Abstract
Inherited bone marrow failure syndromes (IBMFS) encompass a group of rare genetic disorders characterized by bone marrow failure, non-hematologic multisystemic comorbidities, disease defining congenital anomalies, and a susceptibility to myelodysplastic syndrome, acute myeloid leukemia, and in some instances solid tumors. The most common IBMFS include Fanconi anemia, Shwachman-Diamond syndrome, Diamond-Blackfan anemia, and telomere biology disorders/ dyskeratosis congenita. Allogeneic hematopoietic stem cell transplant (HCT) is a well-established curative treatment to correct the hematological manifestations but does not halt or reverse the nonhematological complications and may hasten them. With advances in HCT and in our ability to care for patients with IBMFS, an increasing number of survivors are making it imperative to not only diagnose but also treat late effects from the pre-, peri-, and post-HCT course and complications relating to the natural history of the syndrome. As the field of HCT evolves to allow for the incorporation of alternate graft sources, for expansion of donor options to include unrelated and mismatched donors, and for use of reduced-intensity conditioning or reduced toxicity myeloablative regimens, we have yet to determine if these advances modify the disease-specific course. While long-term outcomes of these patients are often included under one umbrella, this article seeks to address disease-specific post-HCT outcomes within IBMFS.
Learning Objectives
Examine the disease-specific post-HCT outcomes of patients with IBFMS to determine the impact of HCT on long-term disease outcomes
Propose screening recommendations for individual IBMFS post-HCT
Introduction
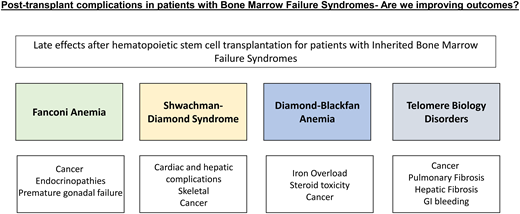
Inherited bone marrow failure syndromes (IBMFS) are a heterogeneous group of genetic disorders clinically characterized by congenital anomalies, bone marrow failure, and a predisposition myelodysplastic syndrome (MDS) with evolution to acute myeloid leukemia (AML) and other solid tumors. Fanconi anemia (FA), Shwachman-Diamond syndrome (SDS), Diamond-Blackfan anemia (DBA), and telomere biology disorders/dyskeratosis congenita (TBD/DC) are the most common IBMFS with defined clinical characteristics. Allogeneic hematopoietic stem cell transplant (HCT) is the only curative option for the hematological impairment and for halting the progression to myeloid malignancies. While it can affect the hematological course, HCT does not improve and may hasten progression to long-term complications of the underlying disorder, including solid tumors. Although historical HCT outcomes for IBMFS have been challenging, the overall practice of HCT has improved owing to detailed multidisciplinary approaches, advancements in disease-defining screening recommendations, and refinement of HCT approaches using alternative donor and graft manipulation. However, the lack of long-term follow-up with these newer approaches makes predicting the impact on the late-effect course challenging, for which there are already minimal data. Individuals who receive an HCT may have significantly inherently worse biological disease, confounding our understanding of these outcomes. Here we offer insight into post-HCT complications and offer supplemental screening tools for disease-specific toxicity (Tables 1–5).
General IBMFS post-HCT screening recommendations
| Late effect/organ system . | Disease-specific abnormality/concern . | Recommendations . |
|---|---|---|
| Audiology | • Risk for hearing loss | • Audiology screening if ear anomalies identified or if high risk of impairment from treatment course |
| Ophthalmology | • Vision changes, cataracts, lacrimal duct stenosis | • History and physical for changes in vision, lacrimal duct stenosis, cataracts, etc |
| Neurocognitive | • Difficulties in school/employment/home settings | • If neurological dysfunction is identified on screening history, perform diagnostic neuropsychological testing and consider referral for school support/employment guidance |
| Endocrinology | • Thyroid impairment • Growth and puberty • Fertility | • Annual TSH, T4 screening • Close height/weight monitoring, evaluation of bone health per bone mineral density recommendations • Tanner staging, physical exam, and long-term screening for premature ovarian/testicular insufficiency: FSH, LH, AMH, inhibin B |
| Bone mineral density | • Impaired bone mineral density/osteopenia with risk for fragility fractures | • Vitamin D monitoring and repletion • DXA scan posttransplanti and close monitoring in those who develop GVHD requiring systemic treatment |
| Pulmonary | • Impairment of lung function | • Annual history and physical screening • PFT every 6-12 months with referral if decline in lung function |
| Renal | • Chronic kidney disease with and without congenital anomalies • Hypertension | • Hypertensive screening at each visit or annually • Urine protein: creatinine, cystatin C urinalysis annually |
| Iron overload | • Iron overload impacting cardiac, liver, and endocrine organs | • Serum ferritin screening until resolution of overload • Consider T2-weighted MRI if screen positive or significant transfusion history • Phlebotomize posttransplant to target normal LIC if possible |
| Psychosocial/lifestyle | • Family planning/genetic counseling • Access to support networks, mental health professionals, and overall screening | • Support for patients living with chronic conditions • Family counseling with referral to genetics for those wishing to conceive • Lifestyle counseling with avoidance of smoking, limiting sun exposure/application of SPF, healthy diet • Revaccination with a focus on HPV with high malignancy risk • Referral to mental health specialist as needed |
| Late effect/organ system . | Disease-specific abnormality/concern . | Recommendations . |
|---|---|---|
| Audiology | • Risk for hearing loss | • Audiology screening if ear anomalies identified or if high risk of impairment from treatment course |
| Ophthalmology | • Vision changes, cataracts, lacrimal duct stenosis | • History and physical for changes in vision, lacrimal duct stenosis, cataracts, etc |
| Neurocognitive | • Difficulties in school/employment/home settings | • If neurological dysfunction is identified on screening history, perform diagnostic neuropsychological testing and consider referral for school support/employment guidance |
| Endocrinology | • Thyroid impairment • Growth and puberty • Fertility | • Annual TSH, T4 screening • Close height/weight monitoring, evaluation of bone health per bone mineral density recommendations • Tanner staging, physical exam, and long-term screening for premature ovarian/testicular insufficiency: FSH, LH, AMH, inhibin B |
| Bone mineral density | • Impaired bone mineral density/osteopenia with risk for fragility fractures | • Vitamin D monitoring and repletion • DXA scan posttransplanti and close monitoring in those who develop GVHD requiring systemic treatment |
| Pulmonary | • Impairment of lung function | • Annual history and physical screening • PFT every 6-12 months with referral if decline in lung function |
| Renal | • Chronic kidney disease with and without congenital anomalies • Hypertension | • Hypertensive screening at each visit or annually • Urine protein: creatinine, cystatin C urinalysis annually |
| Iron overload | • Iron overload impacting cardiac, liver, and endocrine organs | • Serum ferritin screening until resolution of overload • Consider T2-weighted MRI if screen positive or significant transfusion history • Phlebotomize posttransplant to target normal LIC if possible |
| Psychosocial/lifestyle | • Family planning/genetic counseling • Access to support networks, mental health professionals, and overall screening | • Support for patients living with chronic conditions • Family counseling with referral to genetics for those wishing to conceive • Lifestyle counseling with avoidance of smoking, limiting sun exposure/application of SPF, healthy diet • Revaccination with a focus on HPV with high malignancy risk • Referral to mental health specialist as needed |
These recommendations have been adapted from published guidelines.1-4
AMH, anti-Mullerian hormone; DXA, dual energy X-ray absorptiometry analysis; FSH, follicle-stimulating hormone; HPV, human papillomavirus; LH, luteinizing hormone; LI, liver iron content; MRI, magnetic resonance imaging; PFT, pulmonary function testing; SPF, sun protection factor; T4, thyroxine; TSH, thyroid stimulating hormone.
Swauger S, Sabulski A, Hornung L, Wasserman H, Myers KC, Howell JC. Bone health outcomes at 1 year after hematopoietic stem cell transplantation in a heterogeneous pediatric population. Transplant Cell Ther. 2022;28(1):44.e1-44.e6. doi:10.1016/j.jtct.2021.08.019.
FA post-HCT screening recommendations
| Late effect/organ system . | Disease-specific abnormality . | Recommendations . |
|---|---|---|
| Cancer screening | • SCC: head, neck, anogenital region • Nonmelanoma skin cancer: basal cell carcinoma and SCC • Breast cancer • CNS tumors • Wilms tumor | • ENT evaluation of the head and neck region every 6-12 months starting at age 10 years with possibility of nasolaryngoscopy • Dermatological evaluation yearly • Gynecological exam annually starting at puberty • Pap smear starting at 18 years or sexual activity • Encourage HPV vaccination • Additional surveillance and genetic counseling for those with FANCD1 (BRCA2)—leukemia, brain tumors, Wilms tumor, breast cancer; FANCJ (BACH1), FANCN (PALB2), FANCO (RED51C)—familial breast cancer genei,ii • Patients with total-body irradiation or chest RT require screening mammography starting at age 25 or 8 years after radiation exposure (no later than 40 years)1 • Chronic GVHD screening |
| Endocrinology | • Hypothyroidism • Glucose dysregulation: insulin resistance/diabetes • Growth hormone deficiency/bone health • Metabolic syndrome | • Annual TSH, free T4 • Oral glucose tolerance testing, insulin levels annually • Growth evaluation, BMI, and bone health, 25-hydroxy vitamin D levels, DXA scan annually • Dyslipidemia screening |
| Renal | • Renal anomalies | • Close follow-up on renal-specific anomalies |
| Reproductive-gonadal | • Genitourinary malformations • Premature ovarian and testicular insufficiency | • Follow-up with urology on genitourinary-specific anomalies • Tanner staging, physical exam, and long-term screening for premature ovarian/testicular insufficiency: FSH, LH, AMH, inhibin B • Fertility counseling services |
| Audiology | • Conductive hearing loss | • Audiology screening and referral for hearing-assistive devices with abnormalities |
| Late effect/organ system . | Disease-specific abnormality . | Recommendations . |
|---|---|---|
| Cancer screening | • SCC: head, neck, anogenital region • Nonmelanoma skin cancer: basal cell carcinoma and SCC • Breast cancer • CNS tumors • Wilms tumor | • ENT evaluation of the head and neck region every 6-12 months starting at age 10 years with possibility of nasolaryngoscopy • Dermatological evaluation yearly • Gynecological exam annually starting at puberty • Pap smear starting at 18 years or sexual activity • Encourage HPV vaccination • Additional surveillance and genetic counseling for those with FANCD1 (BRCA2)—leukemia, brain tumors, Wilms tumor, breast cancer; FANCJ (BACH1), FANCN (PALB2), FANCO (RED51C)—familial breast cancer genei,ii • Patients with total-body irradiation or chest RT require screening mammography starting at age 25 or 8 years after radiation exposure (no later than 40 years)1 • Chronic GVHD screening |
| Endocrinology | • Hypothyroidism • Glucose dysregulation: insulin resistance/diabetes • Growth hormone deficiency/bone health • Metabolic syndrome | • Annual TSH, free T4 • Oral glucose tolerance testing, insulin levels annually • Growth evaluation, BMI, and bone health, 25-hydroxy vitamin D levels, DXA scan annually • Dyslipidemia screening |
| Renal | • Renal anomalies | • Close follow-up on renal-specific anomalies |
| Reproductive-gonadal | • Genitourinary malformations • Premature ovarian and testicular insufficiency | • Follow-up with urology on genitourinary-specific anomalies • Tanner staging, physical exam, and long-term screening for premature ovarian/testicular insufficiency: FSH, LH, AMH, inhibin B • Fertility counseling services |
| Audiology | • Conductive hearing loss | • Audiology screening and referral for hearing-assistive devices with abnormalities |
These recommendations have been adapted from published guidelines.1-4
AMH, anti-Mullerian hormone; BMI, body mass index; DXA, dual energy X-ray absorptiometry analysis; ENT, otolaryngology; FANC, Fanconi anemia complementation; FSH, follicle-stimulating hormone; HPV, human papillomavirus; LH, luteinizing hormone; RT, radiation therapy; T4, thyroxine; TSH, thyroid stimulating hormone.
Woodward ER, Meyer S. Fanconi anaemia, childhood cancer and the BRCA genes. Genes. 2021;12(10):1520. doi:10.3390/genes12101520.
Zierhut HA, Bartels DM. Waiting for the next shoe to drop: the experience of parents of children with Fanconi anemia. J Genet Couns. 2012;21(1):45-58.
SDS post-transplant screening recommendations
| Late effect/organ system . | Disease-specific abnormality . | Recommendations . |
|---|---|---|
| Cancer screening | • Potential risk for future solid tumor | • Close monitoring with lifestyle modifications, eg, avoidance of smoking and excessive alcohol intake • Encourage SPF usage • Encourage regular self-exams and general population cancer screening recommendations • Follow general population screening recommendations • Patients with total-body irradiation or chest RT require screening mammography starting at age 25 or 8 years after radiation exposure (no later than 40 years)1 |
| Skeletal | • Rib cage deformities, scoliosis, short stature | • Bone mineral density evaluation • Growth hormone evaluation • Consultation with orthopedics for bracing/other interventions |
| GI/pancreatic | • Nutritional deficiencies secondary to pancreatic insufficiency | • Pancreatic dysfunction may become subclinical over time but those who are symptomatic may require long-term pancreatic enzyme replacement and close monitoring of stool output, nutrition, and growth |
| Cardiac | • Baseline cardiac dysfunction compounded by regimen-related toxicity | • Baseline echocardiogram and exposure-modified frequency post-HCT; referral to cardiology if abnormalities identified |
| Hepatic | • Potential for hepatic dysfunction | • Close monitoring of liver enzymes |
| Late effect/organ system . | Disease-specific abnormality . | Recommendations . |
|---|---|---|
| Cancer screening | • Potential risk for future solid tumor | • Close monitoring with lifestyle modifications, eg, avoidance of smoking and excessive alcohol intake • Encourage SPF usage • Encourage regular self-exams and general population cancer screening recommendations • Follow general population screening recommendations • Patients with total-body irradiation or chest RT require screening mammography starting at age 25 or 8 years after radiation exposure (no later than 40 years)1 |
| Skeletal | • Rib cage deformities, scoliosis, short stature | • Bone mineral density evaluation • Growth hormone evaluation • Consultation with orthopedics for bracing/other interventions |
| GI/pancreatic | • Nutritional deficiencies secondary to pancreatic insufficiency | • Pancreatic dysfunction may become subclinical over time but those who are symptomatic may require long-term pancreatic enzyme replacement and close monitoring of stool output, nutrition, and growth |
| Cardiac | • Baseline cardiac dysfunction compounded by regimen-related toxicity | • Baseline echocardiogram and exposure-modified frequency post-HCT; referral to cardiology if abnormalities identified |
| Hepatic | • Potential for hepatic dysfunction | • Close monitoring of liver enzymes |
These recommendations have been adapted from published guidelines.1-4
SPF, sun protection factor.
DBA post-HCT screening recommendations
| Late effect/organ system . | Disease specific abnormality . | Recommendations . |
|---|---|---|
| Cancer screening | • Colon cancer • Osteogenic sarcoma | • Colonoscopies starting at 1 year after HCT and every 5 years if normali • Awareness and education of risk of osteogenic sarcoma • Patients with total-body irradiation or chest RT require screening mammography starting at age 25 or 8 years after radiation exposure (no later than 40 years)1 |
| Ophthalmological | • Cataracts | • Evaluation for vision changes/cataracts in patients pretreated with steroids |
| Endocrinology | • Iron overload depositing in endocrine organs | • Evaluation of all endocrine organs: growth hormone, TSH, free T4, diabetes screening, parathyroid, gonadal axis screening on an annual basis |
| Neurocognitive | • Impact on neurodevelopment and educational performance | • Screening for neurodevelopmental issues particularly in patients with craniofacial abnormalities |
| Iron overload | • Iron overload burden increased in DBA compared with rest of IBMFS | • T2-weighted MRI of the heart and liver • Monitor liver function testing and consider referral to hepatology if abnormal • Phlebotomizing per Table 1 |
| Bone mineral density | • Fractures/osteopenia secondary to steroid use | • Screening with DXA scan prior to HCT and every 2-3 years if abnormal post-HCT |
| Obstetrics | • Risk for premature delivery | • Discussion with obstetrician for pregnancy planning |
| Late effect/organ system . | Disease specific abnormality . | Recommendations . |
|---|---|---|
| Cancer screening | • Colon cancer • Osteogenic sarcoma | • Colonoscopies starting at 1 year after HCT and every 5 years if normali • Awareness and education of risk of osteogenic sarcoma • Patients with total-body irradiation or chest RT require screening mammography starting at age 25 or 8 years after radiation exposure (no later than 40 years)1 |
| Ophthalmological | • Cataracts | • Evaluation for vision changes/cataracts in patients pretreated with steroids |
| Endocrinology | • Iron overload depositing in endocrine organs | • Evaluation of all endocrine organs: growth hormone, TSH, free T4, diabetes screening, parathyroid, gonadal axis screening on an annual basis |
| Neurocognitive | • Impact on neurodevelopment and educational performance | • Screening for neurodevelopmental issues particularly in patients with craniofacial abnormalities |
| Iron overload | • Iron overload burden increased in DBA compared with rest of IBMFS | • T2-weighted MRI of the heart and liver • Monitor liver function testing and consider referral to hepatology if abnormal • Phlebotomizing per Table 1 |
| Bone mineral density | • Fractures/osteopenia secondary to steroid use | • Screening with DXA scan prior to HCT and every 2-3 years if abnormal post-HCT |
| Obstetrics | • Risk for premature delivery | • Discussion with obstetrician for pregnancy planning |
These recommendations have been adapted from published guidelines.1-4
DXA, dual energy X-ray absorptiometry analysis; MRI, magnetic resonance imaging; T4, thyroxine; TSH, thyroid stimulating hormone.
Lipton JM, Molmenti CL, Hussain M, et al. Colorectal cancer screening and surveillance strategy for patients with Diamond Blackfan anemia: preliminary recommendations from the Diamond Blackfan Anemia Registry. Pediatr Blood Cancer. 2021;68:e28984. doi:10.1002/pbc.28984.
DC post-HCT screening recommendations
| Late effect/organ system . | Disease-specific abnormality . | Recommendations . |
|---|---|---|
| Cancer screening | • HNSCC • Gynecological tumors/anal cancer | • Encourage HPV vaccination • Yearly gynecological evaluation for Pap smear and HPV screening • Oral and skin exams for cancer screening every 6-12 months • Patients with total-body irradiation or chest RT require screening mammography starting at age 25 or 8 years after radiation exposure (no later than 40 years)1 |
| Liver | • Liver cirrhosis/fibrosis | • Baseline liver labs yearly • Early assessment of iron overload and aggressive phlebotomy/chelation as needed after HCT • Elastography-based ultrasound with concerns • Early referral for liver consult with concerns |
| Pulmonary | • Pulmonary fibrosis • Pulmonary AVMs | • Close monitoring with screening history and physical • PFTs yearly with early referral to pulmonology with decline in pulmonary function • Imaging studies as required with a focus on avoiding unnecessary radiation exposure • Consider xenon MRI if available at local centeri • Bubble echocardiogram for AVM surveillance |
| Esophageal/GI | • Esophageal stenosis • Risk of GI bleed due to telangiectasias/varices | • Screening for stenosis with dilatation as needed • Endoscopies with GI bleed concerns |
| Reproductive | • Urethral stenosis | • Follow-up with urology for dilations |
| Bone health | • Osteopenia • Avascular necrosis | • Screening with DXA scan prior to HCT and every 2-3 years if abnormal post-HCT |
| Late effect/organ system . | Disease-specific abnormality . | Recommendations . |
|---|---|---|
| Cancer screening | • HNSCC • Gynecological tumors/anal cancer | • Encourage HPV vaccination • Yearly gynecological evaluation for Pap smear and HPV screening • Oral and skin exams for cancer screening every 6-12 months • Patients with total-body irradiation or chest RT require screening mammography starting at age 25 or 8 years after radiation exposure (no later than 40 years)1 |
| Liver | • Liver cirrhosis/fibrosis | • Baseline liver labs yearly • Early assessment of iron overload and aggressive phlebotomy/chelation as needed after HCT • Elastography-based ultrasound with concerns • Early referral for liver consult with concerns |
| Pulmonary | • Pulmonary fibrosis • Pulmonary AVMs | • Close monitoring with screening history and physical • PFTs yearly with early referral to pulmonology with decline in pulmonary function • Imaging studies as required with a focus on avoiding unnecessary radiation exposure • Consider xenon MRI if available at local centeri • Bubble echocardiogram for AVM surveillance |
| Esophageal/GI | • Esophageal stenosis • Risk of GI bleed due to telangiectasias/varices | • Screening for stenosis with dilatation as needed • Endoscopies with GI bleed concerns |
| Reproductive | • Urethral stenosis | • Follow-up with urology for dilations |
| Bone health | • Osteopenia • Avascular necrosis | • Screening with DXA scan prior to HCT and every 2-3 years if abnormal post-HCT |
These recommendations have been adapted from published guidelines.1-3
AVM, arteriovenous malformation; DXA, dual energy X-ray absorptiometry analysis; GI, gastroenterology; HNSCC, head and neck squamous cell carcinomas; HP, human papillomavirus; MRI, magnetic resonance imaging.
Walkup LL, Myers K, El-Bietar J, et al. Xenon-129 MRI detects ventilation deficits in paediatric stem cell transplant patients unable to perform spirometry. Eur Respir J. 2019;53(5):1801779. doi:10.1183/13993003.01779-2018.
Fanconi anemia (FA)
FA is an inherited chromosomal breakage disorder characterized by bone marrow failure, congenital anomalies, susceptibility to clonal evolution to MDS/AML, and squamous cell carcinoma (SCC). The Fanconi core complex along with its associated protein complexes are required to ensure timely DNA repair.5 Mutations in this complex lead to excessive DNA damage and a high risk for transformation to malignancies. The National Cancer Institute IBMFS cohort studies6,7 reported a cumulative incidence of severe BMF of 70% by age 50 years and 20% overall solid tumor risk by 65 years. The observed to expected rate (O/E) for AML was >200-fold, surpassing other IBMFS. Elevated O/E ratios unique to FA included vulvar cancer and brain tumors, with 2098-fold and 64-fold, respectively, making long-term outcomes more challenging given the limited tolerance for chemotherapy and radiation. Comparing cancer risk for transplanted vs untransplanted patients, the O/E ratio was 55 to 19 and with an earlier median age at presentation, making posttransplant cancer surveillance high priority.
HCT for FA was initiated in the late 1970s to 1980s, with excess regimen-related toxicity and mortality secondary to high-dose alkylating agents like cyclophosphamide and the use of total-body irradiation given the underlying hypersensitive to DNA cross-linking agents.8,9 The following 30 years of advancement have been summarized,10,11 highlighting the introduction of fludarabine, a powerful adjunct to dose de-escalation of total-body irradiation/cyclophosphamide, reducing graft failure and improving overall survival.12 Recently, there has been an effort to eliminate use of radiation therapy.13 In vivo T-cell depletion has been routinely used, but other graft manipulation strategies using CD34+ enrichment techniques with and without T-cell add-back or T-cell receptor alpha/beta depletion14 have been evaluated to eliminate the risk for acute and chronic graft- versus-host disease (GVHD) while minimizing risk of graft rejection. There is a strong association with chronic GVHD and SCC; however, it remains unclear if busulfan will improve the incidence or delay development of SCCs. Alternate mismatch donor options have been extensively explored, as has the use of cord blood transplant.15 T-cell replete grafts with dose-reduced posttransplant cyclophosphamide have been investigated but require dose optimization.16,17 Future therapy with antibody-based stem cell ablation or gene therapy using gene-corrected autologous hematopoietic stem cells (HSCs)18,19 may offer a less toxic approach, but long-term data is needed. Gene therapy may be promising, as repaired HSCs may offer selective advantage in vivo and allow for genetic reversion,20 but it raises the question whether residual nonrevertant stem cells will impact durability of hematopoiesis21 and lead to risk for MDS/AML, which would necessitate separate screening recommendations.
Outcomes for HCT in FA have improved significantly, and novel approaches may improve long-term toxicity; however, follow-up data are limited, especially in adults. Currently, we are not able to separate the progression of disease phenotypes from those associated or exaggerated by HCT. Endocrinopathies including thyroid dysfunction, glucose intolerance, growth and bone density abnormalities, and gonadal dysfunction22-24 are common irrespective of transplant status but may be exacerbated by HCT. Disease- and treatment-related complications related to congenital abnormalities, endocrinopathies, and most importantly increased cancer susceptibility make lifelong monitoring imperative (Table 2).
Shwachman-Diamond syndrome (SDS)
SDS is a rare IBMFS classically caused by mutations in the Shwachman-Bodian-Diamond syndrome (SBDS) gene on centromeric region of chromosome 7 (7p12-7q11), but recently other genes, including DNAJC21, EFL1, and SRP54, have been associated with an SDS-like phenotype. These genes are well defined in ribosomal biogenesis but contribute to the pleiotropic phenotype. SDS is a multisystem disorder characterized by multilineage cytopenias and susceptibility to MDS and AML, skeletal malformations, pancreatic exocrine insufficiency contributing to malnutrition and poor growth, and various dysmorphic features.25 The prognosis of patients who develop MDS or AML is dismal, and efforts have focused on identifying clonal changes before clinical transformation occurs. Presence of deletion 20q (del(20)(q12)) and isochromosome 7 (i(7)(q10)) are well established as compensatory mechanisms to improve hematopoiesis,26-28 which in isolation do not necessitate HCT. Other cytogenetic changes and distinct clonal somatic changes in TP5329 confer worse prognosis and can predict progression to myeloid malignancies. Yearly marrow surveillance or earlier in the presence of evolving cytopenias, cytogenetic changes, or molecular aberrations consistent with leukemic clones is recommended such that preemptive HCT can be pursued.
HCT for SDS has been effective before development of MDS or AML, but balancing preexisting organ dysfunction and comorbidities is essential. Myeloablative conditioning (MAC) regimens are required to eliminate the abnormal marrow and facilitate donor engraftment. However, the associated toxicity, specifically cardiac and hepatic, may be too great for patients with SDS,30-33 suggesting that the defects in ribosomal biogenesis are not only limited to the bone marrow but also impact other organ systems.
Historical HCT outcomes for SDS are poor, with high rates of transplant-related toxicities, increased incidence of graft failure, and infection burden, along with an increased risk for cardiac, liver, and pulmonary complications. Cesaro et al.34 reported transplant-related mortality (TRM) of 35%, which was higher in those receiving total-body irradiation–containing regimens (~70%) than in those without. Recent updates from the European Society for Blood and Marrow Transplantation35 indicate that while outcomes for BMF have improved, outcomes with malignancy remain poor, with overall survival (OS) of 71% vs 29%, along with high rates of TRM. Advancements in conditioning regimens should lead to consideration of RIC or reduced-toxicity regimens. Outcomes for RIC regimens report minimal TRM,36 but long-term follow-up is limited.
Treosulfan, which has a more predictable pharmacokinetic profile, may be better incorporated for SDS regimens to reduce TRM and limit hepatoxicity.37 Long-term studies are needed to evaluate HCT survivor outcomes, especially in known areas of sensitivity such as cardiac and liver disease, to best tailor long-term monitoring (Table 3).
Patients with SDS may be predisposed to solid tumors, but the exact relative risk is poorly known. Isolated fatal development of solid tumors of the ovary, breast, and esophagus in the fifth decade of life have been reported,6,7,38 but association with HCT has not been possible given small sample size.
Diamond-Blackfan anemia (DBA)
DBA is a rare congenital red cell aplasia disorder characterized by normocytic or macrocytic anemia, reticulocytopenia in the absence of erythroid precursors in the marrow, and a predisposition to malignancy. It is accompanied by congenital anomalies affecting many systems, including cardiac, renal, musculoskeletal, and craniofacial defects.39 DBA is primarily inherited in an autosomal dominant fashion, with most mutations involving ribosomal protein subunits; however, 50% of the mutations are de novo. Twenty-five percent of mutations involve the RPS19 gene, but newer mutations have been recognized in GATA1 (X-linked) and TSR2 (X-linked). ADA2 and biallelic mutations with EPO confer a DBA-like phenotype with erythroblastopenia.40
The mainstay of treatment for DBA is chronic steroids sufficient to maintain a response at low doses, and chronic transfusions with chelation for those who do not. As outcomes for HCT in DBA improve, a growing number of patients who are unresponsive to steroids are electing to undergo HCT and forgo chronic transfusions with chelation. The OS and event-free survival with MAC regimens have improved post-2010 independent of donor type, likely attributed to improvement in chelation practices and pre-/post-HCT subspecialty care.41,42 While HCT mitigates the risk of MDS/AML, the increasing use of reduced-intensity HCT43 may change this with potential for mixed chimerism. Although HCT represents the only curative option to correct the hematological features, posttransplant monitoring is imperative as patients still face concerns for iron overload, endocrinopathies, and solid tumor risk44,45 (Table 4). It is critical that iron overload be assessed and abated with chelation prior to pursuing HCT46 and further addressed post-HCT with phlebotomy to avoid long-term cardiac and hepatic toxicity, as deaths due to cardiac iron overload can occur as late as 5 to 7 years after transplant.1-3,47 Long-term steroid use may impact bone mineral density and growth and require long-term ophthalmologic follow-up.1
Importance must be placed on cancer screening, which is unique to DBA of lung, colorectal, and osteogenic sarcoma. The incidence of malignancies per longitudinal DBA registry data48 showed an O/E ratio of 4.8 (95% CI 3.2%-6.9%). In a post-HCT subanalysis, the O/E for colorectal cancer (n = 2) was 346.9 (95% CI 42.0%-1252.0%) and osteosarcoma (n = 1) was 257.8 (95% CI 6.5%-1436.2%). The O/E compared with overall cancer risk may suggest higher risk in the transplanted group, but this is a small sample size and requires further long-term analysis.
Telomere biology disorders/dyskeratosis congenita (TBD/DC)
DC is a rare hereditary multisystem TBD. It is characterized by a triad of oral leukoplakia, reticular skin changes, and abnormal nails, which are now recognized as outward manifestations of a systemic disease process. Patients with DC have shortened telomeres for age (less than first percentile for age) and often present with 1 or a combination of BMF with potential for evolution to MDS/AML, pulmonary fibrosis, liver cirrhosis or fibrosis, esophageal stenosis, enteropathy, avascular necrosis of the joints, and head and neck SCC.49 To date, more than 18 genes have been identified that account for 70% of the diagnoses with variable expression pattern. By 40 years of age, 50% to 90% of patients have at least single-lineage cytopenia due to accelerated telomere attrition contributing to hematopoietic failure over time.6,7 HCT is the only curative method to correct the hematological deficiency, but it does not cure underlying tissue involvement and may accelerate it. Temporizing measures with transfusion support is indicated with severe cytopenias but can contribute to allosensitization and iron burden.
HCT regimens for DC historically involved MAC regimens, which resulted in hepatic and pulmonary fibrosis, veno-occlusive disease, and a high early and late mortality rate.50 Transition to RIC regimens has improved outcomes51-53 along with the push toward radiation- and alkylator-free approaches.54,55 Despite the adoption of RIC regimens, several case reports show deaths related to gastrointestinal bleeding post-HCT possibly related to erosive duodenitis, esophageal varices, or ectasia likely secondary to portal hypertension or endothelial dysregulation, highlighting that RIC regimens pose risk for complications.56,57 A consortium study58 reported 14 patients with TBD, including several without history of HCT, who were refractory to angiodysplasia treatment, underscoring a propensity for GI bleeding and additional mortality in this patient population.
The early success of RIC regimens should still prompt long-term monitoring, as a review59 of 109 patients with a combination of MAC and RIC regimens showed 5-year and 10-year survival estimates of 57% and 23%, respectively, highlighting significant late mortality. Early deaths were attributed to infection, nonengraftment, and hemorrhage, whereas deaths >1 year were attributed to pulmonary, liver, and vascular complications. The incorporation of MAC regimens may contribute to the long-term toxicity, or this may indicate that patients with BMF have a worse DC phenotype. Longitudinal studies evaluating RIC-based regimens with the goal of preventing late mortality and identifying cancer risk will take more time; further collaboration is needed given the rarity of the disease.60
Cancer predisposition is notable in the DC population, with 20% of patients developing malignancy by age 65 with a higher risk in posttransplant compared with untransplanted patients, 30 vs 4.2.6,7 The reported O/E ratio was significantly elevated at 11-fold for all cancer sites, with a striking O/E for tongue cancer of 1154-fold. Risk of MDS/AML is substantial at 2500-fold but is largely mitigated by HCT.
Conclusions
IBMFS are a heterogeneous group of rare disorders classified by the overarching hematological manifestations in the setting of extensive disease-specific complexities. HCT remains the only curative treatment option to correct the hematological impairment or halt progression to MDS/AML, but this begs the question of whether we are truly improving patient outcomes or insidiously affecting the natural disease course. We have made significant strides as a transplant community through optimizing HCT regimens, often to prevent graft rejection, minimize acute and chronic GVHD, and enhance short-term OS at the forefront. However, significant limitations remain due to the drought of knowledge of late effects, which is needed to guide prospective trials. Patients with marrow failure or progression to MDS/AML necessitating HCT may inherently have a more severe phenotype, but without cohort studies comparing transplanted versus untransplanted patients, the natural history will be challenging to decipher. The Center for International Blood and Marrow Transplant Research estimates that by 2023, there will be 502000 HCT survivors of which 14% would have been younger than 18 years at the age of transplantation.61 This emphasizes the need to understand long-term health consequences that patients may face due to the underlying disease process, pre-HCT therapy, the HCT process, or a combination. We propose future directions as guidance for subsequent studies.
Future directions
Longitudinal international collaborative efforts are needed to pursue multi-institutional clinical trials with uniform disease-specific approaches and to allow for pooling of retrospective data to lengthen follow-up and establish more information on late effects. With newer regimens proposed, disease-specific long-term outcomes should be prioritized in study aims.
Consensus meetings at regular intervals to review new disease-specific data will allow for timely updates on long-term monitoring recommendations. Data-driven publications will assist with bridging care gaps that occur as patients transition from pediatric to adult providers. This will also allow nontransplant community-based physicians to understand the unique and complex health challenges.
Further study via international collaboration of large cohorts including both transplanted and untransplanted patients is needed to ascertain whether disease manifestations may mimic late effects or are further exacerbated by the transplant process. This may include genotype-phenotype correlation to further establish long-term guidance tailored to the individual disease processes.
Additional future studies should focus on less-toxic conditioning regimens and minimization of toxicity pre-HCT, including gene therapy.
Conflict-of-interest disclosure
Zahra Hudda: No conflict of interest.
Kasiani C. Myers: Research funding from Incyte for an industry sponsored investigator initiated clinical trial and also funding from Elixirgen Therapeutics for an industry sponsored trial.
Off-label drug use
Zahra Hudda: There is discussion of off-label drug use throughout. The majority of pediatric medications are used off-label and many of these transplants are in pediatric patients.
Kasiani C. Myers: There is discussion of off-label drug use throughout. The majority of pediatric medications are used off-label and many of these transplants are in pediatric patients.