Abstract
Hematopoietic cell transplantation (HCT) can cure blood dyscrasias and reduce the risk of hematologic cancers in patients with inherited bone marrow failure syndromes (IBMFS). However, because of its high mortality rate, HCT is generally reserved until patients with IBMFS manifest life-threatening cytopenias or myeloid malignancy, at which point outcomes are poor. Screening tests that accurately predict transformation and enable timely intervention are lacking. These unknowns and risks limit the use of HCT in patients with IBMFS, sometimes until significant disease-related sequelae have occurred. A major goal for IBMFS is to reduce cellular therapy–related complications to the point that earlier intervention can be considered before significant transfusion exposure, occurrence of comorbidities, or malignant transformation. In recent decades, disease-specific allogeneic HCT trials have yielded significant improvements in outcomes in IBMFS conditions, including Fanconi anemia and dyskeratosis congenita. This is in large part due to marked reductions in conditioning intensity to address the increased sensitivity of these patients to cytotoxic chemotherapy and radiation. The success of these approaches may also indicate an ability to leverage intrinsic fitness defects of hematopoietic stem and progenitor cells across IBMFS disorders. Now with advances in tracking somatic genetic evolution in hematopoiesis and tailored minimal intensity conditioning regimens, this question arises: is it time for preventative HCT for IBMFS?
Learning Objectives
Describe determinants of HCT timing for IBMFS
Compare disease-specific factors and approaches for HCT among IBMFS
CLINICAL CASE
A boy born at 32 weeks' gestation with a history of intrauterine growth retardation and esophageal strictures requiring dilation was found to have moderate pancytopenia at age 8 years. Bone marrow evaluation showed hypocellularity but no evidence of myelodysplastic syndrome/acute myeloid leukemia (MDS/AML). Referral and evaluation for inherited bone marrow failure syndromes (IBMFS) were notable for skin pigmentation abnormalities, oral leukoplakia, and dystrophic nails, leading to a diagnosis of dyskeratosis congenita (DC). The diagnosis was confirmed using a newly available flow cytometry–based fluorescence in situ hybridization telomere length test, showing mean lymphocyte telomere length less than the first percentile for age. A genetic workup for known telomere biology disorder–associated genes was negative. In further evaluation, he was found to have cerebellar hypoplasia, dysarthria, lacrimal duct obstruction, and a markedly abnormal DLCO of 50% on pulmonary function testing. His sibling was a full human leukocyte antigen match but showed mean lymphocyte telomere length at the first percentile. He had multiple 10/10 HLA-matched unrelated donors (MUD), but reported outcomes for hematopoietic cell transplantation (HCT) for DC were poor, with a 100-day mortality ∼25%. Androgen therapy was thus initiated when the boy was 9 years old, with a substantial response in his hemoglobin and platelets, obviating the need for transfusions for the next 6 years. This was, however, complicated by hyperlipidemia and suppression of puberty. During this time, he was found to have compound heterozygous mutations in the RTEL1 gene, consistent with his diagnosis. His parents and sibling were carriers.
Diagnosis and HCT decision-making in IBMFS
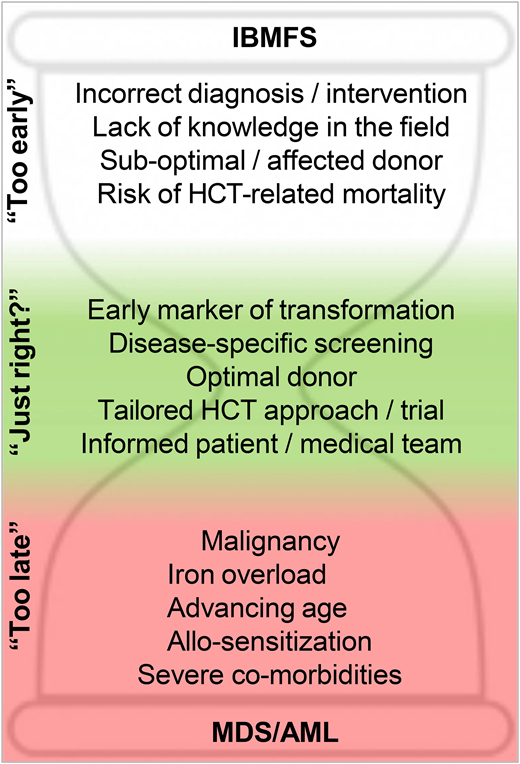
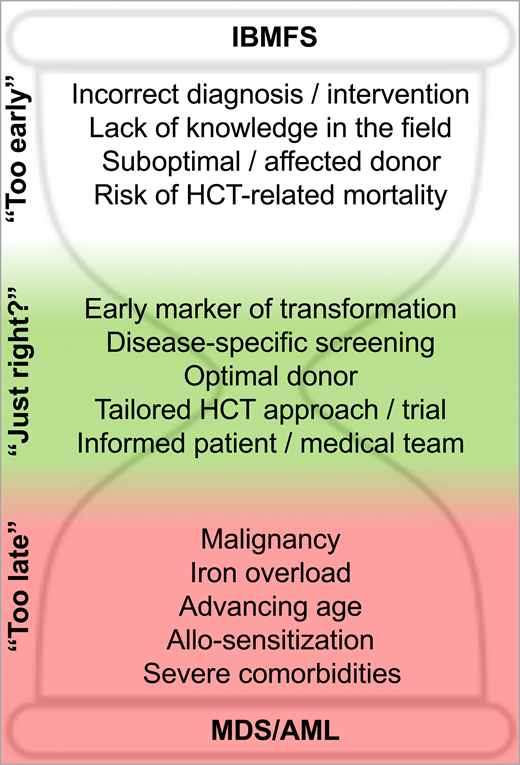
Genetic discoveries in recent decades have transformed our understanding of IBMFS. Unlike the case described, some patients do not follow the textbook presentation. The use of gene panels and functional tests has greatly increased diagnostic capability and enabled grouping based on the underlying biology rather than clinical phenotype alone. For patients presenting with bone marrow failure (BMF), accurate and timely diagnosis of an acquired vs inherited disorder is critical for decision-making,1,2 with different therapeutic interventions for their cytopenias depending on this categorization. These include immunosuppressive therapy (cyclosporine A/antithymocyte globulin), eltrombopag, androgens, corticosteroids, or HCT. The choice of frontline approach(es) depends on factors beyond diagnosis, however, and in many cases the evidence base underlying these choices is still not well established. Although successful HCT would cure the blood dyscrasias in most IBMFS, treatment-related mortality across the board can be estimated to be at least 10%, and often higher. Therefore, many factors need to be taken in account to determine the feasibility and timing of HCT for IBMFS, including patient status, donor suitability, and prior experience in the treating center and field (Figure 1).
With respect to patient hematologic status, cytopenias including severe neutropenia (defined as an absolute neutrophil count <500/µL) and transfusion-dependent thrombocytopenia or anemia are considered indications to move to HCT because of accumulating infection risk, iron overload, and allosensitization. Patients with IBMFS and evidence of MDS or AML based on adverse cytogenetics and blast count also require HCT for cure, with or without prior cancer-directed therapy. More moderate cytopenias and low-grade myelodysplastic syndrome constitute “gray zones” requiring careful consideration of HCT vs observation or other treatments, depending on estimates of disease evolution, treatment response, and expected toxicities. For example, androgens have been shown to increase blood counts in approximately two-thirds of patients with Fanconi anemia (FA) or DC within 3 months of treatment.3,4 However, the degree and durability of response are unpredictable, and side effects that vary based on the age and gender of the patient limit adherence. Finally, patient-specific features, including age, disease-related comorbidities, and infection status, are carefully weighed to determine HCT candidacy and timing.
Regarding donor factors, as for HCT in general, the use of HLA-matched family donors is preferred but complicated in IBMFS by the need to evaluate and triage family members for the possibility of subclinical disease.5 The complexity increases when a genetic etiology has not been firmly established, or when the clinical significance of carrier status is unclear, as seen in the sibling in our case who had borderline telomere length and a heterozygous RTEL1 mutation. Thus, in equivocal cases, well-matched unrelated bone marrow donors might be prioritized instead of related donors. Except for FA, data are relatively sparse on haploidentical transplants and alternative graft sources in IBMFS disorders, limiting their appeal.
Beyond patient and donor factors, HCT candidacy and counseling for IBMFS patients have relied heavily on anecdotal experience and local practice. For most IBMFS other than FA, the literature is composed primarily of retrospective studies with small patient numbers, often reflective of different HCT “eras” and disease categorizations (eg, based on clinical phenotypes), and with variable regimens and supportive care that may not accurately reflect current practices. It is not uncommon for centers to have limited experience in HCT for particular IBMFS disorders, with the outcomes of 1 or a few prior patients influencing local decision-making.
With these considerations in mind, there is a clear need for prospective trials in IBMFS to guide HCT and other therapeutic interventions. Ideally, the eligibility criteria of a disease-specific protocol could thus be used to help determine HCT timing. In the case described, lacking an open prospective trial, androgen therapy was initially chosen by the team based on bleak retrospective HCT data in DC. This approach temporized cytopenias and allowed progress in the field that impacted later decision-making.
CLINICAL CASE (continued)
The patient underwent yearly surveillance for DC, including bone marrow exams, which consistently showed severe hypocellularity but no morphologic or cytogenetic evidence of transformation. Somatic mutation screening by next-generation sequencing became available during this time and was performed but did not show any abnormalities in bone marrow DNA. At 15 years of age, the patient's platelet count and hemoglobin waned, and the oxymetholone dose was increased with partial effect, but hyperlipidemia worsened, requiring statin therapy. He was reevaluated for allogeneic MUD HCT and deemed eligible for a new minimal intensity prospective trial tailored for DC, but the family deferred due to the relatively small number of patients treated on the protocol to date.
By 18 years of age, the patient's platelets had decreased to ∼20 K/µL and hemoglobin to ∼8 g/dL on high doses of oxymetholone, and he required platelet transfusions for procedures. He was found to have a positive contrast echocardiogram suggestive of pulmonary arteriovenous shunting. His bone marrow exams continued to show hypocellularity ∼5%-10% without morphologic, cytogenetic, or somatic mutation abnormalities indicative of MDS/AML. He was again evaluated for HCT on the minimal intensity disease-specific protocol, which by this time had treated several additional patients with up to 5 years of follow-up. The HCT team, patient, and family contemplated the waning androgen response, ongoing MDS/AML risk, progressive systemic disease, and quality of life. He remained eligible for the HCT protocol and participation was offered. After careful consideration, the now-adult patient decided to proceed with MUD HCT in the year between high school and college.
Surveillance and earlier HCT intervention in IBMFS
Surveillance and screening tests in general are only useful if (1) there is a reliable early marker; (2) there is a predictable, adverse disease outcome; and (3) there is an effective intervention to prevent or treat the outcome, relative to the side effects of the intervention itself.
Surveillance for cancer risk and disease-specific comorbidities
IBMFS are highly heterogenous in their hematologic presentation and progression not only between but also within biologically linked disease categories. After diagnosis, many patients with IBMFS find themselves in one of the “gray zones” of HCT timing, with only mild-moderate cytopenias and uncertain trajectories. Several factors might push the equation toward earlier intervention, including MDS/AML risk, progression of nonhematologic disease, and individual decision-making (Table 1).
IBMFS and risks/surveillance impacting HCT timing
| Disorder . | MDS/AML risk . | Solid tumor risk . | Other surveillance . |
|---|---|---|---|
| Fanconi anemia | + | + | |
| Dyskeratosis congenita/ telomere diseases | + | + | Immune deficiency Liver disease Lung disease |
| Shwachman-Diamond syndrome | + | +/– | Immune deficiency |
| Diamond Blackfan anemia | +/– | +/– | Iron overload |
| GATA2 deficiency | + | Immune deficiency | |
| SAMD9/SAMD9L syndromes | + | Immune deficiency | |
| RUNX1 | + | ||
| ANKRD26 | + | ||
| ERCC6L2 | + | ||
| MECOM | + | ||
| DDX41 | + |
| Disorder . | MDS/AML risk . | Solid tumor risk . | Other surveillance . |
|---|---|---|---|
| Fanconi anemia | + | + | |
| Dyskeratosis congenita/ telomere diseases | + | + | Immune deficiency Liver disease Lung disease |
| Shwachman-Diamond syndrome | + | +/– | Immune deficiency |
| Diamond Blackfan anemia | +/– | +/– | Iron overload |
| GATA2 deficiency | + | Immune deficiency | |
| SAMD9/SAMD9L syndromes | + | Immune deficiency | |
| RUNX1 | + | ||
| ANKRD26 | + | ||
| ERCC6L2 | + | ||
| MECOM | + | ||
| DDX41 | + |
Several forms of IBMFS, including FA, DC, Shwachman- Diamond syndrome (SDS), and GATA2 deficiency, carry high risks of transformation to MDS/AML,6-8 up to thousands of times higher than the general population.9 The goal of early detection of MDS/AML underlies the recommendations for bone marrow screening in these patients.10,11 While bone marrow morphology, blast count, cytogenetics, and fluorescence in situ hybridization have been used to evaluate for transformation to MDS/AML, it is unclear whether these measures or the frequency at which they are assessed has a meaningful impact on improving outcomes. From anecdotal experience, MDS/AML certainly can occur within a year-long interval without abnormalities detected in the peripheral blood or on the preceding bone marrow exam.
One promising approach to address this gap is next- generation sequencing for somatic mutations in the blood and bone marrow of patients with IBMFS. On a clinical basis, somatic mutations are identified by targeted capture and sequencing of MDS/AML-associated genes to detect clonal changes with high sensitivity, with implications for disease stratification and therapy.12 Similar analyses are now being conducted in IBMFS patients on a research basis, with early results indicating that somatic genetic changes may be adaptive to the underlying germline lesion, or alternatively may serve as early markers of malignant transformation.13 However, the analyses are still in the early stages, and the utility of somatic genetic analysis in clinical decision-making for IBMFS remains to be tested, ideally in prospective trials. In the case presented, somatic mutation testing was arguably introduced prematurely, prior to establishing its performance characteristics for MDS/AML screening in DC. Importantly, the prospective trial protocol remained silent on its use for determining eligibility.
Beyond MDS/AML, IBMFS carry risks of nonhematologic sequelae that may complicate or exclude eligibility for HCT. Notable examples include pulmonary and liver disease in DC, solid cancers in DC and FA, and cardiac and liver iron overload from chronic red cell transfusions in Diamond Blackfan anemia (DBA). Several reports suggest inferior HCT outcomes with increasing age, possibly due to such comorbidities.14-16 Thus, patients with IBMFS require comprehensive screening beyond the hematopoietic system in order to anticipate and identify a window of opportunity for interventions. For example, patients with telomere biology disorders may present with a simultaneous need for lung or liver transplant and HCT and may be excluded from either intervention due to increased risks. Optimizing outcomes in these cases requires rethinking the typical parameters of transplant timing, via close collaboration, alignment, and often advocacy by organ transplant and HCT teams. As children with IBMFS transition to adulthood, their own wishes and priorities may become clearer, sometimes differing from those of their parents. Ideally, they have been engaged in their care and thus better able to make informed choices,17 as in the case at hand, in which the patient decided his HCT timing upon reaching adulthood.
Earlier intervention with minimal intensity HCT
HCT is the only known intervention expected to prevent MDS/AML in IBMFS. To realize the benefit of improved surveillance, the outcomes of HCT in the “gray zone” or BMF stage of hematologic disease must be improved. Features of an ideal HCT regimen for IBMFS include (1) ensuring full engraftment and eliminating host HSPCs with their cell-intrinsic risk of MDS/AML; (2) minimizing acute toxicity and treatment-related mortality (TRM); (3) avoiding acceleration of other disease-specific organ pathology and cancer risk. Conditioning intensity is driven by doses of alkylating agents and/or radiation and correlates with higher engraftment, but also with organ toxicity, TRM, and accelerated carcinogenesis. While the relative risks of these trade-offs of conditioning intensity are difficult to quantify, Khan et al applied mathematical modeling to estimate event-free survival (EFS) of patients with FA after “pre-emptive” HCT (ie, before the onset of severe BMF or MDS).18 Indeed, significant improvements in EFS were predicted if HCT was performed in early childhood, and if TRM and acceleration of solid tumors were minimized.18
Therefore, based on these parameters, successfully reducing conditioning intensity while maintaining engraftment would be expected to improve long-term IBMFS outcomes and enable preemptive or “preventative” HCT. Fortunately, a track record of substantially reduced-intensity conditioning in IBMFS has been established via disease-specific studies in the past 4 decades. The 3 examples provided here are illustrative and not exhaustive and primarily aim to encourage additional prospective studies in IBMFS based on their success (Table 2).
Prospective, multicenter HCT trials for IBMFS
| Disorder(s) . | Trial . | Regimen . | Donors . | Graft/GVHD ppx . | N (IBMFS) . | 1° graft . | OS . | Ref . |
|---|---|---|---|---|---|---|---|---|
| Fanconi anemia | NCT00987480 | PK-targeted busulfan; FLU 140 mg/m2; CY 40 mg/kg; rATG 10 mg/kg | HLA 7/8 or better URD; HLA 4/8 to 7/8 RD | CD34-selected PBSC CSA | 34 (non-MDS) | 100% | 85% | 21 |
| SDS, GATA2, DBA, other IBMFS | NCT00919503 (completed) NCT04965597 (ongoing) | Treosulfan 42 g/m2 FLU 150 mg/m2 | MRD or MUD | Unmanipulated BM or PBSC TAC/MTX | 10 (non-PNH) | 100% | 100% | 27 |
| Dyskeratosis congenita/telomere biology disorders | NCT01659606 | FLU 180 mg/m2 C1H 1 mg/kg | HLA 7/8 or better URD or RD | Unmanipulated BM CSA or TAC/MMF | 20 | 95% | 90% | 26 |
| Disorder(s) . | Trial . | Regimen . | Donors . | Graft/GVHD ppx . | N (IBMFS) . | 1° graft . | OS . | Ref . |
|---|---|---|---|---|---|---|---|---|
| Fanconi anemia | NCT00987480 | PK-targeted busulfan; FLU 140 mg/m2; CY 40 mg/kg; rATG 10 mg/kg | HLA 7/8 or better URD; HLA 4/8 to 7/8 RD | CD34-selected PBSC CSA | 34 (non-MDS) | 100% | 85% | 21 |
| SDS, GATA2, DBA, other IBMFS | NCT00919503 (completed) NCT04965597 (ongoing) | Treosulfan 42 g/m2 FLU 150 mg/m2 | MRD or MUD | Unmanipulated BM or PBSC TAC/MTX | 10 (non-PNH) | 100% | 100% | 27 |
| Dyskeratosis congenita/telomere biology disorders | NCT01659606 | FLU 180 mg/m2 C1H 1 mg/kg | HLA 7/8 or better URD or RD | Unmanipulated BM CSA or TAC/MMF | 20 | 95% | 90% | 26 |
1° graft, primary engraftment rate; BM, bone marrow; BU, busulfan; C1H, alemtuzumab; CSA, cyclosporine A; CY, cyclophosphamide; FLU, fludarabine; HLA, human leukocyte antigen; MMF, mycophenolate mofetil; MRD, matched related donor; MTX, methotrexate; N, number treated; OS, overall survival; PBSC, peripheral blood stem cell; PK, pharmacokinetic; PNH, paroxysmal nocturnal hemoglobinuria; ppx, prophylaxis; rATG, rabbit antithymocyte globulin; RD, related donor; TAC, tacrolimus; URD, unrelated donor.
Fanconi anemia
Tailoring HCT intensity in IBMFS has its origins in attempts to mitigate the heightened toxicity to alkylator and radiation exposure manifested by patients with FA.19 Remarkably, cyclophosphamide was able to be reduced by up to ten times that of conventional doses and radiation exposure also substantially reduced, while engraftment rates were preserved and survival was improved.20 The evolution of reduced-toxicity regimens in FA has been reviewed, with highlights including the introduction of fludarabine to enable successful MUD HCT without radiation, and T-cell–depleted grafts to minimize graft-versus-host disease (GVHD).15 Notably, in a multi-center prospective trial, reduced-dose busulfan was evaluated in radiation-free unrelated donor HCT using CD34+-selected grafts and yielded 1-year overall survival in the BMF cohort of 85% (Table 2).21 Taken together, the international experience in FA over the past 4 decades improved 5-year overall survival to >90% for young patients with BMF and reduced graft rejection and GVHD to <10%, setting the stage for preventive transplants in select patients and clinical settings.15
Dyskeratosis congenita
The success of tailored approaches in FA stimulated disease-specific HCT trials in DC, as it was apparent early on that these patients also suffered high TRM ∼25% within 4 months post-transplant.22,23 Regimens incorporating fludarabine to reduce alkylator and radiation exposure, and alemtuzumab for in vivo T-cell depletion to prevent GVHD were described in single- center studies, improving alternative donor HCT outcomes but with ∼70% overall survival.24,25 A prospective HCT trial for BMF in DC patients initiated in 2012 (NCT01659606) asked whether myeloid engraftment could be achieved without using radiation or DNA alkylating agents. The multicenter study utilized fludarabine and alemtuzumab conditioning alone, based on the theory that the immune suppression may be sufficient in a BMF disorder in which host hematopoietic stem and progenitor cells with impaired replicative capacity due to short telomeres might be outcompeted by healthy donor cells. An interim report of 20 patients treated between 2012 and 2018 demonstrated primary engraftment in 95% of patients and overall survival of 90%.26 The study reached its accrual goal of 40 patients over the course of 10 years and will be reported soon. Long-term follow-up is planned to define the natural history in DC after HCT without exposure to agents that would accelerate organ toxicity and carcinogenesis.
Treosulfan for IBMFS
Alongside attempts to reduce toxicity by minimizing or eliminating alkylators, alternative agents may hold promise in IBMFS. Treosulfan is a pro-drug of alkylating agents that are effective in myeloablation and immunosuppression, but with more predictable pharmacokinetics and potentially decreased organ toxicity. In a prospective, multicenter trial, Burroughs et al reported 100% engraftment and 100% survival in 10 patients with IBMFS (3 SDS, 4 DBA, 2 GATA2, and 1 undefined) who underwent MSD or MUD HCT after conditioning with treosulfan, fludarabine ± rabbit anti-thymocyte globulin.27 All patients had improvements in cytopenia(s) and achieved transfusion independence. Notably, 2 patients had cytogenetic abnormalities (17p deletion in SDS and trisomy 8 in GATA2), which resolved on bone marrow examination post-HCT. Treatment-related toxicities were minimal, including no liver toxicity despite pre-HCT iron overload in all the patients with DBA. The ability to minimize nonhematopoietic toxicity while providing some degree of myeloablation with treosulfan extends the possibility of earlier HCT to patients with higher cellularity or evidence of clonal changes without overt MDS/AML. This encouraging study has been expanded in a multicenter blood & marrow transplant clinical trials network prospective trial (NCT04965597) to evaluate treosulfan-based conditioning in 40 patients with a range of IBMFS.
CLINICAL CASE (continued)
The patient underwent MUD HCT on the radiation- and alkylator- free protocol for DC. He developed hemoptysis during conditioning with alemtuzumab and fludarabine and required oxygen but recovered with supportive care. He proceeded to have an uneventful HCT course with full engraftment of donor cells and normalized peripheral blood counts. He developed mild steroid-responsive gut GVHD but no other complications. He began college the following winter. In the years that followed, his engraftment and hematologic parameters remained intact, but he developed avascular necrosis requiring multiple joint replacements and radiographic evidence of pulmonary fibrosis without hypoxia.
Conclusions and future directions
This case demonstrates the changing equation around HCT for a patient with DC, BMF, and risk for MDS/AML over 10 years of his life. Noncurative therapy with androgens bought time, during which a disease-specific minimal intensity prospective HCT trial was developed and successfully undertaken. His acute pulmonary complications may indicate a sensitivity that would have complicated higher-intensity conditioning. The patient's MDS/AML risk post-HCT is now considered negligible, but he continues to develop non-hematologic manifestations of DC.
As exemplified, significant progress in minimal intensity HCT for IBMFS has been achieved through disease-specific and prospective studies over 4 decades, with important lessons and considerations for the future (Table 3):
The implementation of screening tests for MDS/AML and nonhematopoietic disease progression in IBMFS should ideally be coupled to prospective trials with carefully determined eligibility criteria. In this manner, prospective trials in IBMFS will inform the efficacy of HCT approaches in specific clinical contexts and help guide HCT timing.
Because of the slow accrual expected and the long-term follow-up needed for meaningful interpretation of HCT for IBMFS, it is expected that such trials will take years to complete. Thus, multi-institutional studies should be promoted. As needed, eligibility criteria should be amended early on to align as closely as possible with real-world experience to be most useful in practice once the trial is complete.
Increased awareness and testing are identifying more patients with de novo presentations of MDS/AML, particularly adults, who have an underlying IBMFS.28,29 Therefore, while preventative HCT for IBMFS is a goal, there remains a need to improve HCT strategies for hematologic malignancy in IBMFS.
As shown in our case, it is difficult to ascertain definitively how minimal-intensity HCT impacts nonhematologic sequelae in IBMFS, highlighting the importance of integrating long-term follow-up into prospective trials.30,31
Considerations for optimal HCT trial design for IBMFS
| • Prioritize prospective, multicenter trials |
| • Articulate diagnostic categorization and hematologic disease status based on molecular features, in eligibility/stratification criteria |
| • Assess adherence to eligibility criteria across centers and ensure timely revision thereof to reflect real-world experience (ie, minimize “picking and choosing”) |
| • Include broad age ranges |
| • Account for disease-specific comorbidities |
| • Develop strata encompassing MDS/AML, including de novo presentations |
| • Consider accessibility of diagnostics (eg, genetic and functional testing) and interventions (eg, graft type/manipulation) at different centers/different parts of the world |
| • Develop trials in collaboration with patient advocacy groups |
| • Include quality-of-life and long-term follow-up measures |
| • Prioritize prospective, multicenter trials |
| • Articulate diagnostic categorization and hematologic disease status based on molecular features, in eligibility/stratification criteria |
| • Assess adherence to eligibility criteria across centers and ensure timely revision thereof to reflect real-world experience (ie, minimize “picking and choosing”) |
| • Include broad age ranges |
| • Account for disease-specific comorbidities |
| • Develop strata encompassing MDS/AML, including de novo presentations |
| • Consider accessibility of diagnostics (eg, genetic and functional testing) and interventions (eg, graft type/manipulation) at different centers/different parts of the world |
| • Develop trials in collaboration with patient advocacy groups |
| • Include quality-of-life and long-term follow-up measures |
Conflict-of-interest disclosure
Suneet Agarwal has disclosed the following financial relationship: owned stock in Rejuveron Telomere Therapeutics.
Off-label drug use
Suneet Agarwal: All drug use mentioned in this article should be considered off-label except eltrombopag for severe aplastic anemia.