Abstract
Allogeneic stem cell transplantation plays a central role in the management of fit adults with high-risk acute myeloid leukemia (AML) in first complete morphologic remission (CR1). Advances in both donor selection and transplant technology have both dramatically increased accessibility of transplant and led to significant reductions in transplant-related mortality over the past 2 decades. There has, however, been no concomitant reduction in the risk of disease relapse, which remains the major cause of transplant failure. Pivotal to the design of innovative strategies with the potential to reduce relapse risk is accurate identification of patients at the highest risk of disease recurrence. Multiple retrospective studies have identified an increased risk of disease relapse in patients allografted for AML in CR1 with evidence of pretransplant measurable residual disease (MRD). The prognostic significance of pretransplant MRD has been confirmed recently in prospective analyses. The optimal management of patients with evidence of pretransplant MRD remains a matter of conjecture with regard to 2 key issues. First, should the presence of pretransplant MRD delay a decision to proceed to transplant, allowing time for delivery of additional MRD-directed therapy prior to transplant? Second, to what extent can the intensity of the conditioning regimen or the magnitude of the graft-vs-leukemia effect be manipulated to improve the outcome of such patients?
Learning Objectives
Understand the prognostic impact of pretransplant measurable residual disease (MRD) status on transplant outcome in patients allografted for acute myeloid leukemia in first complete morphologic remission
Characterize strategies with the potential to improve transplant outcomes in MRD+ patients
CLINICAL CASE
A 58-year-old woman presents with pancytopenia. A bone marrow aspirate confirms a diagnosis of acute myeloid leukemia (AML). Cytogenetic examination demonstrates a complex karyotype, and next-generation sequencing (NGS) analysis demonstrates mutations in RUNX1 and ASXL1. She has a history of well-controlled maturity-onset diabetes and has been taking oral hypoglycemic agents for the previous 5 years. Until 2 months before presentation, she was walking 3 miles a day and is a carer for her husband, who has Parkinson disease. She achieves a morphologic complete remission after 1 course of CPX351 induction chemotherapy. She has no matched sibling donors, but a 10/10 matched unrelated donor is identified, and she is referred for transplant after a second course of CPX351. Pretransplant workup investigations are normal, and her Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) score is 1. A bone marrow examination confirms morphologic complete remission (CR). Multiparameter flow cytometry–based measurable residual disease (MFC-MRD) quantitation demonstrates 0.2% MRD.
Introduction
Allogeneic stem cell transplantation (allo-SCT) is an increasingly important component of the treatment algorithm in fit adults with high-risk AML.1 The advent of reduced intensity conditioning (RIC) regimens coupled with increased donor availability has permitted the extension of allo-SCT to fit patients with AML up to the age of 75 years.2 At the same time, improvements in supportive care and graft-vs-host disease (GVHD) prophylaxis have reduced transplant toxicity such that a 1-year transplant-related mortality (TRM) of 15% or less can now be predicted in fit adults transplanted using a well-matched sibling or unrelated donor. It has long been recognized that allo-SCT represents the most effective form of antileukemic therapy, consequent both upon dose intensification and the genesis of a potent and manipulable graft-vs-leukemia (GVL) effect, and registry analyses confirm a reduction in relapse risk in the region of 60% to 70% in patients in first complete morphologic remission (CR1) who proceed to transplant relative to those consolidated with intensive chemotherapy.3 The magnitude of the reduction in relapse risk conferred by an allograft appears to be similar regardless of disease biology. Importantly, the impact of disease biology and MRD status on relapse risk in patients treated with intensive chemotherapy (IC) alone can now be predicted with increasing accuracy.1 At the same time, the impact of the use of an alternative donor or patient comorbidities on TRM is now better understood.
A structured and increasingly personalized discussion concerning the benefits and risks of allo-SCT therefore now forms the cornerstone of therapeutic decision-making in fit adults newly diagnosed with AML in CR1.4 A decision whether to proceed to transplant is based on (1) the predicted risk of disease relapse if a CR1 patient receives IC alone as consolidation and (2) the predicted TRM of an allograft. It follows that patients with a predicted risk of relapse greater than 40% to 45% have the potential to derive a survival benefit if transplanted in CR1 (Table 1). In patients with a higher risk of relapse, a decision to proceed to transplant in the presence of comorbidities or using a suboptimally matched alternative donor may be justified despite the higher predicted TRM. It is, however, important to remember that the decision whether to proceed to transplant or not is often nuanced, particularly in older patients, in whom there may be subtle comorbidities or carer responsibilities.
Which patients with AML in CR1 should be considered for an allogenic stem cell transplant
| Characteristic . | MRD after induction cycle 2 . | Estimated risk of relapse following consolidation with . | Maximal tolerated NRM prognostic scores for allogeneic SCT to be considered . | ||
|---|---|---|---|---|---|
| Intensive chemotherapy, % . | Allogeneic SCT, % . | HCT-CI score . | 2-year NRM, % . | ||
| Favorable | Negative | 30-40 | 15-20 | NA (not advisable to proceed) | |
| Positive | 70-80 | 30-40 | ≤3-4 | <35 | |
| Intermediate | Negative | 50-60 | 25-30 | ≤2 | <20 |
| Positive | 70-80 | 30-35 | ≤3-4 | <35 | |
| Adverse | NA | >90 | 40 | ≤3-4 | <35 |
| Characteristic . | MRD after induction cycle 2 . | Estimated risk of relapse following consolidation with . | Maximal tolerated NRM prognostic scores for allogeneic SCT to be considered . | ||
|---|---|---|---|---|---|
| Intensive chemotherapy, % . | Allogeneic SCT, % . | HCT-CI score . | 2-year NRM, % . | ||
| Favorable | Negative | 30-40 | 15-20 | NA (not advisable to proceed) | |
| Positive | 70-80 | 30-40 | ≤3-4 | <35 | |
| Intermediate | Negative | 50-60 | 25-30 | ≤2 | <20 |
| Positive | 70-80 | 30-35 | ≤3-4 | <35 | |
| Adverse | NA | >90 | 40 | ≤3-4 | <35 |
NA, not advisable; NRM, non-relapse mortality.
The prognostic importance of pretransplant MRD in determining transplant outcome in patients allografted for AML in CR1
Central to the design of new strategies with the potential to reduce the risk of disease relapse is an increased understanding of both the biology of transplant relapse and the clinical factors that predict relapse risk.5,6 Sequential molecular and single-cell analyses have highlighted the importance of both clonal heterogeneity and evolution at diagnosis and in response to chemotherapy as determinants of relapse risk. Registry studies have identified a number of clinical factors that predict the risk of disease relapse in patients allografted for AML in CR1. These include disease biology as defined by presentation karyotype and mutational status, pretransplant disease status, the intensity of the conditioning regimen, and the degree and duration of posttransplant immunosuppression.7 Recent studies have confirmed the importance of disease biology in determining relapse risk, which is as high as 60% to 70% in patients allografted for AML associated with a TP53 or FLT3 ITD mutation. Taken together, these data have played an important role in identifying a population of patients at a particularly high risk of disease relapse and informing personalized transplant decision-making, and they also increasingly underpin the design of new transplant strategies.
It is well established that disease status at transplant is an important determinant of relapse risk and transplant outcome in patients allografted for AML. Thus, although a proportion of patients with primary refractory AML can be salvaged by an allograft, the presence of active disease at the time of transplant is associated with a substantially increased risk of disease relapse in comparison with patients transplanted in a morphologic CR.8,9 The development of reproducible MRD technologies has confirmed the importance of disease load in patients allografted in CR1. The ability of MFC-MRD and molecular MRD assessed by quantitative polymerase chain reaction and, more recently, NGS-based technologies to reliably quantitate leukemic burden in patients in morphologic CR has been extensively studied in the context of prospective trials and has refined risk stratification in patients treated with IC.10 Multiple retrospective studies have also highlighted the prognostic significance of the presence of pretransplant MRD in patients allografted for AML in CR1.11,12 The clinical relevance of such studies has, however, been blunted not only by the fact that the effect size reported in different retrospective studies was highly variable but also that the precise timing of the pretransplant MRD analysis was either highly variable or not specified. Coupled with the potential attendant selection bias inevitable in retrospective studies, it was difficult to confidently draw conclusions from these data. Future prospective studies comparing emerging MRD strategies such as NGS-MRD with MFC-MRD and, where appropriate, molecular MRD technologies prior to stem cell transplantation will be important given the fact that the prognostic significance of MRD varies according to the clinical context specifically with regard to both the intensity and the number of prior cycles of intensive chemotherapy.
More recently, a number of prospective data sets have correlated pretransplant MRD status with relapse risk and overall survival (OS) posttransplant (Figure 1). In the NCRI FIGARO trial, older adults with AML or high-risk myelodysplasia in CR were randomized to receive either a standard RIC regimen (most patients received a FB2 regimen) or an augmented sequential FLAMSA-Bu regimen.13 Quantitation of pretransplant MFC-MRD in the 4 weeks prior to transplant was stipulated for all trial patients. OS and cumulative incidence of relapse were similar in both study arms, and no benefit of a FLAMSA-Bu regimen was observed even in patients who were pretransplant MRD+. In randomly assigned patients, pretransplant MRD positivity was associated with an increased risk of disease relapse (2-year cumulative incidence of relapse 41% vs 20%, P = .01) and a borderline reduction in 2-years OS (70% vs 51%, P = .05). Of note, however, patients with evidence of MRD pretransplant still had a greater than 50% 2-year OS. In the NCRI AML 17 trial, the impact of pretransplant MRD status on patient outcome was evaluated in 107 adults allografted for NPM1+ AML.14 The 2-year OS was 83% in patients with no evidence of pretransplant MRD vs 13% in patients defined as having “high” levels of MRD (>200 NPM1 transcripts per 105 ABL in the peripheral blood and >1000 copies in the bone marrow). In patients defined as having “low” levels of MRD who did not fulfill the above criteria, the presence of a FLT3 mutation was associated with a worse outcome. These data must be interpreted in the context of the excellent transplant outcomes reported by the French ALFA group in patients allografted for NPM1+ AML who had demonstrated a suboptimal MRD response to induction chemotherapy.15 Finally, the pivotal US CTN 0901 trial, which compared outcomes after a myeloablative conditioning (MAC) or RIC allograft in patients with AML in CR1, peripheral blood samples obtained pretransplant were used to quantitate MRD levels using an innovative NGS technology.16 In this study, the presence of pretransplant MRD was associated with an increased risk of relapse but only in patients transplanted using a RIC regimen. NGS detection of a FLT3 mutation pretransplant appeared to be associated with a particularly high risk of relapse, although the number of patients in this and other molecular subgroups was inevitably small. While certainly supporting the use of a MAC regimen in patients who have detectable MRD pretransplant, if clinically deliverable, interpretation of these data is complicated by the paradoxically low relapse rate observed in the MAC arm and very high relapse rate in the RIC arm in this study. Taken together, these prospective data sets, while providing important information with the potential to inform clinical decision-making, also highlight the critical importance of future randomized trials examining (1) the impact of pretransplant MRD status and mutational status on transplant outcome and (2) the impact of specific transplant interventions aimed at improving outcomes in MRD+ patients.
Impact of pretransplant MRD status on outcome in patients allografted for AML in CR1: results of 2 prospective randomized trials. (A) Results of the USCTN 0901 trial demonstrating the impact of the presence of pretransplant MRD status on outcome in patients receiving a MAC or RIC regimen.16 (B) Impact of pretransplant MRD status on cumulative incidence of relapse in patients transplanted in the FIGARO trial.13 CI, confidence interval; HR, hazard ratio.
Impact of pretransplant MRD status on outcome in patients allografted for AML in CR1: results of 2 prospective randomized trials. (A) Results of the USCTN 0901 trial demonstrating the impact of the presence of pretransplant MRD status on outcome in patients receiving a MAC or RIC regimen.16 (B) Impact of pretransplant MRD status on cumulative incidence of relapse in patients transplanted in the FIGARO trial.13 CI, confidence interval; HR, hazard ratio.
Should patients with detectable MRD pretransplant receive additional chemotherapy before proceeding to transplant?
The compelling evidence that the presence of pretransplant MRD is associated with an increased risk of disease relapse has led to the proposition that such patients will benefit from additional chemotherapy aimed at reducing the MRD load prior to transplant. There are, however, no prospective randomized data to support such an approach, and indeed there are well-grounded concerns that it may compromise the subsequent safe delivery of an allograft. However, 2 recent randomized studies of induction chemotherapy provide tantalizing support for the benefit of such an approach. In the pivotal randomized study of CPX-351 vs DA as induction chemotherapy in older adults with secondary AML, the survival benefit of CPX-351 was most apparent in patients who proceeded to transplant.17 Similarly, in the RATIFY trial, the survival benefit associated with the addition of midostaurin to DA in adults with newly diagnosed AML associated with a FLT3 ITD mutation was most marked in allografted patients.18 Although neither of these studies measured MRD levels, and it remains unclear whether the improved transplant outcome observed in the experimental arms of these studies was consequent upon a reduction in TRM or relapse, they support formal examination of the benefits of additional course(s) of chemotherapy in MRD+ patients prior to transplant in prospective randomized trials. There remain, however, a number of important caveats concerning the routine adoption of such a strategy in patients who are MRD+ pretransplant. First, the extent to which the adverse prognostic impact of pretransplant MRD is consequent upon underlying adverse disease biology remains unresolved, and it is of interest that in a recent analysis of outcome in adults older than 60 years transplanted in CR1 using a RIC regimen, pretransplant MRD status did not add to the prognostic information provided by karyotyping and NGS analysis.19 Second, it remains a distinct possibility that a significant number of patients will fail to benefit from a potentially curative allograft either because of immediate complications of the additional chemotherapy or because further chemotherapy contributes to an increased transplant toxicity. Finally, additional courses of chemotherapy may drive clonal evolution and the emergence of mutations in genes such as TP53, which are associated with a higher risk of relapse posttransplant.20
CLINICAL CASE (Continued)
Practical considerations with the potential to improve outcomes in high risk AML
The patient was allografted using a FB2 conditioning regimen that incorporated pretransplant ATG and posttransplant cyclosporine (CsA) as GVHD prophylaxis. Posttransplant CsA levels were monitored thrice weekly with the aim of maintaining a CsA level between 150 and 200 µg/L during the immediate posttransplant period. A CsA taper was commenced on day 60. The patient was recruited to the AMADEUS trial (NCT04173533), a randomized comparison of CC486 maintenance in patients allografted for AML/high-risk myelodysplasia. A repeat bone marrow aspirate at 1 year posttransplant demonstrated that the patient was in a cytogenetic and MRD remission.
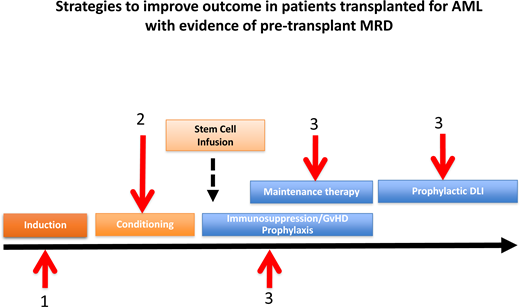
Strategies to improve outcome in patients allografted in CR1 with evidence of pretransplant MRD and future directions
Advances in transplant technology, notably the advent of RIC regimens, coupled with accumulating evidence of the magnitude of the GVL effect exerted after both MAC and RIC allografts, has led to an increased recognition that the antileukemic activity of allo-SCT in high-risk AML is highly manipulable.21,22 This recognition tempers the interpretation of retrospective analyses of transplant registry data in which there are no details of either the conditioning or GVHD prophylaxis regimen, thus complicating interpretation of the benefit of transplant. As importantly, a number of clinical strategies are now aimed at “dialing up” the antileukemic activity of an allograft. Broadly, these include approaches directed at (1) eradication of pretransplant MRD as discussed above, (2) optimization of the conditioning regimen such that antileukemic activity is increased without a concomitant increase in transplant toxicity, and (3) optimization of a GVL effect posttransplant (Visual Abstract). In fit adults, there is evidence from the US CTN 0901 trial that a MAC regimen is associated with improved outcomes in patients with evidence of MRD pretransplant.16 Randomized data have demonstrated the Flu/Bu4 MAC regimen is associated with a lower 100-day TRM compared with Bu/Cy in adults under the age of 55 years, and this regimen is now widely used in this setting.23 It remains the case, however, that many patients with evidence of pretransplant MRD are ineligible for a MAC regimen, either on the grounds of age or comorbidity, and for these patients, the optimal RIC regimen has not been defined. Both Flu/Bu2 and Flu/Mel regimens are widely used and appear to be associated with broadly equivalent outcomes, although there has been no prospective randomized comparison. The sequential FLAMSA-Bu regimen has shown promising activity in early-phase trials, but the recent NCRI FIGARO trial failed to demonstrate improved outcomes in older adults with high-risk AML, regardless of pretransplant MRD status.13 Approaches to optimize a GVL effect posttransplant (Table 2) include the following:
Minimizing posttransplant immunosuppression
Commencement of an early posttransplant taper of immunosuppression
Delivery of prophylactic donor lymphocyte infusion in patients deemed to be at a particularly high risk of relapse
Elective posttransplant maintenance with either targeted drugs or agents with a broader antileukemic activity
Clinical interventions with the potential to decrease relapse in patients allografted for high-risk AML in the Clinical Case
| Maneuver . | Clinical context . |
|---|---|
| Minimize posttransplant immunosuppression | There is compelling evidence that the risk of relapse is correlated with the intensity of posttransplant immunosuppression. Regular monitoring of posttransplant CsA levels was performed in this patient with the aim of optimizing CsA levels posttransplant. |
| Early immunosuppression taper | No consensus exists concerning the optimal timing of a taper of CsA/tacrolimus in patients allografted for AML in CR1. Such a decision is influenced by a number of factors that include: • Predicted risk of disease relapse • Donor stem cell source • The degree of patient: donor HLA disparity • GVHD prophylaxis regimen adopted—specifically, the use of pretransplant ATG or alemtuzumab • History of GVHD In patients with high-risk AML transplanted using an ATG/alemtuzumab-based GVHD prophylaxis with no history of GVHD, consideration should be given to commencing a rapid (over less than 2 months) CsA/tacrolimus taper on or before day +60. Randomized trials of an early vs late taper of immunosuppression strategy are required. |
| Posttransplant maintenance | Randomized trials demonstrate improved outcomes in patients allografted for Flt3+ AML who receive sorafenib maintenance.24,25 Many patients find it difficult to tolerate sorafenib maintenance, and the results of a recently completed randomized evaluation of posttransplant maintenance with the Flt3 inhibitor gilteritinib are awaited. The patient entered the AMADEUS trial—a randomized comparison of maintenance therapy with CC486, which is an oral preparation of azacitidine. Key considerations in the design of an effective maintenance strategy are (a) patient selection, (b) tolerability of the maintenance agent, (c) when to start maintenance, and (d) duration of therapy. |
| Prophylactic DLI | The only other strategy with the potential to reduce the risk of relapse considered by the transplant team was the administration of prophylactic DLI. This is typically considered in patients with evidence of mixed T-cell chimerism or posttransplant MRD but is associated with a significant risk of GVHD. There are no compelling randomized data to support this strategy, and the results of the recently recruited Pro-DLI trial are awaited. |
| Maneuver . | Clinical context . |
|---|---|
| Minimize posttransplant immunosuppression | There is compelling evidence that the risk of relapse is correlated with the intensity of posttransplant immunosuppression. Regular monitoring of posttransplant CsA levels was performed in this patient with the aim of optimizing CsA levels posttransplant. |
| Early immunosuppression taper | No consensus exists concerning the optimal timing of a taper of CsA/tacrolimus in patients allografted for AML in CR1. Such a decision is influenced by a number of factors that include: • Predicted risk of disease relapse • Donor stem cell source • The degree of patient: donor HLA disparity • GVHD prophylaxis regimen adopted—specifically, the use of pretransplant ATG or alemtuzumab • History of GVHD In patients with high-risk AML transplanted using an ATG/alemtuzumab-based GVHD prophylaxis with no history of GVHD, consideration should be given to commencing a rapid (over less than 2 months) CsA/tacrolimus taper on or before day +60. Randomized trials of an early vs late taper of immunosuppression strategy are required. |
| Posttransplant maintenance | Randomized trials demonstrate improved outcomes in patients allografted for Flt3+ AML who receive sorafenib maintenance.24,25 Many patients find it difficult to tolerate sorafenib maintenance, and the results of a recently completed randomized evaluation of posttransplant maintenance with the Flt3 inhibitor gilteritinib are awaited. The patient entered the AMADEUS trial—a randomized comparison of maintenance therapy with CC486, which is an oral preparation of azacitidine. Key considerations in the design of an effective maintenance strategy are (a) patient selection, (b) tolerability of the maintenance agent, (c) when to start maintenance, and (d) duration of therapy. |
| Prophylactic DLI | The only other strategy with the potential to reduce the risk of relapse considered by the transplant team was the administration of prophylactic DLI. This is typically considered in patients with evidence of mixed T-cell chimerism or posttransplant MRD but is associated with a significant risk of GVHD. There are no compelling randomized data to support this strategy, and the results of the recently recruited Pro-DLI trial are awaited. |
ATG, anti-thymocyte globulin; DLI, donor lymphocyte infusion; HLA, human leukocyte antigen.
Two randomized trials have demonstrated that posttransplant maintenance with sorafenib can reduce the risk of disease relapse and improve survival in adults transplanted for FLT3 + AML, and maintenance strategies using targeted therapies are being developed in patients with IDH1 mutations and TP53 mutations.24,25 An alternative approach is to use a well-tolerated agent with broader antileukemic activity as a maintenance therapy. Randomized trials using subcutaneous azacitidine have failed to demonstrate evidence of improved survival but may reflect relatively poor tolerability of this agent posttransplant.26 A phase 3 trial of oral azacitidine (CC486) maintenance (AMADEUS) is currently approaching full recruitment (NCT04173533). The benefit of combined venetoclax and subcutaneous azacitidine maintenance posttransplant is being examined in the VIALE-T trial (NCT04161885), which has recently opened to recruitment.
Further refinement of our understanding of the impact of both disease biology and pre- and posttransplant MRD status on relapse risk will permit the more accurate identification of patients at the highest risk of disease relapse. This, coupled with the emergence of novel agents, such as the Bcl-2 inhibitor venetoclax, and immunotherapeutic strategies using chimeric antigen receptor T cells and bispecific antibodies, will underpin the design of new strategies with the potential to reduce the risk of disease relapse. Accelerated delivery of such studies is, however, pivotally dependent on the further development of transplant trials networks, such as the US BMT CTN and the UK IMPACT initiative, in order to accelerate the generation of practice informing randomized trial data to patient benefit.
Conflict-of-interest disclosure
Charles Craddock has received honoraria from Novartis, Celgene, BMS, Abbvie, and Astellas and research support from Celgene.
Off-label drug use
Charles Craddock: nothing to disclose.