Abstract
Patients with advanced liver diseases frequently acquire profound alterations in their hemostatic system. Simultaneous changes in procoagulant and anticoagulant systems result in a reset in the hemostatic balance with a relatively neutral net effect, although there are notable hypocoagulable and hypercoagulable features in the hemostatic system in patients with liver disease. Laboratory and clinical studies have demonstrated that patients have a relatively well-preserved hemostatic system even though routine diagnostic tests of hemostasis (prothrombin time, platelet count) suggest a bleeding tendency. Routine diagnostic tests of hemostasis are unsuitable to assess the hemostatic status of patients with liver disease, as these tests are insensitive for the concurrent prohemostatic and antihemostatic changes in these patients. These tests are, however, frequently requested in patients with liver disease, as they are well established indicators of severity of liver disease. This paper will discuss commonly used diagnostic and research-type hemostatic tests and will outline how test results should be interpreted in patients with liver disease.
Learning Objectives
To identify limitations of common laboratory tests performed in patients with complex hemostatic alterations as a result of liver disease
To differentiate use of hemostatic tests in patients with liver disease as markers of disease severity from use in prediction of bleeding
CASE
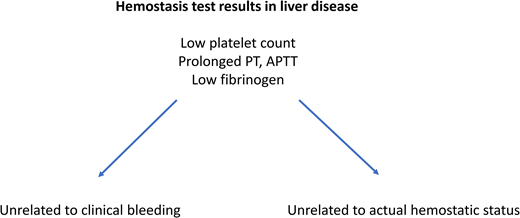
A patient with chronic liver disease related to alcohol abuse is hospitalized for acute decompensation with massive ascites. The patient has severe liver disease, evidenced by a model of end-stage liver disease score of 20 and a Child-Pugh score C10. A paracentesis is scheduled, but the treating physician is concerned about the patient's coagulopathy with an international normalized ratio of 1.8, a plasma fibrinogen level of 1.4 g/L, and a platelet count of 61 000/µL.
Why assess hemostasis in severe liver disease?
Advanced chronic liver disease is commonly associated with substantial changes in the hemostatic system. Frequent findings include thrombocytopenia, low plasma levels of coagulation factors, inhibitors of coagulation, and proteins involved in the fibrinolytic system. The decreased platelet count and decreased plasma levels of hemostatic proteins likely relate to a combination of compromised synthetic failure of the diseased liver, decreased platelet production in part related to decreased thrombopoietin production, consumption of platelets and hemostatic proteins as a result of low-grade activation of the hemostatic system, and sequestration of platelets in an enlarged spleen. The net result of the complex hemostatic changes in these patients is surprisingly benign (Figure 1). Due to a simultaneous decline in both prohemostatic and antihemostatic factors, the hemostatic balance appears to remain in balance, as evidenced by laboratory studies using advanced hemostatic tests and clinical observations.1 Specifically, such laboratory studies included 3 main categories. One category was studies of platelet adhesion from whole blood to adhesive proteins, such as collagen, under conditions of flow.2 These studies have shown that highly elevated levels of the platelet adhesive protein von Willebrand factor (VWF) compensates for the decreased platelet count.
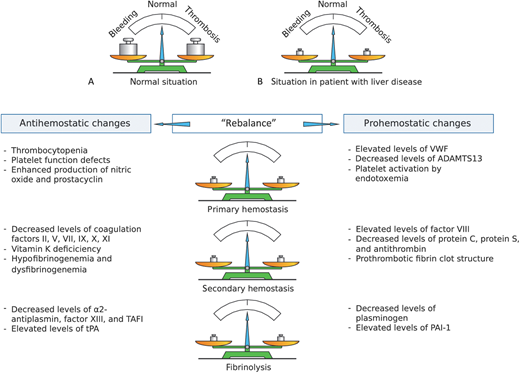
Schematic presentation of the rebalanced hemostatic system in patients with liver disease. In healthy individuals (A), the hemostatic system is in solid balance. In patients with liver disease (B and table) both prohemostatic and antihemostatic changes result in a “rebalance” of the hemostatic system. This new balance is characterized by specific hypocoagulable and hypercoagulable features. ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; PAI-1, plasminogen activator inhibitor-1; TAFI, thrombin activatable fibrinolysis inhibitor; tPA, tissue plasminogen activator; VWF, von Willebrand factor. Reprinted from van den Boom & Lisman with permission.46
Schematic presentation of the rebalanced hemostatic system in patients with liver disease. In healthy individuals (A), the hemostatic system is in solid balance. In patients with liver disease (B and table) both prohemostatic and antihemostatic changes result in a “rebalance” of the hemostatic system. This new balance is characterized by specific hypocoagulable and hypercoagulable features. ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; PAI-1, plasminogen activator inhibitor-1; TAFI, thrombin activatable fibrinolysis inhibitor; tPA, tissue plasminogen activator; VWF, von Willebrand factor. Reprinted from van den Boom & Lisman with permission.46
The second category of laboratory studies was thrombomodulin-modified thrombin generation tests.3 Such assays are sensitive for levels of procoagulant proteins and for levels of anticoagulant drivers, including the protein C system and antithrombin. Due to a simultaneous decline in procoagulant and anticoagulant proteins, thrombomodulin-modified thrombin generation test results are normal or even show enhanced thrombin generation in patients with liver disease. Additional studies have demonstrated that low levels of procoagulant proteins are compensated for by low levels of protein C and antithrombin and elevated levels of factor VIII.4-6
The third category of studies is plasma-based fibrinolytic tests that demonstrate normal fibrinolytic potential in many patients with liver disease due to simultaneous changes in profibrinolytic and antifibrinolytic proteins.7 Clinical observations in support of the concept of rebalanced hemostasis in liver disease include low transfusion requirements in many contemporary liver transplantation programs; also included is the notion that clinically relevant bleeds in patients with cirrhosis are often independent of hemostatic failure and are instead driven by portal hypertension (eg, variceal bleeds) or mechanical injury to blood vessels (eg, bleeding following invasive procedures) (Figure 2).8-11 Importantly, these nonhemostatic bleeds require different strategies to treat or prevent. As in the case described herein, there is a seemingly paradoxical disconnect between routine diagnostic tests of hemostasis that suggest a bleeding tendency, the rebalanced hemostatic status identified by more sophisticated tests of hemostasis, and clinical bleeding which is often unrelated to hemostatic failure.
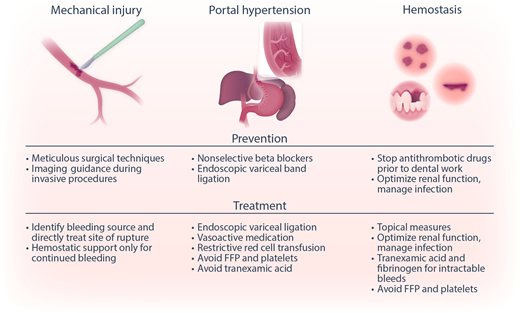
Categories of bleeding in liver disease. Bleeding in patients with liver disease may be due to mechanical injury (by inadvertent laceration of a vessel during surgery or a minor invasive procedure), portal hypertension-related causes (eg, variceal bleeding), or hemostatic failure (bleeding following dental extraction, bruising, and bleeding from puncture wounds). Shown are strategies to prevent and treat these different types of bleeding complications. Reprinted from Lisman with permission.47 FFP, fresh frozen plasma.
Categories of bleeding in liver disease. Bleeding in patients with liver disease may be due to mechanical injury (by inadvertent laceration of a vessel during surgery or a minor invasive procedure), portal hypertension-related causes (eg, variceal bleeding), or hemostatic failure (bleeding following dental extraction, bruising, and bleeding from puncture wounds). Shown are strategies to prevent and treat these different types of bleeding complications. Reprinted from Lisman with permission.47 FFP, fresh frozen plasma.
Recent clinical guidance documents suggest that routine diagnostic tests of hemostasis are not helpful in predicting spontaneous or procedure-related bleeding in patients with advanced chronic liver disease.8-11 Some of these documents even advise not using such tests prior to invasive procedures. However, this advice is ignored in clinical practice for 2 important reasons. First, routine diagnostic tests of hemostasis are useful indicators of severity of liver disease. The prothrombin time (PT) or international normalized ratio (INR) are part of clinical scores for the severity of liver diseases such as the Child-Pugh and model of end-stage liver disease scores, as they are indicators of the synthetic capacity of the liver. The platelet count is a well-established indicator of severity of portal hypertension,12 which relates to the decrease in platelet count caused by portal hypertension–related splenomegaly, which itself increases platelet sequestration in the spleen. The PT/INR and platelet count are thus helpful in staging patients with liver disease, and longitudinal assessment of these tests can be used to monitor disease progression. Second, it is commonly perceived that baseline PT/INR values and platelet counts are useful to guide hemostatic management in case bleeding occurs.
Thus, hemostatic testing is commonly used to help assess severity of liver disease and to establish baseline values in case patients bleed during follow-up. In the presented case, however, there is concern that the abnormal INR and platelet count indicate a bleeding risk, even though there is generous evidence that these tests are unsuitable to assess the hemostatic status in patients with liver disease. Although the concept of rebalanced hemostasis proposes that patients with liver diseases, even those that are critically ill,13 remain in hemostatic balance, there may be conditions in which the hemostatic balance in individual patients becomes hypocoagulable.14-16 Such conditions include acute kidney injury, infection, and development of acute-on-chronic liver failure.17 There is very limited evidence that, in these cases, the development of a net hypocoagulable state increases the risk for hemostasis-related bleeding.16,18
In this paper, I will discuss how routine diagnostic tests of hemostasis should be interpreted in patients with liver disease. In addition, I will examine whether more advanced hemostatic tests are helpful in assessing hemostatic capacity in these patients.
What do routine tests of hemostasis in patients with severe liver disease tell us?
This section will discuss the interpretation of routine diagnostic hemostasis tests in patients with liver disease, their limitations, and the relation to bleeding. Table 1 summarizes key aspects of this section.
Summary of how routine diagnostic hemostasis tests should be interpreted in liver disease patients
| Laboratory test . | Sensitive for . | Does not assess . | Utility in liver disease . |
|---|---|---|---|
| PT/INR | Factors VII, X, V; prothrombin, and fibrinogen | Other procoagulant factors and anticoagulant proteins | Reflects synthetic capacity of the liver but has no relation to hemostatic status or bleeding |
| APTT | Prekallikrein; high-molecular weight kininogen; factors XII, XI, IX, VIII, X, and V; prothrombin; and fibrinogen | Factor VII and anticoagulant proteins | Has no relation to hemostatic status or bleeding |
| Fibrinogen | Fibrinogen level and function | Polymerisation defects due to increased sialic acid content, altered physical properties of the fibrin clot | Relation between low fibrinogen and bleeding in liver disease unclear |
| Platelet count | Platelet number | Compensation of thrombocytopenia by high VWF levels | Reflects severity of portal hypertension; relation between low platelet count and bleeding risk debated |
| Laboratory test . | Sensitive for . | Does not assess . | Utility in liver disease . |
|---|---|---|---|
| PT/INR | Factors VII, X, V; prothrombin, and fibrinogen | Other procoagulant factors and anticoagulant proteins | Reflects synthetic capacity of the liver but has no relation to hemostatic status or bleeding |
| APTT | Prekallikrein; high-molecular weight kininogen; factors XII, XI, IX, VIII, X, and V; prothrombin; and fibrinogen | Factor VII and anticoagulant proteins | Has no relation to hemostatic status or bleeding |
| Fibrinogen | Fibrinogen level and function | Polymerisation defects due to increased sialic acid content, altered physical properties of the fibrin clot | Relation between low fibrinogen and bleeding in liver disease unclear |
| Platelet count | Platelet number | Compensation of thrombocytopenia by high VWF levels | Reflects severity of portal hypertension; relation between low platelet count and bleeding risk debated |
APTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; VWF, von Willebrand factor.
PT/INR
The PT is a functional coagulation test that assesses the functional activity of coagulation factors V, VII, X, prothrombin, and fibrinogen. The PT or INR will thus be prolonged when there are qualitative or quantitative defects in one or more of these 5 procoagulant proteins. This test thus is useful to diagnose isolated or combined deficiencies or defects in these coagulation factors, which, for example, include hereditary factor deficiencies or acquired defects related to vitamin K antagonist use.
Crucially, the PT is insensitive for variations in anticoagulant proteins, which include tissue factor pathway inhibitor, proteins C and S, and antithrombin. Per definition, therefore, the PT cannot be used to assess global hemostatic status. In patients with advanced liver disease, a prolonged PT is a useful indicator of hepatic synthetic function but not of global hemostatic capacity due to the simultaneous decline in procoagulant and anticoagulant proteins in these patients.19 Indeed, a recent meta-analysis, which included 29 studies with a total of 13 276 patients, demonstrated no relation between the PT/INR and procedural bleeding risk in patients with cirrhosis.20
Activated partial thromboplastin time
Similar to the PT, the activated partial thromboplastin time (APTT) is sensitive for a discrete number of procoagulant proteins (prekallikrein; high-molecular weight kininogen; factors XII, XI, IX, VIII, X, V; prothrombin; and fibrinogen), and a prolongation of the APTT will signal a qualitative or quantitative defect in either of these factors. Notably, an isolated deficiency of 1 of the contact factors (prekallikrein, high-molecular weight kininogen, factor XII) is not associated with clinical bleeding, even though the APTT is prolonged by these deficiencies. In patients with advanced liver disease, the APTT is commonly prolonged, but not as severely as the PT. High levels of coagulation factor VIII (FVIII) are the reason the APTT is only mildly prolonged. Unlike most coagulation proteins that are synthesized in hepatocytes, FVIII is synthesized in both intrahepatic and extrahepatic endothelial cells.21 Chronic endothelial cell activation with highly elevated plasma levels of the FVIII carrier protein von Willebrand factor (VWF) result in elevated FVIII levels in patients with liver disease. Like the PT, the APTT is insensitive to anticoagulant proteins and therefore does not adequately represent hemostatic capacity in patients with liver disease; hence, the APTT should not be used to assess bleeding risk.
Fibrinogen
Fibrinogen levels vary widely in patients with liver disease. In mild liver disease, fibrinogen levels may be increased,22 which likely relates to the fact that fibrinogen is an acute phase protein. In more advanced liver disease, fibrinogen levels decrease on average, but the range of fibrinogen levels is much wider than in healthy individuals.23 In patients with advanced cirrhosis, a subset has normal fibrinogen levels, whereas other patients have fibrinogen levels that are clearly below reference ranges. It is, however, unusual to find fibrinogen levels lower than 1 g/L.
It has been argued that low fibrinogen levels in critically ill patients with cirrhosis contribute to clinical bleeding.24 However, although there is a relation between fibrinogen levels and bleeding risk, this relation may be indirect. Most cases of bleeding in critically ill cirrhosis patients are related to portal hypertension,25 and the relation between low fibrinogen and portal hypertensive bleeds may thus reflect a relation between severity of disease and bleeding rather than a relation between hemostatic failure and bleeding. Indeed, a recent retrospective study demonstrated that (prophylactic) administration of cryoprecipitate with the aim to increase plasma fibrinogen levels did not reduce bleeding or mortality in critically ill cirrhosis patients.26 It is therefore questionable whether low fibrinogen levels in patients with cirrhosis signal a clinically relevant hemostatic defect. Importantly, it has been demonstrated that the quality of fibrin clots in patients with cirrhosis is normal to thrombogenic, despite reduced fibrinogen plasma levels.27 Thrombogenic posttranslational modifications in the fibrinogen molecule, notably oxidation, have been proposed to promote thrombogenicity of fibrin clots in these patients.27
Platelet count
Thrombocytopenia is a common finding in patients with cirrhosis, but it is unusual for the platelet count to drop below 50 000/µL, except in patients who are critically ill. Whether thrombocytopenia in these patients is accompanied by a platelet function defect is unclear: some studies find platelet function defects,28 whereas others find hyperfunctional platelets in cirrhosis.29,30
Although thrombocytopenia in the absence of an underlying liver disease may be associated with a bleeding risk, the situation is more complex in patients with cirrhosis. Laboratory studies have demonstrated that the highly elevated levels of VWF in cirrhosis patients compensate for the low platelet count.2
It has been argued that a low platelet count reduces thrombin generation in patients with cirrhosis.31 However, since the thrombin-generating capacity in platelet-poor plasma is preserved or even hypercoagulable, and since platelets are not the only cellular surfaces that can promote thrombin generation, this conclusion may be too simplified.
Pros and cons of global hemostasis tests in patients with severe liver disease
The repertoire of diagnostic or research-type hemostasis tests is much larger than the PT/APTT/fibrinogen/platelet count test panel. Global tests of hemostasis are increasingly used to assess hemostasis or guide clinical management in patients with isolated or complex hemostatic disorders. Such tests include whole blood viscoelastic tests, platelet function tests, thrombin generation tests, and plasma-based fibrinolysis tests. Opportunities and caveats of these tests will be discussed in this section. Findings are summarized in Table 2.
Summary of how global diagnostic or research-type hemostasis tests should be interpreted in liver disease patients
| Laboratory test . | Sensitive for . | Does not assess . | Utility in liver disease . |
|---|---|---|---|
| Whole blood viscoelastic tests | Blood cells, coagulation proteins, fibrinolytic proteins. | VWF levels, protein C system, platelet function defects, role of flow in clot formation. | Better representation of hemostatic capacity compared to routine diagnostic tests, reduces blood product use, likely underestimates hemostatic capacity. |
| Platelet function tests | Platelet function, most tests also for platelet count. | Interplay between coagulation and platelet activation, other blood cells, role of flow (except for PFA-100/200). | Most platelet function tests are not helpful because they are not only sensitive for platelet function but also for platelet count. Flow cytometry–based analyses may have merit in a research setting. |
| Thrombin generation tests | All procoagulant and anticoagulant factors, provided thrombomodulin or another protein C activator is added to the reaction mixture. Platelet count when performed in platelet-rich plasma. | Role of blood cells and endothelial cells in supporting thrombin generation, role of flow. | Promising indicator of coagulation capacity—not yet ready for clinical use. |
| Plasma-based fibrinolysis tests | All fibrinolytic proteins except tissue-type plasminogen activator. | Role of blood cells in fibrinolysis. |
| Laboratory test . | Sensitive for . | Does not assess . | Utility in liver disease . |
|---|---|---|---|
| Whole blood viscoelastic tests | Blood cells, coagulation proteins, fibrinolytic proteins. | VWF levels, protein C system, platelet function defects, role of flow in clot formation. | Better representation of hemostatic capacity compared to routine diagnostic tests, reduces blood product use, likely underestimates hemostatic capacity. |
| Platelet function tests | Platelet function, most tests also for platelet count. | Interplay between coagulation and platelet activation, other blood cells, role of flow (except for PFA-100/200). | Most platelet function tests are not helpful because they are not only sensitive for platelet function but also for platelet count. Flow cytometry–based analyses may have merit in a research setting. |
| Thrombin generation tests | All procoagulant and anticoagulant factors, provided thrombomodulin or another protein C activator is added to the reaction mixture. Platelet count when performed in platelet-rich plasma. | Role of blood cells and endothelial cells in supporting thrombin generation, role of flow. | Promising indicator of coagulation capacity—not yet ready for clinical use. |
| Plasma-based fibrinolysis tests | All fibrinolytic proteins except tissue-type plasminogen activator. | Role of blood cells in fibrinolysis. |
PFA, platelet function analyzer; VWF, von Willebrand factor.
Whole blood viscoelastic tests
Whole blood viscoelastic tests, such as thromboelastography (TEG) and rotational thromboelastography (ROTEM), and variants, such as the ClotPro device and the Quantra, are gaining popularity as rapid point-of-care devices to assess hemostasis in patients with bleeding related to trauma, childbirth, liver disease, and other medical situations associated with bleeding. In liver disease, multiple studies reported the use of kaolin-induced TEG,33,34 while for ROTEM, multiple studies report a panel of EXTEM, INTEM, FIBTEM, and APTEM.35,36 The advantage of these techniques is that clot formation is assessed in whole blood and thus interactions between cellular components and the plasma environment are taken into account. Viscoelastic tests in patients with liver disease give a more accurate representation of hemostatic status than routine diagnostic tests such as the PT and platelet count. When prophylactic transfusion management of patients with liver disease was guided by viscoelastic tests instead of routine diagnostic tests of hemostasis, a dramatic decrease in blood component transfusions was observed in multiple studies.37
Viscoelastic tests have 3 drawbacks that need to be taken into account when interpreting test results in patients with liver diseases. First, the platelet count is a major determinant of clot formation in these assays. However, viscoelastic tests are insensitive for plasma levels of VWF, and the potential compensation of thrombocytopenia by elevated VWF in liver disease patients is not taken into account, leading to an underestimation of hemostatic capacity.38 Second, viscoelastic tests are insensitive for plasma levels of the protein C system, and the compensation of low levels of procoagulant proteins by low levels of protein C and S is not taken into account, again resulting in an underestimation of true hemostatic potential.38 Finally, the strength of the initiator of clot formation may alter the interpretation of viscoelastic test results. For example, in patients with liver disease, TEG test results are more often within normal ranges compared to ROTEM test results, which are frequently hypocoagulable.39,40 This likely relates to the difference in composition of the reagents between tests that lead to slower clot initiation in TEG. This slower clot initiation better allows anticoagulant mechanisms to control coagulation.
Platelet function tests
Platelet function tests are notoriously difficult in patients with liver diseases, as most platelet function tests are strongly influenced by platelet count, which is often decreased in patients with liver disease. In recent reports, researchers have studied ratios of platelet function test results with the platelet count,30 but it is questionable whether there is a truly linear relation between platelet count and platelet function test results.41 Flow cytometry–based platelet function tests are independent of platelet count, but such tests as not yet suitable for use in routine diagnostics.
Thrombin generation tests
The use of thrombin generation tests has revolutionized the assessment of hemostatic status in patients with liver disease. Tripodi and coworkers were the first to report preserved thrombin generating capacity in patients with cirrhosis by using thrombomodulin-modified thrombin generation tests.42 These tests accurately assess the balance between procoagulant and anticoagulant proteins and, in my opinion, these are the best tests currently available to assess plasma coagulation potential. Importantly, thrombin generation tests have been automated, and the Genesia device appears suitable for use in specialized diagnostic laboratories.43 The obvious limitations of the thrombin generation test are the lack of cellular components and the fact that thrombin generation, not clot formation, is the endpoint of the test. Thus, thrombin generation tests should always be interpreted in the context of tests assessing other aspects of hemostasis, such as platelet function, clot formation, and clot stability. Whole blood thrombin generation tests are in development, and initial results in patients with liver disease have been reported.44
Plasma-based fibrinolysis tests
Historically, liver diseases were considered to be associated with a hyperfibrinolytic state. More modern laboratory tests and clinical observations have challenged this dogma. There is a plasma- based fibrinolysis assay in which clot formation is initiated by tissue factor, lysis is induced by tissue-type plasminogen activator, and turbidity measurements are used to monitor clot formation and fibrinolysis.45 This particular assay has been extensively used to profile the fibrinolytic system in patients with liver diseases, and, importantly, has been extensively validated in the context of thrombotic disease.45 Fibrinolytic test results measured with this assay vary widely: normal fibrinolytic capacity is seen in compensated cirrhosis; hyperfibrinolysis, in decompensated patients; and hypofibrinolysis, in critically ill patients with acute-on-chronic liver failure and sepsis or acute liver failure.7 Although a hypofibrinolytic state identified by this test correlates with risk of venous thrombosis, the test is less well characterized in patients with bleeding. The lack of cellular components in the test needs to be taken into account when interpreting the results.
Back to our case
A variety of clinical and research-type laboratory tests are available to assess hemostatic status in patients with liver diseases. However, for most tests, the interpretation of results is not straightforward. The average patient with liver disease appears in hemostatic balance, but specific hypocoagulable and hypercoagulable features can be identified. Which laboratory characteristics predict hemostasis-related bleeding is uncertain, and, as most spontaneous or procedure-related bleeds in patients with liver disease are unrelated to hemostatic failure, prophylactic hemostatic interventions may not be useful for the majority of patients. Importantly, recent clinical guidance documents argue against routine evaluation of hemostasis using routine diagnostic tests (PT/INR, APTT, platelet count, and fibrinogen) to predict bleeding risk prior to procedural intervention in patients with cirrhosis, even in those who are critically ill.10,11 Whether prohemostatic therapy is indicated in patients with extreme alterations in their hemostatic system is uncertain. One study identified APTT values >100 sec, a platelet count <30 000/µL, and fibrinogen levels <0.6 g/L as predictors of bleeding in critically ill cirrhosis patients.24 Whether this association is causal or whether these very abnormal laboratory tests simply signal very severe liver disease is uncertain. Future studies should identify which patients may benefit from hemostatic interventions and which laboratory studies should be used to identify these patients.
Conflict-of-interest disclosure
Ton Lisman: no competing financial interests to declare.
Off-label drug use
Ton Lisman: nothing to disclose.