Abstract
While immune thrombocytopenia often presents with mild bleeding manifestations or surprising findings of thrombocytopenia on routine complete blood counts in patients without symptoms, some patients can present with new thrombocytopenia and life-threatening bleeding. Emergent assessment and treatment are needed to prevent substantial morbidity and even mortality. These patients present to the emergency room with bleeding, and hematologists are subsequently consulted. Understanding the approach to making the diagnosis and excluding other life-threatening illnesses is essential, as is rapid initiation of treatment in the bleeding patient even when the diagnosis of immune- mediated thrombocytopenia is tentative. Using a case-based format, we review how to approach and treat patients presenting with new thrombocytopenia and bleeding.
Learning Objectives
Evaluate adult patients who present with new thrombocytopenia and life-threatening bleeding
Determine the best treatment plan for adults who have newly diagnosed ITP and life-threatening bleeding
Introduction
Adult patients who present with new isolated thrombocytopenia can be challenging to manage, especially if they have more symptoms than mucosal bleeding or petechiae. Immune thrombocytopenia (ITP) is a diagnosis of exclusion, as no test can positively confirm a diagnosis of ITP, yet patients may require urgent or emergent treatment due to bleeding manifestations on presentation. Although bleeding events are rare in patients with primary ITP, they can occur and when they do, they can be life-threatening.1 Past reports from a pooled analysis suggested a 5-year mortality from bleeding of 49% in patients over the age of 60, although this predated the use of thrombopoietin receptor agonist (TPO-RA) and rituximab in the treatment of ITP.2 The approach to a patient with new findings of thrombocytopenia accompanied by bleeding requires a rapid clinical assessment and the institution of treatment aimed at determining the underlying etiology.
Antecedent medical history can be informative, including a history of recent infections, the use of antibiotics known to be associated with thrombocytopenia,3 or a history of disorders associated with ITP such as collagen vascular disorders, especially lupus and rheumatoid arthritis, and inflammatory bowel disorders.4 Here we discuss the approach to the differential diagnosis of new-onset thrombocytopenia associated with bleeding. Standardized definitions of bleeding in patients with ITP, assessment of the need for urgent or emergent treatment, and treatment strategies that can be used up front to mitigate bleeding and restore hemostasis are reviewed.
CLINICAL CASE
A 67-year-old woman presents to the emergency department (ED) with a severe headache. Her past medical history is notable for hypertension, hyperlipidemia, and hypothyroidism due to a remote history of Hashimoto's thyroiditis; she has been taking lisinopril, rosuvastatin, and levothyroxine for over 3 years. She has noted some bruises on her legs but thought they might be due to playing with her grandchildren. She denies any recent infections, including COVID-19 or diarrheal illnesses. Her grandchildren have been healthy. A physical exam in the ED reveals normal vital signs and no acute distress, clear lungs, regular rate and rhythm with no murmur, and no hepatosplenomegaly. There are a few small 1- to 2-cm bruises on bilateral legs, distal lower extremity petechiae she thought were due to an allergy to sunscreen, and a bruise near her watch on her left forearm. A complete blood cell count (CBC) and chemistry panel are sent, and she undergoes noncontrast head computed tomographic imaging. The computed tomographic radiologist pages you with findings of a moderate-sized subdural hematoma. The lab pages with results of the CBC because her platelet count is 3000/µL. Her hemoglobin level is 12.7 gm/dL with a normal mean corpuscular volume (MCV). A chemistry panel reveals normal creatinine and normal transaminases. The ED pages you, the hematologist on call.
Differential diagnosis
In a patient presenting with new thrombocytopenia, the platelet count and presenting symptoms inform the speed of evaluation and treatment. The patient in this case has not only significant thrombocytopenia but also serious bleeding in a critical location that must be addressed now, as compared to a patient who, on routine yearly physical exam, is found to have a platelet count of 40 000/µL and no bleeding manifestations. Presenting symptoms and recent history are important clues that can aid in the diagnosis. She has had no recent fevers, viral infections, gastrointestinal illness with diarrhea, or recent use of antibiotics or new medications, though other symptoms, such as transient neurologic symptoms like her headache, are important. Few disorders other than ITP are associated with a platelet count of less than 10 000/µL, but this platelet count can also be seen in other life-threatening disorders such as immune thrombotic thrombocytopenic purpura (iTTP), atypical hemolytic syndrome (aHUS), or acute leukemia, which are important to rule out. Risks for viral infections such as hepatitis C and HIV should be assessed, as these infections are associated with the development of immune-mediated thrombocytopenia. A history of autoimmune thyroid disease has been associated with ITP while lupus and RA have a strong association with the development of ITP. This patient, given her remote history of Hashimoto's thyroiditis, may be at increased risk for other autoimmune disorders such as ITP. Other disorders such as chronic lymphocytic leukemia and even immune checkpoint inhibitor treatment can be associated with ITP; however, these should be evident by the history, or in the case of chronic lymphocytic leukemia, the CBC.
In addition to the history, a thorough physical exam is needed, as well as laboratory tests, including a review of the peripheral blood smear. With a normal white blood cell count and differential, hemoglobin, and hematocrit, aplastic anemia and hematologic malignancies such as acute leukemia can be excluded. Acute kidney injury, as manifest by elevated creatinine, and new anemia suggest diagnoses other than ITP. The peripheral blood smear should be assessed for schistocytes, the presence of which can indicate iTTP, aHUS, or disseminated intravascular coagulation (DIC). The platelets are usually larger than average with ITP. Although an elevated MPV is indicative of large platelets, it is often not reliably measured or reported when the platelet count is low; however, if previous CBCs are available for review and an elevated MPV was seen, it can lend some degree of certainty to the diagnosis of ITP. A “normal” recent CBC can also exclude the possibility of the sudden presentation of an inherited thrombocytopenia, although this is very unlikely at age 67. The reticulated platelet count, or immature platelet fraction, is a promising assay that can help distinguish between peripheral platelet destruction and decreased marrow platelet production, as the immature platelet fraction is increased in disorders such as ITP but is not routinely reported in many clinical laboratories and has not yet been validated in the differential diagnosis of severe thrombocytopenia.5,6 A review of the peripheral smear is also important to rule out pseudo-thrombocytopenia, although this is unlikely in this patient because she has bruising and bleeding.
Other laboratory tests that can aid in excluding diagnoses such as iTTP and HUS include creatinine, the transaminases, total and direct bilirubin, and the coagulation tests prothrombin time, activated partial thromboplastin time, and fibrinogen. In this patient all these tests are normal, suggesting that this is not iTTP, aHUS, or DIC. Chronic liver failure can contribute to ITP not only through the development of splenomegaly and splenic sequestration but also due to decreased TPO production, as TPO is synthesized in the liver. This patient would have to have experienced a history of chronic liver disease or findings in physical exam and laboratory assessments (such as low albumin) to implicate chronic liver disease as the etiology for thrombocytopenia.
In this older patient, a diagnosis of ITP is highly likely based on the above findings. If the patient were much younger and had a slightly higher platelet count, congenital thrombocytopenia should also be considered in the differential diagnosis. Patients with known chronic ITP can also present with life-threatening bleeding, and the treatment strategies below can be used, although knowledge about the patient's current and prior ITP treatments may help tailor treatment.
Severity of bleeding
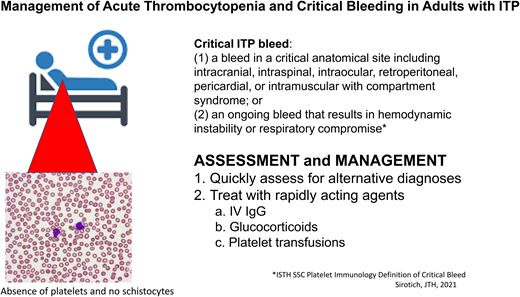
Although the platelet count can drop to very low levels in patients with ITP, significant bleeding events in adults are rare, with intracerebral hemorrhage (ICH) occurring in just 1.4% (95% CI, 0.9-2.1) of adults with chronic ITP and severe non-ICH bleeding in 9.6% (95% CI, 4.1-17.1) with acute or chronic ITP in one meta-analysis.1 This report, like others, uses terms such as “severe” to define bleeding events, yet there has been variability in definitions of bleeding across studies in patients with ITP. To address this lack of standardized definitions, a large panel of experts from multiple specialties as part of the International Society on Thrombosis and Hemostasis Scientific and Standardization Committee on Platelet Immunology recently published a consensus definition of critical bleeding: “A critical ITP bleed was defined as: (a) a bleed in a critical anatomical site including intracranial, intraspinal, intraocular, retroperitoneal, pericardial, or intramuscular with compartment syndrome; or (2) an ongoing bleed that results in hemodynamic instability or respiratory compromise.”7 The authors note that patients with ITP and critical bleeding meeting this definition require treatment. While it is generally thought that critical bleeds rarely occur even with a platelet count less than 20 000/µL, the authors of this standardized definition and others note that factors such as the use of anticoagulants or anatomic lesions can result in critical bleeds at higher platelet count thresholds.
Other less severe forms of bleeding have been variably categorized as minor, including bruising or skin petechiae, mucosal bleeds such as epistaxis or oral bleeding, mild hematuria, or increased hemorrhoid bleeding. Some patients present with bleeding that falls in between minor and critical. In these cases, individual patient assessment is required to determine the urgency for addressing the etiology of thrombocytopenia and instituting treatment. In general, patients with platelet counts greater than 30 000/µL and no bleeding manifestations can be treated as outpatients.8 Factors such as age,9 comorbid disease, including renal insufficiency, and medications affect the risk of bleeding and the need for hospitalization and urgency for treatment.
The patient in this case meets the definition of critical bleeding. She requires not only hospital admission but also urgent treatment to prevent further life-threatening bleeding.
Initial treatment
Treatments for the patient with presumed new-onset or recently diagnosed ITP with critical bleeding require rapid results and are therefore limited to intravenous immunoglobulin (IVIG), steroids, and platelet transfusions (Table 1). In patients with critical bleeding, we use both IVIG and glucocorticoids together. In cases of critical bleeding manifest as ICH, we also use platelet transfusion until IVIG takes effect. These strategies for rapidly increasing the platelet count can also be used for those with critical bleeding and chronic ITP who are on minimal or no treatment.
Initial treatment of critical bleeding in patients with ITP
| Agent . | Mechanism of action . | Onset of effect . | Risks . |
|---|---|---|---|
| IVIG | Interferes with FcR-mediated opsonization of antibody-coated platelets by macrophages | Hours | Volume overload Headache Fever |
| Glucocorticoids | Inhibits macrophage-mediated platelet clearance Decreases IgG production | Days | Elevated blood glucose Hypertension Steroid psychosis |
| Platelet transfusions | Immediate supply of platelets | Immediate | Short duration of effect Transfusion reaction Bacterial infection from contaminated products |
| Tranexamic acid | Stabilizes hemostatic clots | Immediate | Headache Nausea |
| Agent . | Mechanism of action . | Onset of effect . | Risks . |
|---|---|---|---|
| IVIG | Interferes with FcR-mediated opsonization of antibody-coated platelets by macrophages | Hours | Volume overload Headache Fever |
| Glucocorticoids | Inhibits macrophage-mediated platelet clearance Decreases IgG production | Days | Elevated blood glucose Hypertension Steroid psychosis |
| Platelet transfusions | Immediate supply of platelets | Immediate | Short duration of effect Transfusion reaction Bacterial infection from contaminated products |
| Tranexamic acid | Stabilizes hemostatic clots | Immediate | Headache Nausea |
Platelet transfusion
Urban legends about the use of platelet transfusions in ITP are many; however, platelet transfusion is the fastest method to increase the platelet count. Unlike other disorders such as iTTP or HIT, in which there is concern that platelet transfusions will exacerbate the underlying disease process, there is no such concern with ITP. The problem is that the duration of effect is short, as antibody-mediated clearance of both autologous and transfused platelets generally occurs. A 1-hour posttransfusion platelet count is important not only to gauge the need for further transfusions but to help confirm that peripheral platelet destruction is occurring, supporting the diagnosis of ITP. Common strategies to combat the rapid platelet clearance in patients with ITP include continuous platelet transfusion,10 transfusion of 2 bags of apheresis platelets or 2 bags of pooled donor platelets every 5 to 6 hours (one author's strategy, JMC), or concomitant platelet transfusions with the administration of IVIG.11 Platelet transfusions carry a risk of common transfusion-associated reactions, they must be ABO compatible, and they can lead to bacterial contamination because they require storage at 20 °C to 24 °C.
While platelet transfusions are appropriate in this setting, they should be used to achieve hemostasis (>20,000/μL) and not to achieve an artificial threshold for intervention (such as a request for a platelet count of 100,000/μL to go to surgery). If patients do not respond one or two units of transfused platelets, repeat transfusion of additional units is unlikely to be effective and puts the patient at higher risk of transfusion-associated complications. Platelet transfusion is best considered part of a multi-modality hemostatic strategy, along with antifibrinolytics, while waiting for glucocorticoids and IVIG to work.
Intravenous immunoglobulin
IVIG works in ITP by blocking crystallizable fragment–mediated opsonization of antibody-coated platelets, the usual mechanism of peripheral platelet destruction in ITP.12 Its effects can be rapid, with an increase in platelet count seen as early as 24 hours after administration. It is usually accompanied by a continued increase over the next 24 to 48 hours. The original dosing scheme was 0.4 gm/kg/d for up to 5 days; however, similar results were found when given as 1 gm/kg/day for 1 or 2 doses.13 We usually use the larger dose of 1 gm/kg/d, and if there is no or minimal improvement in the platelet count 24 hours later, we give a second dose of 1 gm/kg/d. However, in older patients or those with impaired cardiac or renal function, the lower dose may prevent acute volume overload. As with the 1-hour posttransfusion platelet count, which can support the diagnosis of ITP, a response to IVIG can help confirm the diagnosis of ITP.14
Glucocorticoids
Glucocorticoids are the mainstay of ITP treatment for newly diagnosed patients who do not require admission and can be managed in the outpatient setting, but they can also be used in those with bleeding. The onset of action is slower than with IVIG, although response at 2 days has been reported, and an increase in the platelet count in response to glucocorticoids alone usually occurs between 5 and 10 days. Much has been written about the type of glucocorticoid and dosing strategy—pulse dexamethasone at 40 mg/d for 4 days, a fixed dose of IV prednisolone at 1 gm/d, or a weight-based dose of oral prednisone of 0.5 to 2.0 mg/kg/d—with a number of randomized controlled trials (RCTs) comparing dexamethasone and prednisone in patients with ITP without critical bleeding, including 1 meta-analysis.15 Dexamethasone has been found to yield higher platelet counts at 7 days, although there does not appear to be a difference at 30 days. For patients with ICH or other critical bleeding, a dose of 1 gm IV methylprednisolone is warranted, with subsequent doses based on response. For less severe bleeding, pulse dexamethasone is associated with a higher platelet count at 7 days, which may simply reflect the higher steroid dose at the outset. Side effects, however, need to be considered and managed, including infection, blood glucose in patients with diabetes, and steroid psychosis with high doses in the elderly.
Subsequent treatments
It is hoped that a combination of the above treatments will be rapidly effective in those that present with critical bleeding due to ITP. In cases refractory to these treatments, other approaches may be needed as long as the diagnosis is certain; aside from continued reassessment to consider alternatives, this usually requires a bone marrow examination to ensure that the megakaryocytes are at least normal in number.
Thrombopoietin receptor agonists (TPO-RA) mimetics have significantly improved the treatment options for patients with chronic ITP but are currently considered by the American Society of Hematology guidelines for the management of ITP to be second-line therapy for those not responsive to glucocorticoids after 3 months.8 The use of TPO-RA for newly diagnosed ITP is more controversial, with little data available to support up-front use and certainly not as stand-alone initial therapy as it can take 1 to 3 weeks to see effects. If a patient with newly diagnosed ITP is refractory to initial treatment with IVIG, corticosteroids, and transfusion or has a very short duration of the increase in platelet counts, we consider starting TPO-RA after 2 to 3 days of treatment in patients who also have critical bleeding. We start at the maximum dose in patients with ITP and critical bleeds as one can more easily titrate the dose down than wait to see effects and then increase after 1 or more weeks of treatment. Although any of the available TPO-RA approved by the US Food and Drug Administration should work, romiplostim as a parenteral agent is more frequently available in inpatient formularies at this time, and it is more easily titrated than the oral agents eltrombopag and avatrombopag. Also, in a sick patient giving it parenterally eliminates any question of absorption. Romiplostim typically has a faster onset of action than eltrombopag, making it more suitable for use in ITP with life-threatening bleeding.
Rituximab, an anti-CD 20 monoclonal antibody, can result in remission of ITP. It works by targeting B lymphocytes, resulting in cell death and therefore decreasing the production of antiplatelet antibodies. It is considered a second-line treatment for ITP by the American Society of Hematology.8 It takes a while for rituximab to be effective, with no difference seen at 30 days in the RCT of patients treated with glucocorticoids plus rituximab vs glucocorticoids alone.16 Thus, it is not useful in the initial emergent management of patients with ITP and critical bleeding.
Anti-D immunoglobulin is not frequently used in adult patients. The patient must be Rh positive. It works by causing red cell destruction and therefore interfering with reticuloendothelial uptake of platelets; however, its effects are not easily controlled. Moderate to severe hemolysis can occur that can result in cardiovascular instability and acute kidney injury due to free hemoglobin, although its effects may be additive with IVIG.
Splenectomy has been used in patients with acute ITP wishing to avoid other treatments, in those who are refractory to initial treatments, and in those with chronic ITP not responding or losing response to treatment. The platelet count going into surgery can be low; surgeons report that once the splenic artery is clamped, the platelet count rises rapidly as the reticuloendothelial destruction of platelets ceases. However, over time the reticuloendothelial activity in other organs can take over and ITP can recur. The shortest duration of effect of splenectomy seen by 1 author is 6 weeks, with a rise in platelet count to over 400 000/µL, only to drop below 20 000/µL 6 weeks later. Emergency splenectomy can be an option for some patients with ITP and critical bleeding if they are candidates for anesthesia. Emergency splenectomy may be appropriate for ITP patients with life-threatening bleeding who do not respond to initial therapies.
Although vincristine was a mainstay of therapy in the 1970s and 1980s, it is rarely used currently in the management of ITP. Its main drawback was neurologic side effects with multiple doses, especially in the elderly, as well as a good but only transient response. However, it is platelet sparing and can be effective when other treatments have proved ineffective when used for only 1 or at most 2 doses, as was recently described in cases of ITP associated with SARS-CoV-2 vaccines.17 Early addition of vincristine (1−1.5 mg) has been studied and found to be safe and effective in emergency treatment of ITP. Vincristine has rapid onset of action and may augment response to IVIG and steroids if these agents do not initially rapidly raise the platelet count.18
Adjunctive hemostatic treatments
As with any form of bleeding, antifibrinolytic agents would appear to have efficacy at preventing clot dissolution. Although the use of tranexamic acid in RCTs for bleeding in trauma and after cesarean birth have shown positive results, RCTs have demonstrated no efficacy in cases of gastrointestinal bleeding, ICH, or bleeding due to thrombocytopenia associated with hematologic malignancies.19-22
Although tranexamic acid and aminocaproic acid are not well-studied in ITP patients with life-threatening bleeding, their use has become commonplace. Their safety has been established in numerous settings, so their benefit likely outweighs their risk in ITP patients. When used, patients with life-threatening hemorrhage should receive an intravenous bolus (e.g., epsilon- aminocaproic acid 5 grams) followed by a continuous drip until hemostasis is achieved (e.g., 1 gram per hour for 8 hours). If prothrombin time, activated partial thromboplastin time, and fibrinogen are normal, there is no role for factor concentrates, such as prothrombin complex concentrates, that are not activated. Similarly, activated recombinant factor VII likely has no role in treating bleeding associated with ITP, as evidenced in a case report from Germany in 2021 in which despite using all modalities of treatments, including rVIIa, fatal bleeding could not be prevented.23
Summary
Patients who present with thrombocytopenia and critical bleeding require urgent assessment and management. Excluding other diagnoses and rapidly instituting treatment for those with isolated thrombocytopenia with initial use of IVIG, glucocorticoids, and platelet transfusions may prevent serious morbidity and fatal outcomes. Early recognition, exclusion of alternative diagnoses, and rapid institution of fast-acting treatment agents are required to successfully treat these patients.
Conflict-of-interest disclosure
Steven Fein: speakers bureau for Amgen and Sobi. Jean Connors: Nothing to disclose.
Off-label drug use
Steven Fein and Jean Connors: Rituximab used for treatment of ITP.