde cholangiopancreatography with sphincterotomy |
| Percutaneous | Paracentesis Thoracocentesis | Percutaneous liver biopsy Tunneled ascitic/pleural drain placement Cranial/spinal surgery# |
| Vascular | Peripheral/central venous catheterization Transjugular liver biopsy | Transjugular intrahepatic portosystemic shunt Transcatheter arterial chemoembolization |
| Other | Dental procedures including extractions Skin biopsy | Intraocular procedures# |
Classification based on major bleeding >1.5% or on minor bleeding associated with high risk of significant organ damage/death.
Very high-risk procedures.
Reproduced with permission from Elsevier Inc.6
Patient-specific factors for periprocedural bleeding risk
In patients with established cirrhosis, the severity of liver synthetic failure and/or portal hypertension may modulate bleeding risk. As highlighted above, the risk of bleeding is higher in critically ill patients, largely due to increased variceal bleeding. Procedural bleeds remain infrequent in these patients. Whilst procedural liver synthetic failure leads to profound changes on routine laboratory testing, with prolonged PT, prolonged INR, and/or thrombocytopenia, as reviewed in detail by Lisman, these parameters poorly predict bleeding tendency.10 Data demonstrating this lack of association has been available for over 40 years; Ewe reported no association between PT and liver bleeding time for liver biopsies performed under direct laparoscopic vision in 1981.11 In the more recent prospective observational study, detailed hemostatic profiling took place, including a bleeding questionnaire, evaluation of individual coagulation factors, platelet count, platelet function (measured with PFA-100), thromboelastometry (TEM), thrombin generation, and clot lysis assays; the authors found no association between heemostatic profile and hematoma formation on planned postprocedural ultrasound.9 A small prospective study of patients with decompensated cirrhosis (n = 72) found an association between maximum amplitude <30 mm measured with thromboelastography (TEG) and major bleeding (n = 3).12 Clauss fibrinogen and functional measures of fibrinogen were not measured, but there was no significant difference in platelet count between patients who bled and those who did not. Low fibrinogen has been reported inconsistently as associated with bleeding in observational studies, and it is possible this is simply another marker of severity of liver disease rather than a risk factor for bleeding.13,14 While there are data to suggest viscoelastic hemostatic assays (eg, TEG/TEM) can reduce inappropriate blood component transfusion,15 the single study incorporating a no-intervention arm demonstrated that the ‘no-intervention’ approach was the most cost-effective without compromising safety in low bleeding risk procedures.16 Further limitations of TEG/TEM include the lack of sensitivity to protein C and von Willebrand Factor, the lack of validated thresholds to guide transfusion, and the lack of clinically meaningful endpoints in published studies to date.17 Further evaluation of Clauss fibrinogen and TEG/TEM, including functional fibrinogen in larger prospective cohorts, are required to confirm any potential clinical utility as a predictor of bleeding.
Acute kidney injury (AKI) commonly complicates acute decompensation of CLD, affecting up to a third of patients admitted to hospital, and is reported to be associated with an increased risk of procedural bleeding.18,19 Acute kidney injury is associated with platelet dysfunction, reduced factor XIII, and endothelial dysfunction, which may all contribute to an increased bleeding risk.20 Active infection/sepsis is thought to increase risk of bleeding based on published reports of a heparin-like effect, platelet dysfunction, and/or hyperfibrinolysis.20,21 However, some patients exhibit hypofibrinolysis and/or hypercoagulability.22 In more recent clinical studies of acutely ill patients with cirrhosis, there are conflicting data on the relationship between AKI, sepsis, and bleeding risk.2,3,12 In acutely ill patients with cirrhosis, particularly those with sepsis, alternate causes for deterioration in hemostatic parameters (thrombocytopenia and hypofibrinogenemia) should be considered.
Prohemostatic therapies to address abnormal hemostatic parameters
Fresh frozen plasma
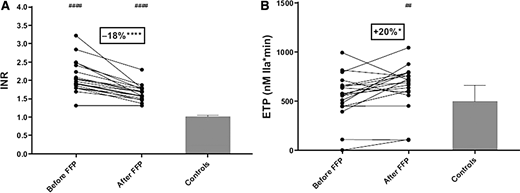
Fresh frozen plasma is often considered (and used) in the periprocedural setting in attempts to correct the prolonged PT/INR.25,26 However, as discussed earlier in this article (and in detail by Lisman10 ), the PT/INR does not reflect the complex hemostatic changes in liver disease and does not predict procedural bleeding. Laboratory studies clearly demonstrate that administration of therapeutic doses of FFP (10-20 mL/kg) do not improve the INR (with few patients achieving an INR <1.5), and that FFP administration increases hypercoagulability measured by thrombin generation (Figure 2)27,28 and markers of in vivo activation of coagulation.28 Additionally, there is evidence that FFP can be harmful, as it associated with significant risks of both transfusion-associated acute lung injury and circulatory overload.29,30 This risk is further illustrated by the demonstrated association between increased portal pressures and FFP administration, which may paradoxically increase the potential for harm of bleeding (eg, in endoscopic variceal band ligation).31 This association is supported by retrospective data from a multicenter study of variceal bleeding management that found increased mortality and 5-day rebleed risk in patients receiving FFP.32
Therapeutic doses of fresh frozen plasma (FFP) on international normalized ratio (INR) and endogenous thrombin potential (ETP). (A) INR and (B) ETP measured in the presence of thrombomodulin in 19 patients with chronic liver disease and a prolonged INR before and after FFP administration, compared with controls (n = 20). Bars represent median ± interquartile ranges. *P < .05. ****P < .0001, before vs after FFP transfusion. ##P < .01, patients vs controls. ####P < .0001, patients vs controls. Adapted from with permission from Elsevier Inc.28
Therapeutic doses of fresh frozen plasma (FFP) on international normalized ratio (INR) and endogenous thrombin potential (ETP). (A) INR and (B) ETP measured in the presence of thrombomodulin in 19 patients with chronic liver disease and a prolonged INR before and after FFP administration, compared with controls (n = 20). Bars represent median ± interquartile ranges. *P < .05. ****P < .0001, before vs after FFP transfusion. ##P < .01, patients vs controls. ####P < .0001, patients vs controls. Adapted from with permission from Elsevier Inc.28
Prothrombin complex concentrate and recombinant factor VIIa
Both prothrombin complex concentrate (PCC) and recombinant factor VIIa (rFVIIa) may appear to be attractive alternatives to FFP, particularly as smaller administration volumes avoid the risk of circulatory overload. A systematic review identified low-quality data to suggest the efficacy of PCC in correcting PT/INR compared with FFP.33 However, PCC use did not result in fewer bleeding events than FFP use; furthermore, thromboembolic events were associated only with PCC. In vitro spiking experiments in plasma from patients with CLD demonstrate a stronger prothrombotic effect of PCC than of FPP on thrombin generating capacity, and this effect increased in parallel with progressive liver disease severity.34 A recent small, retrospective multicenter observational study highlighted significant deterioration in hemostatic markers in a proportion of patients receiving PCC, particularly those with ACLF, raising concerns that its use might precipitate disseminated intravascular coagulation.35 rFVIIa has been evaluated in randomized controlled trials in patients with CLD and variceal bleeding and had no impact on outcomes.36,37 Similarly, studies in liver transplantation found that rFVIIa had no impact on clinical outcomes or volume of red cell transfusion.38,39 Additionally, in vitro laboratory data demonstrate only a minor effect on increasing thrombin generating capacity.34 Neither PCC nor rFVIIa should be used for prevention of bleeding in the periprocedural setting.
Cryoprecipitate/fibrinogen concentrate
There are limited data on the role of fibrinogen supplementation in the periprocedural setting. Laboratory data demonstrate interindividual variation, with some individuals exhibiting increased fibrin clot thrombogenicity despite low fibrinogen levels.40 In a retrospective cohort of critically ill patients with CLD and hypofibrinogenemia, there was no association between reduced fibrinogen and death, and cryoprecipitate use was not associated with reduced bleeding or improved survival.41 Retrospective observational cohorts of critically ill patients and liver transplantation suggest an association between cryoprecipitate use and venous thromboembolism.3,42 There is currently insufficient evidence to support the use of cryoprecitipate/fibrinogen concentrate to prevent periprocedural bleeding.
Vitamin K
Vitamin K may be considered for patients with coexisting risk factors for deficiency, such as advanced malnutrition or cholestasis.6 However, routine use in patients with CLD and a prolonged PT/INR is not warranted, as high doses of vitamin K are ineffective in reducing INR in patients with cirrhosis.43
Tranexamic acid
There are no high-quality data for tranexamic acid as prophylaxis against procedural bleeding in patients with CLD. The HALT-IT randomized controlled trial of tranexamic acid versus placebo in patients with gastrointestinal bleeding found no survival benefit or reduction in transfusion requirements; furthermore, the authors reported an increased risk of venous thromboembolism.44 Therefore, tranexamic acid should not be routinely used to reduce the risk of bleeding in the periprocedural setting.
CLINICAL CASE 1 (continued)
In our case, aside from the abnormal hemostatic markers and potential severity of CLD (if confirmed), there were no additional patient-related factors modulating procedural bleeding risk. A transjugular approach to liver biopsy was planned with an experienced operator. No prohemostatic therapy was offered prior to procedure, and the biopsy was performed without complication. A diagnosis of cirrhosis was confirmed, and she proceeded to assessment for liver transplantation.
CLINICAL CASE 2
A 70-year-old woman with stable (Child Pugh A) cirrhosis secondary to previous alcohol misuse is under surveillance for a lung nodule. Recent imaging reveals a significant increase in nodule size, with a positron emission tomogram demonstrating moderate fluorodeoxyglucose uptake. There is concern for underlying malignancy, and a computed tomography–guided percutaneous biopsy is planned. Her full blood count reveals hemoglobin 108 g/L, white cell count 3.44 × 109/L, and platelets 32 × 109/L. Her liver and renal function are normal, as is PT/INR. There is concern about performing the biopsy due to thrombocytopenia (fluctuating from 25-40 × 109/L over the last year). The proceduralist requests a platelet count of 50 × 109/L prior to proceeding.
Percutaneous lung biopsy is considered a high bleeding risk procedure, as excess bleeding will be difficult to control with potentially serious consequences. As discussed earlier in this article (and in detail in Lisman10 ), there is a lack of consistent evidence that thrombocytopenia predicts bleeding in patients with CLD and inadequate evidence to inform a specific platelet threshold for procedural interventions. In a prospective observational study of 363 patients undergoing 852 procedures, platelet count was not associated with an increased risk of procedural bleeding.45 Thrombocytopenia is increasingly reported to reflect severity of portal hypertension rather than bleeding risk. In the aforementioned 211 critically ill patients with CLD, platelet count of <30 × 109/L predicted an increased risk of (predominantly gastrointestinal) bleeding.2 It should be noted that thrombocytopenia with a platelet count of <30 × 109/L is infrequently seen in patients with CLD, and alternate contributing causes should be considered.6 This might include addressing a potential immune component (particularly in patients with autoimmune or viral hepatitis), medication review for agents that can cause thrombocytopenia, and treating for underlying hematinic deficiency.6
The European Association of Liver Disease recommends against routine use of platelet concentrates or thrombopoietin receptor agonists, but with consideration on a case-by-case basis in patients with platelet counts of <50 × 109/L, in the context of a high bleeding risk procedure in which bleeding would be difficult to control.
Platelet transfusion
Platelet transfusions are commonly used in attempts to increase the platelet count in the periprocedural setting in this patient group.25,26 There are conflicting data as to whether thrombocytopenia increases bleeding risk in the periprocedural setting1,2,12,45 ; the degree of uncertainty is due to data quality and indiscriminate use of platelet transfusion in earlier observational series. It is important to recognize that in patients with CLD and hypersplenism, platelet transfusion often has minimal impact on raising the platelet count, may have a prothrombotic effect,28 and exposes the patient to risks of receiving transfusions.29
Thrombopoietin receptor agonists
Lusutrombopag and avatrombopag have been evaluated in randomized controlled trials of patients with CLD and thrombocytopenia undergoing procedural intervention and are now licensed for this indication. Recommended dosing schedules are shown in Table 2. Both agents were found to significantly increase platelet count and reduce platelet transfusion compared with placebo. However, there are important limitations to these studies: 1) the importance of the primary endpoint (platelet count) is uncertain and 2) participants predominantly underwent procedures with a low bleeding risk. Therefore, the role of thrombopoietin receptor agonists in reducing clinically relevant bleeding remains uncertain.46,47 Also of note, an earlier study of eltrombopag in this setting was terminated due to safety concerns with increased portal vein thrombosis in the eltrombopag arm. This finding may have been confounded by lack of pretreatment imaging to exclude preexisting portal vein thrombosis.48 Portal vein thrombosis was not increased in the studies of lusutrombopag or avatrombopag, in which participants were required to have abdominal imaging confirming the absence of portal vein thrombosis (+/− reduced portal venous flow, <10 cm/s) 2 to 4 weeks prior to randomization.49,50
Licensed dosing for thrombopoietin-agonists for patients with cirrhosis and platelet count <50 × 109/L prior to invasive procedures
| TPO-R agonist . | Baseline platelet count* ( × 109/L) . | Dose . | Procedure date# . |
|---|---|---|---|
| Lusutrombopag | <50 | 3 mg once daily for 7 days | Day 9-14 |
| Avatrombopag | <40 | 60 mg once daily for 5 days | Day 10-13 |
| Avatrombopag | 40-49 | 40 mg once daily for 5 days | Day 10-13 |
| TPO-R agonist . | Baseline platelet count* ( × 109/L) . | Dose . | Procedure date# . |
|---|---|---|---|
| Lusutrombopag | <50 | 3 mg once daily for 7 days | Day 9-14 |
| Avatrombopag | <40 | 60 mg once daily for 5 days | Day 10-13 |
| Avatrombopag | 40-49 | 40 mg once daily for 5 days | Day 10-13 |
TPO-R agonist, thrombopoietin-receptor agonist.
If platelet count <30 × 109/L, consider and correct other causes of thrombocytopenia.
Number of days following initiation of TPO-R agonist.
CLINICAL CASE 2 (continued)
The proceduralist did not feel an expectant approach was appropriate. There were no alternate causes/contributors to thrombocytopenia. The patient had previously received a platelet transfusion prior to a high risk bleeding procedure with no improvement in platelet count. She was treated with lusutrombopag (3 mg daily for 1 week) and had a good response; the platelet count was 89 × 109/L on the day of percutaneous biopsy. There were no bleeding complications.
Clinical practice guidelines and future research
Recent clinical practice guideline recommendations for management of hemostasis in the periprocedural setting are summarized in Table 3. There is agreement across major societal guidance bodies from hematology, hepatology, and interventional radiology that FFP should not be used to correct prolonged PT/INR prior to procedures irrespective of procedural bleeding risk, nor should thrombocytopenia be corrected prior to low bleeding risk procedures. There is less certainty regarding the need to correct thrombocytopenia prior to high bleeding risk procedures, and this is reflected by variation in the guideline recommendations.4-8,51 Research recommendations proposed by EASL to address knowledge gaps in this domain are summarized in Table 4.
Comparison of selected major societal guideline recommendations for correction of hemostatic parameters prior to high bleeding risk procedures
| . | BSIR 20228 . | EASL 20225 . | ISTH 20216 . | AGA 20217 . | AASLD 20204 . | SIR 201951 . |
|---|---|---|---|---|---|---|
| PT/INR | Do not correct | Do not correct | Do not evaluate | Do not correct | Do not correct | INR >2.5* |
| Platelets | Consider if <50 × 109/L | Case by case for <50 × 109/L | Do not correct† | Do not correct | Do not correct | >30 × 109/L |
| Fibrinogen | Consider if <1.2 g/l | Correction discouraged | Do not evaluate | No recommendation | Do not correct | >1 g/L |
| . | BSIR 20228 . | EASL 20225 . | ISTH 20216 . | AGA 20217 . | AASLD 20204 . | SIR 201951 . |
|---|---|---|---|---|---|---|
| PT/INR | Do not correct | Do not correct | Do not evaluate | Do not correct | Do not correct | INR >2.5* |
| Platelets | Consider if <50 × 109/L | Case by case for <50 × 109/L | Do not correct† | Do not correct | Do not correct | >30 × 109/L |
| Fibrinogen | Consider if <1.2 g/l | Correction discouraged | Do not evaluate | No recommendation | Do not correct | >1 g/L |
AASLD, American Association for the Study of Liver Disease; AGA, American Gastroenterology Association; EASL, European Association for the Study of Liver Diseases; BSIR, British Society of Interventional Radiology; INR, international normalized ratio; ISTH, International Society of Thrombosis and Haemostasis; PT, prothrombin time; SIR, Society of Interventional Radiology.
Give vitamin K if INR >2.5; do not use fresh frozen plasma or prothrombin complex concentrate.
Consider correction prior to planned elective very-high-risk procedures.
Research needs in periprocedural management of hemostasis as proposed by European Association for Study of the Liver5
| Large prospective observational collaborative studies to • establish the incidence of procedural bleeding • evaluate associations between anemia +/- thrombocytopenia and procedural bleeding • assess relationship between fibrinogen deficiency and procedural bleeding • explore the impact of a hyperfibrinolytic state on procedural bleeding |
| Evaluate the role of viscoelastic tests in predicting procedural bleeding in patients undergoing high-risk procedures (including adequate numbers of patients with progressive disease severity [ie, compensated, acute decompensation, acute on chronic liver failure]) |
| Randomized, placebo-controlled trials in patients with severe thrombocytopenia undergoing high-risk procedures, to assess the role of 1) platelet transfusions and 2) thrombopoietin-receptor agonists, with clinically significant bleeding as the primary endpoint |
| Clinical trials to evaluate antifibrinolytics in the periprocedural setting, particularly in patients with known/suspected hyperfibrinolysis |
| Large prospective observational collaborative studies to • establish the incidence of procedural bleeding • evaluate associations between anemia +/- thrombocytopenia and procedural bleeding • assess relationship between fibrinogen deficiency and procedural bleeding • explore the impact of a hyperfibrinolytic state on procedural bleeding |
| Evaluate the role of viscoelastic tests in predicting procedural bleeding in patients undergoing high-risk procedures (including adequate numbers of patients with progressive disease severity [ie, compensated, acute decompensation, acute on chronic liver failure]) |
| Randomized, placebo-controlled trials in patients with severe thrombocytopenia undergoing high-risk procedures, to assess the role of 1) platelet transfusions and 2) thrombopoietin-receptor agonists, with clinically significant bleeding as the primary endpoint |
| Clinical trials to evaluate antifibrinolytics in the periprocedural setting, particularly in patients with known/suspected hyperfibrinolysis |
Conclusion
It is important to recognize that the majority of commonly performed procedures in patients with advanced CLD are associated with a low procedural bleeding risk. While prolonged PT/INR and/or thrombocytopenia are commonly encountered, they are not reliable indicators of hemostasis or procedural bleeding risk in this patient group. Accordingly, FFP and/or platelet transfusion should not be used routinely to prevent periprocedural bleeding in patients with ACLD.
Conflict-of-interest disclosure
Lara N. Roberts: no competing financial interests to declare.
Off-label drug use
Lara N. Roberts: Prothrombin complex concentrates, recombinant factor VIIa, and fibrinogen concentrate are not recommended in the periprocedual hemostatic management of patients with liver disease.