Abstract
Hemophagocytic lymphohistiocytosis (HLH) is one of the life-threatening emergencies that a hematologist may be called upon to diagnose and manage. It is a hyperinflammatory process that develops in patients with genetic abnormalities, hematologic malignancies, chronic inflammatory states, or infections. The main clinical challenges are recognizing HLH, determining whether the immune response is aberrant or appropriate, and deciding upon therapy. Patients may present with fever, central nervous system symptoms, cytopenias, or elevated liver enzymes.
Recognizing HLH is challenging because its features overlap with numerous systemic disorders, thus requiring a high level of suspicion and timely investigations to confirm the diagnosis and detect the underlying trigger. Once HLH is diagnosed, careful consideration of immunosuppressive therapy's potential benefit versus harm is necessary. Such therapy can sometimes be tailored to the underlying trigger. In the acute setting, the competing pressures of completing a thorough diagnostic process (including evaluation for the presence of lymphoma and infection) and the need for expedited treatment must be balanced. During the management of an HLH patient, continuous vigilance for the presence of as-yet unrecognized disease triggers, monitoring response, and identifying emerging complications is critical. This review will discuss the recognition and management of HLH in the inpatient setting.
Learning Objectives
Recognize the HLH syndrome in the inpatient setting
Identify HLH disease triggers and their appropriate treatments
Determine when to start immunosuppressive therapy for suspected HLH
CLINICAL CASE
A 45-year-old healthy man presented with a 3-day history of fever of up to 38.2°C, headache, and gait instability. His general physical exam was unremarkable, and neurologic examination revealed normal motor, sensory, and cerebellar functions. Complete blood count and serum chemistries were normal, and the C reactive protein (CRP) level was elevated at 9 mg/dL. A brain CT scan was normal. Lumbar puncture showed clear cerebrospinal fluid (CSF) with 10 white blood cells, 90% mononuclear cells, no red blood cells, and an elevated protein level of 63 mg/dL. A presumptive diagnosis of aseptic meningoencephalitis was made.
Magnetic resonance imaging revealed an increased pontine signal, suggesting an inflammatory process. Syphilis, West Nile virus, HIV, herpes virus, and SARS-coronavirus type 2 infections were excluded. Autoimmune and paraneoplastic CSF antibody panels were negative. The patient's headache worsened, with no response to intravenous hydration, caffeine, or analgesic medications. Empiric therapy with methylprednisolone 1 g/d led to rapid clinical improvement. Upon corticosteroid cessation, the temperature increased to 39.2°C, and the headache recurred, accompanied by saddle anesthesia. The CRP increased to 18 mg/dL; new cytopenias developed: platelets were 135 k/µL and hemoglobin was 8 g/dL. Creatinine increased to 1.5 mg/dL, triglycerides were elevated at 661 mg/dL, and ferritin was 1275 mg/dL.
Differential diagnosis and initial evaluation
HLH is a hyperinflammatory syndrome characterized by a unique constellation of clinical and laboratory features: fever, neurologic symptoms, splenomegaly, cytopenias, elevated triglycerides, decreased fibrinogen, elevated liver enzymes, and increased ferritin and soluble CD25 (sCD25). These may present in combination or individually, which may be particularly pertinent in instances of unexplained fever or liver enzyme elevations. Patients with HLH are frequently among the most severely ill in the hospital, often encountered in intensive care units with multiorgan dysfunction. The nonspecific nature of disease phenotypes in HLH makes the distinction from other causes of severe multisystem disorders, particularly sepsis, difficult.1 Characteristically, HLH patients have an unremitting fever accompanied by severe cytopenias, particularly thrombocytopenia. Early assessment of ferritin and soluble CD25, typically markedly increased in HLH, may expedite the diagnosis. Recently, a specific T-cell phenotypic signature has been suggested to discriminate between HLH and sepsis: CD38/HLA-DR bright CD8+ T cells were increased in a cohort of children with familial HLH but not in children with a confirmed diagnosis of bacterial sepsis; the presence of as few as 7% of cells with this unique phenotype was sufficient to distinguish between children with HLH from those with sepsis, although in HLH levels are usually higher.2-4 HLH may mimic several conditions that must simultaneously be considered possible mimics or true triggers of HLH (Table 1). In a rapidly deteriorating HLH patient, prompt evaluation of these conditions is necessary. Novel serologic biomarkers, such as C-X-C motif chemokine ligand 9 (CXCL9)5 and interleukin 18,6 are emerging as markers with the potential to distinguish between HLH and clinical syndromes with overlapping features.
The differential diagnosis of hemophagocytic lymphohistiocytosis (HLH), including triggering and mimicking conditions
| Malignant . | Infectious . | Inflammatory . | Storage/metabolic . | Vascular . | Iatrogenic . |
|---|---|---|---|---|---|
| BCL HL TCL CLL MDS AML ALL CML Myelofibrosis HCC | EBV CMV HIV Leishmania COVID-19 Atypical infections* West Nile virus Tuberculosis Aspergillus Bacterial sepsis | SJIA Still's disease SLE ALPS Sarcoidosis GCA Kawasaki disease PIMS Kikuchi disease | Gaucher's disease LPI Wolman's disease Lysosomal acid lipase deficiency GSD type 1 Niemann-Pick disease | TTP HUS HELLP DIC | CAR-T BITES ICB DRESS |
| Malignant . | Infectious . | Inflammatory . | Storage/metabolic . | Vascular . | Iatrogenic . |
|---|---|---|---|---|---|
| BCL HL TCL CLL MDS AML ALL CML Myelofibrosis HCC | EBV CMV HIV Leishmania COVID-19 Atypical infections* West Nile virus Tuberculosis Aspergillus Bacterial sepsis | SJIA Still's disease SLE ALPS Sarcoidosis GCA Kawasaki disease PIMS Kikuchi disease | Gaucher's disease LPI Wolman's disease Lysosomal acid lipase deficiency GSD type 1 Niemann-Pick disease | TTP HUS HELLP DIC | CAR-T BITES ICB DRESS |
Atypical infections: Rickettsia, Leptospira, Bartonella, Brucella, and Ehrlichia.
ALPS, autoimmune lymphoproliferative syndrome; AML, acute myeloid leukemia; BCL, B-cell lymphoma; BITE, bispecific T-cell engager; CART, chimeric antigen receptor T cell therapy; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; DIC, disseminated intravascular coagulation; DRESS, drug reaction with eosinophilia and systemic symptoms; EBV, Epstein Bar virus; GCA, giant cell arteritis; GSD, glycogen storage disease; HCC, hepatocellular carcinoma; HELLP, emolysis, elevated liver enzymes and low platelet count; HL, Hodgkin lymphoma; HUS, hemolytic uremic syndrome; ICB, immune checkpoint blockade; LPI, lysinuric protein intolerance; MDS, myelodysplastic syndrome; PIMS, pediatric inflammatory multisystem syndrome; SLE, systemic lupus erythematosus; SJIA, systemic juvenile arthritis; TCL, T-cell lymphoma; TTP, thrombotic thrombocytopenic purpura.
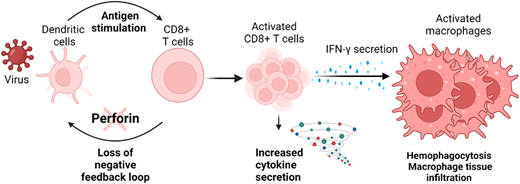
HLH is not an autoimmune disorder, but rather an aberrant inflammatory response characterized by excessive and maladaptive T-cell activation. Hence, when a critically ill patient has a widespread inflammatory response, it is imperative to establish a conceptual framework that distinguishes between an appropriate immunologic response, such as one to a serious bacterial or viral infection, and a disordered and unregulated response, which characterizes HLH. Seminal advances in understanding the molecular and cellular underpinnings of HLH have been critical in this regard. The mechanism of dysregulated immune response in HLH (Figure 1) was first described in a mouse model of the genetic version of the disease, familial HLH (F-HLH). The underlying genetic defects of lymphocyte cytotoxicity cause inadequate control of T-cell activation and failure to clear infections, particularly viral. This results in persistent CD8+ T–cell activation with massive cytokine elaboration, commonly called a cytokine storm. Preeminent among these is interferon-gamma (IFN-γ), the main driver of the disease phenotype. IFN-γ activates macrophages associated with hemophagocytosis and tissue infiltration and damage.7,8
The pathogenesis of familial hemophagocytic lymphohistiocytosis (HLH). Patients with familial HLH (F-HLH) often have defects of the perforin cytotoxic pathway. Experimental studies have demonstrated that this pathway provides critical feedback regulating CD8+ T–cell activation. Defects result in excessive antigen presentation by dendritic cells, leading to excessive T-cell activation and overproduction of IFN-gamma, which results pathologic systemic macrophage activation. Thus, clinical HLH results from a primary defect of immune regulation leading to largely IFN-γ driven immunopathology. Created with BioRender.com
The pathogenesis of familial hemophagocytic lymphohistiocytosis (HLH). Patients with familial HLH (F-HLH) often have defects of the perforin cytotoxic pathway. Experimental studies have demonstrated that this pathway provides critical feedback regulating CD8+ T–cell activation. Defects result in excessive antigen presentation by dendritic cells, leading to excessive T-cell activation and overproduction of IFN-gamma, which results pathologic systemic macrophage activation. Thus, clinical HLH results from a primary defect of immune regulation leading to largely IFN-γ driven immunopathology. Created with BioRender.com
HLH is commonly defined when a patient meets 5 or more of the 8 enrollment criteria from the HLH-2004 study, developed as entry criteria for a clinical trial in F-HLH.9 The appropriateness of these criteria for diagnosing other types of HLH is uncertain.10 Furthermore, while some of these features are driven by inappropriate immune activation in F-HLH,7 their presence in the context of cancer may represent nonimmunologic effects of the malignant clone itself, such as infiltration of the marrow or spleen. Thus, some patients fulfilling the HLH-2004 criteria mimic F-HLH, and not only may such patients not benefit from HLH-directed therapy, but they may even be harmed by it.11 Regardless of whether therapy may be helpful, if it obscures a critical diagnosis, such as prompt identification of malignancy, then it is potentially harmful.
How to approach a patient with suspected HLH
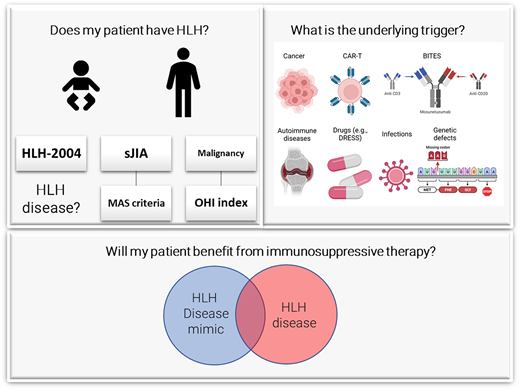
Once HLH is suspected, the provider should pose 3 questions:
Does my patient have HLH syndrome?
What is the underlying cause (genetic?) and trigger (infection, malignancy)?
Will my patient benefit from immunosuppressive therapy?
Does my patient have the HLH syndrome?
All patients fulfilling the required number of HLH diagnostic features of HLH-2004 diagnostic criteria9 or HScore12 (Table 2) may potentially have HLH syndrome. However, not all individuals fulfilling these criteria have a hyperinflammatory process and may not benefit from immune suppression. Such patients should be considered to have an HLH mimic instead of HLH disease. For example, while a patient with spontaneous familial HLH or another with macrophage activating syndrome (MAS) associated with a rheumatologic disease would be considered to have HLH disease, a patient with disseminated tuberculosis with the requisite number of features resulting from an appropriate immune response would best be thought of as having an HLH mimic. Thus, establishing an HLH diagnosis begins with recognizing HLH syndrome followed by exclusion of HLH disease mimics (Figure 2).11
Conceptualizing hemophagocytic lymphohistiocytosis (HLH) syndrome. HLH syndrome includes all conditions meeting HLH diagnostic criteria. This syndrome includes conditions of hyperinflammation (“HLH disease,” conditions close to the upper side) that would benefit from HLH-directed immunosuppressive therapies, and those conditions with adequate inflammation (“HLH disease mimics,” conditions close to the lower side) that would not benefit from such therapy. These conditions can be endogenous (those close to the right) or iatrogenic (those close to the left). DRESS, Drug Reaction with Eosinophilia and Systemic Symptoms; MAS, macrophage activation syndrome.
Conceptualizing hemophagocytic lymphohistiocytosis (HLH) syndrome. HLH syndrome includes all conditions meeting HLH diagnostic criteria. This syndrome includes conditions of hyperinflammation (“HLH disease,” conditions close to the upper side) that would benefit from HLH-directed immunosuppressive therapies, and those conditions with adequate inflammation (“HLH disease mimics,” conditions close to the lower side) that would not benefit from such therapy. These conditions can be endogenous (those close to the right) or iatrogenic (those close to the left). DRESS, Drug Reaction with Eosinophilia and Systemic Symptoms; MAS, macrophage activation syndrome.
| Familial HLH . | Reactive HLH . | Context-specific . | ||
|---|---|---|---|---|
| HLH-20049 If criterion 1 or 2 is fulfilled. 1) A molecular diagnosis consistent with HLH 2) 5 of the 8 criteria: • Fever • Splenomegaly • Cytopenias: 2 out of 3 lineages ○ Hemoglobin <9 g/dL ○ Platelets <100 × 109/L ○ Neutrophils <1.0 × 109/L • Triglycerides ≥265 mg/dL OR Fibrinogen ≤150 mg/dL • Hemophagocytosis in bone marrow or spleen or lymph nodes. No evidence of malignancy • Low or no NK cell activity • Ferritin ≥500 µg/L • sCD25 ≥ 2400 U/mL | H-Score12 >169 points is the optimal diagnostic threshold for HLH. Addition of the points according to: | Malignancy-associated optimized HLH inflammatory (OHI) index19 sCD25 > 3,900 U/mL AND Ferritin >1,000 ng/mL Systemic juvenile arthritis associated with EULAR/ACR/PRINTO for MAS39 Serum ferritin level >684 ng/mL AND Any 2 of the following: • Platelet count ≤181 × 109/L • AST >48 units/L • Triglyceride >156 mg/dL OR Fibrinogen ≤360 mg/dL | ||
| Variable | Categories | Points | ||
| Known underlying immunosuppression* | No | 0 | ||
| Yes | 18 | |||
| Temperature, °F (°C) | <101.1 (<38.4) | 0 | ||
| 101.1–102.9 (38.4-39.4) | 33 | |||
| >102.9 (>39.4) | 49 | |||
| Organomegaly | No | 0 | ||
| Hepatomegaly or splenomegaly | 23 | |||
| Hepatomegaly and splenomegaly | 38 | |||
| Number of cytopenias** | 1 lineage | 0 | ||
| 2 lineages | 24 | |||
| 3 lineages | 34 | |||
| Ferritin, ng/mL (µg/L) | <2000 | 0 | ||
| 2000–6000 | 35 | |||
| >6000 | 50 | |||
| Triglyceride, mg/dL (mmol/L) | <132.7 (<1.5) | 0 | ||
| 132.7-354 (1.5–4) | 44 | |||
| >354 (>4) | 64 | |||
| Fibrinogen, mg/dL (g/L) | >250 (>2.5) | 0 | ||
| ≤250 (≤2.5) | 30 | |||
| AST, U/L | <30 | 0 | ||
| ≥30 | 19 | |||
| Hemophagocytosis features on bone marrow aspirate | No | 0 | ||
| Yes | 35 | |||
| *HIV positive or receiving long-term immunosuppressive therapy (ie, glucocorticoids, cyclosporine, azathioprine). | ||||
| **Defined as hemoglobin ≤9.2 g/dL and/or WBC ≤5×109/L and/or platelets ≤110×109/L. | ||||
| Familial HLH . | Reactive HLH . | Context-specific . | ||
|---|---|---|---|---|
| HLH-20049 If criterion 1 or 2 is fulfilled. 1) A molecular diagnosis consistent with HLH 2) 5 of the 8 criteria: • Fever • Splenomegaly • Cytopenias: 2 out of 3 lineages ○ Hemoglobin <9 g/dL ○ Platelets <100 × 109/L ○ Neutrophils <1.0 × 109/L • Triglycerides ≥265 mg/dL OR Fibrinogen ≤150 mg/dL • Hemophagocytosis in bone marrow or spleen or lymph nodes. No evidence of malignancy • Low or no NK cell activity • Ferritin ≥500 µg/L • sCD25 ≥ 2400 U/mL | H-Score12 >169 points is the optimal diagnostic threshold for HLH. Addition of the points according to: | Malignancy-associated optimized HLH inflammatory (OHI) index19 sCD25 > 3,900 U/mL AND Ferritin >1,000 ng/mL Systemic juvenile arthritis associated with EULAR/ACR/PRINTO for MAS39 Serum ferritin level >684 ng/mL AND Any 2 of the following: • Platelet count ≤181 × 109/L • AST >48 units/L • Triglyceride >156 mg/dL OR Fibrinogen ≤360 mg/dL | ||
| Variable | Categories | Points | ||
| Known underlying immunosuppression* | No | 0 | ||
| Yes | 18 | |||
| Temperature, °F (°C) | <101.1 (<38.4) | 0 | ||
| 101.1–102.9 (38.4-39.4) | 33 | |||
| >102.9 (>39.4) | 49 | |||
| Organomegaly | No | 0 | ||
| Hepatomegaly or splenomegaly | 23 | |||
| Hepatomegaly and splenomegaly | 38 | |||
| Number of cytopenias** | 1 lineage | 0 | ||
| 2 lineages | 24 | |||
| 3 lineages | 34 | |||
| Ferritin, ng/mL (µg/L) | <2000 | 0 | ||
| 2000–6000 | 35 | |||
| >6000 | 50 | |||
| Triglyceride, mg/dL (mmol/L) | <132.7 (<1.5) | 0 | ||
| 132.7-354 (1.5–4) | 44 | |||
| >354 (>4) | 64 | |||
| Fibrinogen, mg/dL (g/L) | >250 (>2.5) | 0 | ||
| ≤250 (≤2.5) | 30 | |||
| AST, U/L | <30 | 0 | ||
| ≥30 | 19 | |||
| Hemophagocytosis features on bone marrow aspirate | No | 0 | ||
| Yes | 35 | |||
| *HIV positive or receiving long-term immunosuppressive therapy (ie, glucocorticoids, cyclosporine, azathioprine). | ||||
| **Defined as hemoglobin ≤9.2 g/dL and/or WBC ≤5×109/L and/or platelets ≤110×109/L. | ||||
AST, aspartate aminotransferase; EULAR/ACR/PRINTO, European League Against Rheumatism/American College of Rheumatology/Pediatric Rheumatology International Trials Organization; NK, natural killer; sCD25, soluble CD25/soluble interleukin-2 receptor alpha; WBC, white blood cell.
Serum ferritin is the most widely available biomarker for HLH and can be measured immediately upon suspicion of the process. However, hyperferritinemia is common in critically ill patients with sepsis, liver disease, and hematological malignancies,13 conditions that may mimic or trigger HLH. Lachman et al recently demonstrated that 4 rather than 5 of the HLH-2004 criteria with optimized values (serum ferritin >3000 µg/L and fever >38.2°C) and an HScore of 168 improve the sensitivity and specificity of HLH diagnosis.14 This application of the HLH diagnostic criteria in critically ill hyperferritinemic patients may facilitate the timely diagnosis of HLH.
Another important test, albeit not widely available globally, is sCD25. This assay and ferritin have a high negative predictive value; when both are normal, hyperinflammation is excluded (Figure 3). The only exception is infants under 1 year of age in whom HLH may present with a low but rising ferritin, necessitating serial measurement.
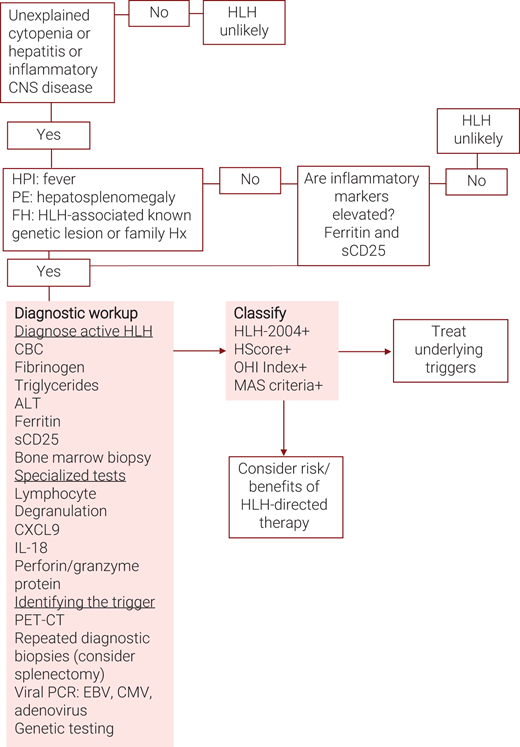
Algorithm for the evaluation of suspected hemophagocytic lymphohistiocytosis (HLH). ALT, alanine transaminase; CBC, complete blood count; CXCL9, C-X-C motif chemokine ligand 9; HPI, history of present illness; PE, physical examination; sCD25, soluble CD25 or soluble interleukin-2 receptor alpha.
Algorithm for the evaluation of suspected hemophagocytic lymphohistiocytosis (HLH). ALT, alanine transaminase; CBC, complete blood count; CXCL9, C-X-C motif chemokine ligand 9; HPI, history of present illness; PE, physical examination; sCD25, soluble CD25 or soluble interleukin-2 receptor alpha.
What is the underlying trigger?
F-HLH should be considered in all children and young adults with HLH (especially in male patients because some defects are X linked). A genetic panel should be sent as soon as possible, but treatment initiation should not be delayed until results are received. Lesions in genes affecting perforin function (UNC13D, STX11, STXBP2, RAB27A, LYST) or effector T-cell function (SH2D1A, ITK, CD27) are associated with HLH, as are those associated with inflammasome activation, such as defects in the XIAP and NLRC4 are also genes. A recent whole exome–based study has expanded the list of potentially HLH-associated genes.15 Adult HLH is not associated with germline mutations but may be associated with clonal hematopoiesis and somatic mutations.16
In adults, malignancies are the most common trigger of HLH and may occur in 3 settings. The first is HLH presenting concurrently with malignancy (M-HLH).17 While the mechanism of this syndrome is unknown, patient outcome is especially poor, with a 5-year overall survival of only 10%-20%. Hematologic malignancies are the most common cancers associated with this form of HLH. They may be particularly difficult to recognize given the clinical similarity between the malignancy and HLH, which frequently begs whether the patients truly have HLH disease or an HLH mimic.18 M-HLH can be accurately identified by the Optimized HLH Inflammatory Index, which comprises the combined elevation of sCD25 > 3900 U/mL and ferritin >1000 ng/mL (Table 2).19 An increased sCD25 to ferritin ratio has been reported to suggest an underlying lymphoma.20 HLH consensus recommendations strongly support positron emission tomography– guided imaging, repetitive tissue sampling, and consultation with a lymphoma reference pathologist in adult patients with HLH (Figure 3).21
In the second scenario, HLH develops after chemotherapy that results in immune compromise, marrow suppression, and/or associated infection; the condition is best thought of as HLH arising in the context of immune compromise (IC-HLH). The final context is iatrogenic HLH resulting from immune-activating therapies (Rx-HLH).22 Rx-HLH is always caused by (intentional) immune hyperactivation and thus should be considered “HLH disease” (Figure 2). These treatment modalities have in common the activation of T-cell responses against a variety of hematologic malignancies and include chimeric antigen receptor (CAR) T cells, bi-specific T-cell engager antibodies, and checkpoint inhibitors, the latter also being associated with Rx-HLH in solid organ tumors.23 Recently, delayed onset Rx-HLH following CD22-directed CART cell administration characterized by extreme hyperferritinemia24 has been described and is termed “immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome (IEC-HS).”25
Infections may either trigger or mimic HLH. Therefore, testing for common HLH-associated viruses by polymerase chain reaction (PCR) should be routine in HLH patients. Epstein-Barr virus (EBV) and cytomegalovirus are two of the most common infectious HLH triggers. EBV is associated with a worse prognosis than other subtypes.26 Hyperinflammation in COVID-19 patients has proven to have common immunologic profiles with HLH disease and may be considered on this spectrum.4 Atypical infections that may lead to cytopenias, elevations of inflammatory markers, and other features of HLH include visceral leishmaniasis; disseminated atypical/tuberculous mycobacteria; histoplasmosis; Ehrlichia, Bartonella, and Brucella species; disseminated adenovirus; and disseminated herpes simplex. In most cases, these infections should be considered as HLH mimics, and specific antimicrobial treatment is preferable to HLH-directed immune suppression because the HLH syndrome does not appear to have a largely immune-mediated etiology in these patients. Importantly, some patients with atypical infections benefit from immunosuppressive therapies.11
HLH in the context of rheumatologic diseases is termed MAS or rheumatologic (R)-HLH and deserves special consideration. Since patients with chronic inflammation have intrinsically elevated platelets and markers of inflammation, the European League Against Rheumatism/American College of Rheumatology/ Paediatric Rheumatology International Trials Organization consortium developed unique diagnostic criteria for patients with juvenile systemic arthritis presenting with MAS (Table 2). Similarly, patients with SLE may have underlying cytopenias making MAS difficult to identify. These patients may present with neutrophilia rather than neutropenia and have other unique clinical symptoms such as arthritis and rash. Evidence from preclinical and clinical studies suggests that interleukin 18 distinguishes MAS from F-HLH.6
Will my patient benefit from immunosuppressive therapy?
After determining that the patient meets the criteria for the HLH syndrome (Table 2), appears to have these features due to a hyperinflammatory process (Figure 2), and has been tested for underlying triggers (Figure 3), the clinician should consider whether the patient would likely benefit from immunosuppressive therapy. Our patient was started on steroids because of an ongoing uncontrolled inflammatory process without a definite diagnosis; though not optimal, this is a very common real-life scenario.
CLINICAL CASE (continued)
Following the elevated ferritin test, sCD25 was obtained and was elevated at 8390 U/mL, and a bone marrow biopsy showed hemophagocytosis. HLH syndrome was established based on fever, elevated sCD25, ferritin, bicytopenia, triglycerides, and hemophagocytosis.
The trigger for HLH was sought: positron emission tomography– guided imaging demonstrated increased splenic18 fluorodeoxyglucose uptake and no lymphadenopathy. Testing for rheumatologic disorders was negative. EBV PCR was elevated with 51,000 copies/mL. A presumptive diagnosis of EBV-associated HLH was made. Genetic testing did not reveal any known F-HLH-associated mutation.
The patient was treated using the HLH-94 protocol; in addition, 3 doses of rituximab as EBV directed therapy were added. After the third dose, virus elimination was confirmed by PCR testing. During treatment, the clinical and laboratory abnormalities gradually resolved.
Approach to therapy
Because patients with F-HLH and often other forms of HLH may deteriorate rapidly, prompt and aggressive therapy is critical in improving outcomes and may require treatment initiation before the conclusion of diagnostic testing.21 While the backbone of therapy is etoposide, dexamethasone, and other immunosuppressive agents, treatment should be individualized according to specific triggers such as the presence of malignancy or active viral infection. Though it may obscure the diagnosis of lymphoma or leukemia, early corticosteroid administration should be considered, particularly if organ dysfunction occurs.27
F-HLH is treated according to the HLH-94 protocol (Figure 428 ): 8 weeks of etoposide and dexamethasone treatment followed by allogeneic hematopoietic stem cell transplantation (HSCT). Patients with refractory or recurrent disease or who cannot tolerate etoposide treatment (eg, because of severe cytopenias) can be treated with emapalumab, an anti-IFN-γ monoclonal antibody. Other targeted therapies are currently being tested in clinical trials. These include the JAK inhibitor ruxolitinib in a phase 2 trial (NCT04551131),29 and a phase 3 trial of Tadekinig alpha, a recombinant interleukin-18 binding protein (r-hIL-18BP) in patients with deleterious NLRC4 and XIAP mutations which are associated with autoinflammatory syndromes (NCT03512314).
Etoposide/dexamethasone therapy for HLH (based on HLH94): initial therapy (weeks 1-8) for patients with familial/ idiopathic HLH. CBC, complete blood count; CNS, central nervous system; HC, hydrocortisone; IgG, immunoglobulin G; IT, intrathecal therapy; IVIG, intravenous immunoglobulin; MTX, methotrexate; PJP, pneumocystis jirovecii pneumoni; PPI, proton pump inhibitors; PT, prothrombin time; PTT, partial thromboplastin time.
Etoposide/dexamethasone therapy for HLH (based on HLH94): initial therapy (weeks 1-8) for patients with familial/ idiopathic HLH. CBC, complete blood count; CNS, central nervous system; HC, hydrocortisone; IgG, immunoglobulin G; IT, intrathecal therapy; IVIG, intravenous immunoglobulin; MTX, methotrexate; PJP, pneumocystis jirovecii pneumoni; PPI, proton pump inhibitors; PT, prothrombin time; PTT, partial thromboplastin time.
Rx-HLH should be treated with anticytokine therapy, which preserves immunotherapy's efficacy, with tocilizumab and anakinra being the preferred agents.25,30 Data regarding the efficacy of emapalumab27 in this setting are emerging.
The optimal treatment for M-HLH treatment is unknown.22 Currently, a 2-phase approach is advised.22,31 First, quench the uncontrolled inflammation using corticosteroids or specific anticytokine therapy, sometimes with etoposide. Evidence is also emerging for ruxolitinib as successful therapy for inflammation control in these patients.32,33 Second, provide tumor-specific antineoplastic therapy, possibly augmented with etoposide, as soon as feasible.
Treatment of IC-HLH requires thorough evaluation to identify possible bacterial, fungal, and viral triggers (including testing for viral triggers with PCR), which, if present and treated expeditiously, may lead to the resolution of the syndrome. In addition, anti-inflammatory therapy with steroids and anticytokine agents is sometimes appropriate.
MAS treatment is based predominantly on corticosteroids and rarely requires etoposide. Recent reports have shown a good response to emapalumab in refractory patients, and a long-term follow-up trial is ongoing.34 Anakinra, a recombinant IL-1 receptor antagonist, and agents targeting IL-6 have been reported as beneficial in MAS patients.35
Allogeneic HSCT is the standard of care for patients with F-HLH, patients with refractory disease, and patients with M-HLH that have no alternate definitive therapy. Reduced-intensity conditioning regimens have reduced toxicities and improved survival rate36 but are often complicated by mixed chimerism.37 Excellent outcomes of HSCT in 21 patients with adult HLH have been reported with a 3-year OS of 75% (95% CI, 51%-89%), suggesting the need to consider this strategy more widely among these patients.38
Conclusions
Without rapid diagnosis and appropriate treatment, the natural course of HLH disease may be fatal. Due to the rarity, diversity, and complexity of the HLH syndrome, diagnosis is difficult and is often delayed. Increasing awareness of HLH in recent years has improved early diagnosis but may carry the risk of overdiagnosis and inappropriate immunosuppression in mimicking conditions. Early measurement of serum ferritin and sCD25 levels, particularly in M-HLH, can expedite diagnosis with a high degree of specificity. Increasing the availability and awareness of these important diagnostic tests, along with comprehensive testing to identify an HLH trigger, such as malignancy or viral infection, is crucial for improving patient outcomes.
Conflict-of-interest disclosure
Adi Zoref-Lorenz: received consulting fees and research support from Sobi.
Martin Ellis: no competing financial interests to declare.
Michael B. Jordan: received consulting fees and research support from Sobi.
Off-label drug use
Adi Zoref-Lorenz: Off-label use of ruxolitinib, emapalumab, and anakinra is discussed.
Martin Ellis: Off-label use of ruxolitinib, emapalumab, and anakinra is discussed.
Michael B. Jordan: Off-label use of ruxolitinib, emapalumab, and anakinra is discussed.