Abstract
TP53 mutations impair the cellular response to genotoxic stress and drive intrinsic resistance to conventional cytotoxic therapies. Clinical outcomes in patients with TP53-mutated myeloid malignancies are poor and marked by high-risk clinical features, such as complex karyotype and prior exposure to leukemogenic therapies, and short survival due to a high risk of relapse after allogeneic transplantation. TP53 mutations are thus included as adverse markers in clinical prognostic models, including European LeukemiaNet recommendations and the Molecular International Prognostic Scoring System for myelodysplastic syndromes (MDS). Recent data indicate that the TP53 allelic state, co-occurring somatic mutations, and the position of the TP53 mutation within the clonal hierarchy define genetic heterogeneity among TP53-mutated MDS and acute myeloid leukemia that may influence clinical outcomes, thereby informing the selection of patients most suitable for transplantation. Further, novel therapeutic methods such as antibody-based agents (monoclonals or dual-affinity retargeting antibodies), cellular therapies (natural killer cells, chimeric antigen receptor T cells), or targeted agents (eprenetapopt) may offer opportunities to modify the approach to pretransplant conditioning or posttransplant maintenance and improve clinical outcomes.
Learning Objectives
Understand the pretransplant factors that may drive poor outcomes in TP53-mutated MDS/AML
Identify emerging opportunities to modify peritransplant management to improve outcomes in patients with TP53-mutated MDS/AML
CLINICAL CASE
A 60-year-old man with pancytopenia was referred to our clinic. He had a past medical history of coronary artery disease, for which he was taking an antiplatelet drug. He reported fatigue and easy bruising. The initial blood work and bone marrow examination are shown in Table 1. Our patient was diagnosed with acute myeloid leukemia (AML) with a single TP53 mutation and a monosomal karyotype.
Case: clinicopathologic characteristics
| Lab . | Unit . | Range . | Bone marrow . | |
|---|---|---|---|---|
| Hemoglobin | 8.1 g/dL | (14.0-17.5) | Morphology | Normal cellular marrow with 20.3% blasts and with dysplastic erythropoiesis (megaloblastosis, abnormalities of the nucleus) and absent megakaryocytes |
| MCV | 96 fL | (80-100) | ||
| Platelets | 8 × 109/L | (150-350) | ||
| WBC count | 1.06 × 109/L | (3.5-10.0) | ||
| Neutrophils | 0.08 × 109/L | Flow cytometry | Immature myeloid population with a phenotype consistent with AML (20%) | |
| Lymphocytes | 0.85 × 109/L | |||
| Eosinophils | 0.02 × 109/L | NGS | TP53 mutation, VAF 69% | |
| Monocytes | 0.08 × 109/L | Karyotype | Monosomal karyotype 47 ~ 50,Y,add(X)(p),add(3)(q),del(5)(q),+6,+8,del(11)(q),-13,-16,add(17)(p11)[19]/46,XY[1] | |
| Blasts | 0.03 × 109/L | |||
| LDH | 340 U/L | (<248) | ||
| Lab . | Unit . | Range . | Bone marrow . | |
|---|---|---|---|---|
| Hemoglobin | 8.1 g/dL | (14.0-17.5) | Morphology | Normal cellular marrow with 20.3% blasts and with dysplastic erythropoiesis (megaloblastosis, abnormalities of the nucleus) and absent megakaryocytes |
| MCV | 96 fL | (80-100) | ||
| Platelets | 8 × 109/L | (150-350) | ||
| WBC count | 1.06 × 109/L | (3.5-10.0) | ||
| Neutrophils | 0.08 × 109/L | Flow cytometry | Immature myeloid population with a phenotype consistent with AML (20%) | |
| Lymphocytes | 0.85 × 109/L | |||
| Eosinophils | 0.02 × 109/L | NGS | TP53 mutation, VAF 69% | |
| Monocytes | 0.08 × 109/L | Karyotype | Monosomal karyotype 47 ~ 50,Y,add(X)(p),add(3)(q),del(5)(q),+6,+8,del(11)(q),-13,-16,add(17)(p11)[19]/46,XY[1] | |
| Blasts | 0.03 × 109/L | |||
| LDH | 340 U/L | (<248) | ||
LDH, lactase dehydrogenase; MCV, mean corpuscular volume; NGS, next generation sequencing; WBC, white blood count.
Introduction
Myeloid malignancies with TP53 alterations form a distinct group defined by limited response to standard therapies and poor overall clinical outcomes. In AML, TP53 mutations are linked to primary refractoriness to intensive induction,1,2 a low likelihood of measurable residual disease (MRD) negativity in cytomorphologic remission,3 increased relapse after complete remission (CR), and shorter overall survival (OS).4-6 In myelodysplastic syndromes (MDS), TP53 mutations are associated with shorter leukemia-free and OS,7,8 even after treatment with DNA methyltransferase inhibitors.9 The clinical effects of TP53 mutations are caused by an impairment in normal cellular stress and DNA damage response pathways, which results in selective survival of TP53-mutated cells after genotoxic insults.
Allogeneic hematopoietic cell transplantation (HCT) is the only potentially curative treatment for many patients with high-risk myeloid malignancies. However, retrospective analyses have demonstrated a wide variability of transplant efficacy based on molecular genetic alterations, including dismal survival outcomes among patients with TP53-mutated MDS and AML.3,10-12 The impact of TP53 mutations on transplant outcomes is driven by an exceptionally high risk of early relapse, suggesting that TP53 mutations drive rapid disease progression that outpaces functional engraftment and the development of an effective graft-versus-leukemia (GVL) effect or cause cell intrinsic or extrinsic effects that subvert GVL activity.
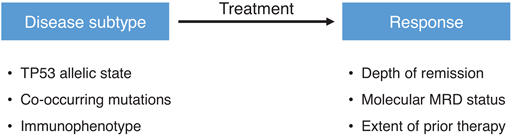
The available data raise a critical question: Is conventional allogeneic HCT an appropriate treatment for patients with TP53-mutated myeloid malignancies given the low probability of a long-term cure and the substantial risk of transplant-related morbidity and mortality? Or perhaps this question is, on its face, overly reductive in its nihilism. Should all TP53 leukemias in all patients really be treated the same, when 10% to 20% of patients with TP53-mutated myeloid malignancies see long-term survival after allogeneic HCT? An enhanced understanding of patient- or disease-related heterogeneity could provide a point of leverage for selecting suitable patients based on pretransplant variables or insights into the development of tailored peritransplant approaches aimed at mitigating relapse risk (Figure 1).
Pretransplant factors that could influence patient selection or outcome.
Potential pretransplant modifiers of clinical outcomes
Genetic heterogeneity among TP53-mutated myeloid malignancies can be broadly defined based on the TP53 allelic state, co-occurring somatic mutations, and position within the clonal hierarchy. Monoallelic TP53 mutations target a single allele while retaining the wild-type allele, whereas multiallelic alterations occur when the second TP53 allele is inactivated by another point mutation, by deletion, or by the copy-neutral loss of heterozygosity. The adverse clinical impact of TP53 mutations in MDS has been reported to be driven by the subset cases with multiallelic TP53 alterations, in which multiallelic TP53 mutations are associated with a complex karyotype, elevated bone marrow blasts, a higher risk of leukemic transformation, and poor OS compared with monoallelic TP53 mutations, whose clinicopathologic characteristics are similar to MDS without TP53 mutations.13
The differential effect of monoallelic and multiallelic TP53 mutations on clinical presentation and outcome may be related to a dose-dependent effect of TP53 inactivation on genome instability. Compared with the monoallelic state, multiallelic TP53 is associated with more chromosomal aberrations and a higher proportion of complex karyotypes. The inability to maintain genome integrity with complete disruption of TP53 may correlate with more clinically significant resistance to genotoxic therapy. In addition, the distribution of somatic comutated genes is different in monoallelic vs multiallelic disease. Whereas the multiallelic state has few concurrent gene mutations, as seen in our case, MDS and AML with monoallelic TP53 mutations are more likely to have concurrent mutations in MDS-associated genes encoding RNA-splicing factors (SF3B1, SRSF2), epigenetic factors (TET2, ASXL1), myeloid transcription factors (RUNX1), and myeloproliferative neoplasm phenotypic drivers (JAK2).
Together, these data indicate that TP53 mutations can arise in at least 2 distinct positions in the myeloid clonal hierarchy. When occurring in a founding clone, a TP53 mutation can initiate clonal advantage, and progression is mediated by subclonal inactivation of the second TP53 allele. In contrast, TP53 mutations can also arise as progression subclones in the context of an established clonal myeloid disease, often in the context of extrinsic selection by cytotoxic therapy.2,10,14,15 In this setting only the lower abundance TP53-mutated subclone possesses the biological characteristics of TP53 inactivation.
Variant allele fraction (VAF) has been used as a proxy for the definitive assessment of the allelic state and clonal hierarchy. As generalizations, a high VAF TP53 mutation implies high clonal abundance and/or multiallelic alteration. In contrast, a lower VAF suggests low-abundance clone/subclone and/or the absence of multiallelic alteration. That said, without knowledge of sample purity (overall clone size or relative subclone abundance) and ploidy (TP53 copy number), the interpretation of VAF relies on inferences based on a cohort-level correlation with outcomes. In the research setting, single-cell DNA sequencing has been reported to identify low-abundance clones with biallelic TP53 alterations well before detection by bulk sequencing methods.16
TP53 mutations are themselves heterogeneous, including hot spot and non–hot spot missense mutations, which account for two-thirds of TP53 mutations, as well as truncating (nonsense, frameshift indels, splice site) mutations. The TP53 mutation subtype has not been definitively linked to specific clinical outcomes, and TP53 missense mutations have been shown to affect p53 pathway function in diverse ways (ie, dominant negative, loss of function, gain of de novo function, etc). TP53-related clinical and molecular factors and relative associations with outcome are summarized in Table 2.
Heterogeneity of TP53-mutated myeloid malignancies and association with relative clinical outcomes
| Variable . | Relatively favorable outcome . | Poor outcome . | Comments/caveats . |
|---|---|---|---|
| Mutation type | Missense | Truncating only | Underpowered; did not account for all transplant-relevant covariates |
| VAF | Low | High | VAF is imprecise → reflects composite of ploidy and purity/clone size; difficult to define a generalized cut point |
| Allelic state | Single | Multi | Only shown in nontransplant MDS; not tested in HCT context |
| Karyotype | Noncomplex | Complex | |
| Response to treatment | MRD-negative CR | MRD-positive, active disease | Conflicting data |
| Clinical ontogeny | - | - | No apparent impact of secondary vs therapy-related vs de novo disease |
| Germline predisposition | - | - | Certain germline leukemia predispositions are associated with development of somatic TP53 mutations (Shwachman-Diamond syndrome, telomere biology disorders, etc). |
| Variable . | Relatively favorable outcome . | Poor outcome . | Comments/caveats . |
|---|---|---|---|
| Mutation type | Missense | Truncating only | Underpowered; did not account for all transplant-relevant covariates |
| VAF | Low | High | VAF is imprecise → reflects composite of ploidy and purity/clone size; difficult to define a generalized cut point |
| Allelic state | Single | Multi | Only shown in nontransplant MDS; not tested in HCT context |
| Karyotype | Noncomplex | Complex | |
| Response to treatment | MRD-negative CR | MRD-positive, active disease | Conflicting data |
| Clinical ontogeny | - | - | No apparent impact of secondary vs therapy-related vs de novo disease |
| Germline predisposition | - | - | Certain germline leukemia predispositions are associated with development of somatic TP53 mutations (Shwachman-Diamond syndrome, telomere biology disorders, etc). |
CLINICAL CASE (Continued)
The patient received intensive induction treatment consisting of cytarabine and daunorubicin (“7 + 3”), which did not result in CR after the first cycle, but he obtained an MRD-negative CR after the second cycle.
In adults under age 65, the clearance of MRD has been associated with improved outcome after HCT with reduced-intensity conditioning (RIC).17 This result has raised the question of whether the achievement of deep MRD-negative remission in patients with TP53-mutated MDS or AML may predict improved outcomes after HCT.18 In patients with MDS or oligoblastic AML who received treatment with DNA methyltransferase inhibitors, the TP53 clearance of TP53 mutations to a VAF lower than 0.05 was associated with improved outcome, particularly in those who underwent subsequent allogeneic HCT.19 In a cohort of older patients with AML who were transplanted in first CR, the molecular clearance of TP53 mutations in remission (VAF <0.001) was not associated with improved leukemia-free survival.3 This question merits continued evaluation with MRD negativity defined using even more sensitive technologies, in patients whose remissions were achieved with new or different induction regimens, or in different patient populations (age, disease subtype, risk groups).
CLINICAL CASE (Continued)
The patient received an allogeneic HCT after myeloablative conditioning (ie, fludarabine, busulfan at 12.8 mg/kg over 4 days) from a matched unrelated donor. No signs of graft- versus-host disease (GVHD) were observed during post-HCT follow-up.
Potential transplant-related modifiers of clinical outcomes
Early disease recurrence is the primary cause of death after allogeneic HCT in patients with TP53 mutations. Relapse after transplantation may result from the rapid reemergence of chemoresistant disease before immune reconstitution and the development of effective alloimmune activity. Alternatively, TP53 alterations in the residual leukemia cells themselves may directly suppress innate immune signaling or modify the expression of key checkpoint molecules, thereby promoting immune evasion, particularly in the context of early immunosuppression.20,21 In addition to optimizing the depth and quality of remissions in TP53-mutated myeloid malignancies through the development of novel induction regimens, the modification of transplant-related factors could further mitigate the risk of relapse. Such strategies might involve the transplant procedure itself (ie, conditioning, donor selection) or changes to posttransplant care, such as the specific enhancement of GVL activity or the addition of antileukemic maintenance therapy (Figure 2).
Opportunities for improving transplant outcomes in patients with TP53-mutated MDS or AML.
Opportunities for improving transplant outcomes in patients with TP53-mutated MDS or AML.
Conditioning intensity and donor choice
High-risk MDS or AML might benefit from intensified conditioning regimens prior to allogeneic HCT to eradicate residual disease. Two prospective randomized phase 3 trials comparing RIC with myeloablative conditioning (MAC) in patients with MDS and AML yielded contradicting results.22,23 In patients with MDS, the European Society for Blood and Marrow Transplantation (EBMT) RICMAC trial reported similar outcomes, including relapse, nonrelapse mortality (NRM), and OS for RIC vs MAC. The BMT CTN 0901 trial, which included patients with MDS and AML, reported a lower incidence of NRM in patients receiving RIC, which was counterbalanced by a higher incidence of relapse compared with MAC, resulting in improved relapse-free survival after MAC.22 In that trial, ultradeep sequencing of 13 genes, including TP53, showed that patients with genomic evidence of disease at transplant were associated with better survival after MAC compared with RIC.17 Although the proportion of TP53-mutated patients without relapse was higher in recipients of MAC, a formal comparison of MAC vs RIC was not performed.17 While these prospective trials did not specifically address TP53 mutations, the beneficial effect of MAC vs RIC has not been shown in a large Center for Blood and Marrow Transplantation Research cohort of 289 patients with TP53-mutated MDS (median OS, 7.5 months vs 9.2 months, respectively; P = .19).10 Similarly, a retrospective EBMT analysis reported no significant difference between RIC vs MAC in 179 patients with TP53-mutated AML.24 Although definitive data are lacking, increasing the intensity of conditioning, which we did in our case, appears to be inadequate to overcome the early relapse of TP53 mutations in MDS and AML.
Optimizing the antileukemic cytotoxic effects of conditioning has been explored by adding venetoclax to a fludarabine- and busulfan-based RIC regimen (FluBu2) in patients with high-risk MDS or AML.25 Increasing doses of venetoclax were added to the FluBu2 regimen, which appeared feasible and safe in this phase 1 trial. Importantly, 12 of the 22 included patients had TP53- mutated myeloid disease. With a relatively short follow-up of 14 months, encouraging responses were observed, with 50% of patients still alive and in remission. Six patients with TP53 mutations were alive at the study evaluation, 3 of whom were in an MRD-negative remission post HCT.25 These data indicate that adding specific drugs to standard combinations of conditioning regimens are feasible and may be associated with promising outcomes. This and other novel approaches altering conditioning regimens need to be considered for future clinical research in these high-risk patients.
The optimal donor in patients with TP53-mutated MDS or AML has not been established. One could argue favoring a donor with greater human leukocyte antigen disparity (eg, mismatched unrelated donors, cord blood grafts, haploidentical donors) over human leukocyte antigen–matched donors, allowing for potentially strong GVL effects. To our knowledge, no studies are available comparing different donor types in patients with TP53 mutations. Retrospective registry data have not identified an independent effect of donor type on the cumulative incidence of relapse or OS in patients with TP53 mutations or chromosome 17p abnormalities.24,26,27 Urgent allogeneic HCT in remission is generally preferred in patients with a high risk of relapse, which should be considered together with the possibility of additional posttransplant cell therapies (eg, donor lymphocyte infusions [DLIs]) when selecting a donor for HCT. Such decision-making needs to be individualized, taking into account the risk of relapse vs the risk of HCT-related mortality.
Posttransplantation interventions
Post-HCT interventions to exploit and preserve the graft-versus-tumor of alloreactive T cells and natural killer cells might reduce relapse and improve outcomes of TP53-mutated MDS or AML. Hypomethylating agents have been associated with enhanced graft-versus-tumor effects by stimulating T-cell responses to overexpressed tumor-associated antigens.28 Although maintenance with azacitidine post HCT was not associated with improved outcomes in a randomized phase 3 study,29 preemptive treatment with azacitidine resulted in reduced or delayed relapse in the RELAZA trials.30,31 These studies, however, did not specifically address residual or emerging TP53 mutations to assess the beneficial effect of azacitidine in patients with TP53 mutations.
A post-HCT combination of hypomethylating treatments and the novel agent eprenetapopt have shown very promising results. Eprenetapopt (APR-246) is a small molecule that restores wild-type p53 in TP53-mutant cells, inducing apoptosis of those cells. Synergistic cytotoxic effects of eprenetapopt and azacitidine have been shown in phase 2 trials including patients with MDS and AML, with encouraging response rates and molecular remissions.32,33 Important data of post-HCT maintenance treatment with eprenetapopt and azacitidine for patients with TP53 mutations have emerged from a recent phase 2 trial.34 The study enrolled 33 patients who were scheduled for 12 cycles of eprenetapopt and azacitidine post HCT. Treatment was completed in 39% of patients, and the median number of cycles given was 7 (range, 1-12). The combination appeared to be safe and well tolerated without a signal of increased GVHD. Encouraging survival was reported, with a 1-year OS of 79% after a median follow-up of 17 months.34 Prospective randomized studies are needed to define the efficacy and optimal duration of this combination in the posttransplant setting.
Allogeneic immunotherapy might be further improved by the early tapering of immunosuppressive drugs and/or prophylactic or preemptive DLIs. Enhancing a GVL effect by DLI has been associated with reduced disease relapse and improved overall outcome in high-risk myeloid malignancies,35,36 but granular data on disease characteristics including TP53 mutations are not available. Post-HCT studies combining hypomethylating agents or histone deacetylase inhibitors with DLI were feasible and associated with low relapse rates,37,38 but studies addressing patients with TP53 mutations are needed.
Alternative approaches to improve post-HCT outcomes might be directed at the microenvironment of patients with TP53-mutated MDS or AML. Recent data indicate that the microenvironment of TP53-mutated disease confers an immune- privileged, evasive phenotype.39 Programmed cell death 1 ligand 1 expression, among others, was significantly increased in hematopoietic stem cells, indicating that restoring that immune- privileged microenvironment by programmed cell death 1 protein blockade might potentiate graft-versus-tumor effects. The usage of programmed cell death 1 protein blockade needs to be carefully studied in the post-HCT setting because of potentiating severe GVHD.
CLINICAL CASE (Continued)
At 7 months after allogeneic HCT, the patient relapsed with 17% blasts and recurring TP53 mutation (VAF, 17%) by bone marrow evaluation. The patient was scheduled by the treating physician for reinduction therapy with azacitidine and venetoclax followed by DLI to induce a GVL effect. Unfortunately, his AML was not responsive to the reinduction treatment, and he passed away after 2 cycles of azacitidine and venetoclax due to bilateral pneumonia.
Further directions
Allogeneic HCT provides long-term survival in a subset of patients with TP53-mutated myeloid malignancies, but the overall outcome remains poor, with OS varying between 10% and 20%. Promising pre-HCT combination treatments in patients with AML are currently being studied, including azacitidine with magrolimab, a monoclonal antibody against the “don't eat me signal” of leukemic cells, in phase 3 trials. Non-HCT cellular therapies are currently emerging, including bispecific antibodies and chimeric antigen receptor (CAR) T-cell therapy. Flotetuzumab, a CD123 × CD3 dual-affinity retargeting antibody, was associated with complete responses in 7 of 15 patients with relapsed/refractory AML, resulting in a median survival of 10.3 months.20 Similarly, CAR T cells targeting leukemic cells (eg, CD123, CD33) have shown their efficacy, but off-target effects are limiting broader application. Recent preclinical data with a CD70-targeted CAR T combined with azacitidine, which increased expression of CD70 antigens on leukemic blasts, were associated with potent in vivo antileukemic activity.40
So do we prescribe allogeneic HCT for patients with TP53 mutations because we can or because we should? Although allogeneic HCT is the only treatment providing a long-term cure, research efforts should focus on the optimal delivery of an allograft in the patient, preferably in a deep MRD-negative remission, while applying optimal conditioning and/or posttransplant interventions.
For those patients with a very high risk of relapse (eg, primary refractory disease, MRD-positive state) and a high risk of NRM, the early use of alternative targeted therapies that harness the innate immune system against leukemia needs further investigation. Such treatments may provide hope for this patient group with dismal outcomes.
Conflict-of-interest disclosure
Jurjen Versluis: no competing financial interests to declare.
R. Coleman Lindsley: consultancy: bluebird bio, Nuprobe, Qiagen, Takeda Pharmaceuticals, Thermo Fisher.
Off-label drug use
Jurjen Versluis: nothing to disclose.
R. Coleman Lindsley: nothing to disclose.