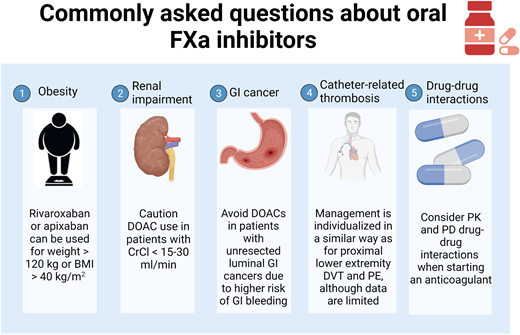
Abstract
Direct oral anticoagulants (DOACs) are commonly used oral factor Xa inhibitors in recent years. However, in some special clinical situations, the appropriate use of these anticoagulants may be of concern. In this article, we address the 5 commonly asked questions regarding their use for the treatment of venous thromboembolism, including in the setting of obesity, renal impairment, gastrointestinal (GI) malignancy, catheter-related thrombosis, and drug-drug interactions. Data on the use of DOACs in the presence of significant obesity or renal failure are mainly observational. Some DOACs are shown to have an increased risk of bleeding in patients with unresected luminal GI malignancy but not others, so selection of appropriate patients is the key. Furthermore, literature on the use of DOACs for catheter-related thrombosis or when drug-drug interactions are of concern is limited, and more research is welcome.
Learning Objectives
Understand the scope of commonly encountered questions in the use of factor Xa inhibitors for the treatment of venous thromboembolism
Learn the evidence and guideline recommendations for appropriate use of factor Xa inhibitors related to these 5 topics
Introduction
Direct oral anticoagulants (DOACs) are among the most commonly used anticoagulants in recent years. Among them, rivaroxaban, apixaban, and edoxaban achieve anticoagulation effects by directly inhibiting factor Xa (FXa) in the coagulation pathway. Different from traditional anticoagulants, such as low-molecular-weight heparin (LMWH) or vitamin K antagonists (VKAs), they are more convenient given their oral administration without the need for regular monitoring. However, specific clinical scenarios can generate questions regarding the optimal utilization of these medications. In this review, we address the 5 commonly asked questions regarding the use of FXa inhibitors for the treatment of venous thromboembolism (VTE), including obesity, renal impairment, gastrointestinal (GI) malignancy, catheter-related thrombosis, and drug-drug interactions. For each topic, we review pertinent literature and associated guidelines, as well as our practice approaches. As dabigatran has a different mechanism of action (an oral thrombin inhibitor), our discussions and recommendations should not be extrapolated to dabigatran.
CLINICAL CASE 1
A 53-year-old man presented with pleuritic chest pain and shortness of breath, and imaging confirmed bilateral pulmonary embolism. He has no history of VTE but has restricted mobility due to progressive multiple sclerosis. His weight is 149 kg, with a body mass index (BMI) of 45 kg/m2. His complete blood count and kidney function are unremarkable. What anticoagulant should be recommended?
Obesity
Obesity is an increasingly prevalent health problem worldwide, recognized by the World Health Organization as a global epidemic.1 Obesity is a known risk factor for many cardiovascular diseases, including atrial fibrillation and VTE, for which anticoagulation is needed. In the era of VKAs, the dose of warfarin increases with increasing weight.2 In addition, fixed-dose LMWH is associated with reduced anti-Xa levels in patients with obesity.3 Therefore, when direct oral anticoagulants (DOACs) became widely used, there were concerns about the efficacy of fixed-dose DOACs in patients with extreme weight. As such, the initial 2016 International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee guidance statement suggested against the routine use of DOACs in patients with weight >120 kg or BMI >40 kg/m2.4 More data have accumulated since then, most of which were from retrospective cohort studies or database analyses. The only randomized controlled trial (RCT) data comparing DOACs with warfarin for the treatment of acute VTE in patients with weight >120 kg or BMI >40 kg/m2 were from the post hoc analysis of the EINSTEIN trial, including 303 patients with such weight/BMI cutoff.5 The rates of recurrent VTE in either rivaroxaban or warfarin groups were low and comparable in this subgroup analysis.
To summarize the available literature, several groups have performed meta-analyses of studies evaluating the use of oral anticoagulants in patients with obesity and VTE (Table 1).6-8 Although each meta-analysis included slightly different sets of studies and analyses differed, overall, there were no concerns of efficacy or safety with DOACs compared with VKAs in the obese population. However, it is worth noting that most of the literature were nonrandomized cohort studies, which were easily affected by residual confounding, and the number of patients with extreme obesity (weight >150 kg or BMI >50 kg/m2) remained relatively small. It is unknown if there is a “ceiling” of weight or BMI for safe DOAC use. Therefore, cautions are needed and discussions with patients about the pros and cons and limitations of current literature are warranted. The ISTH Scientific and Standardization Committee updated its guidance statements in 2021 and now suggests that rivaroxaban or apixaban (not dabigatran or edoxaban) can be used in patients with VTE without restrictions in weight or BMI.9 In contrast to the 2016 guidance, anti-Xa level monitoring is not recommended, given the lack of data supporting its utility. On the other hand, DOACs are not recommended as first therapy in patients after bariatric surgery, based on even scantier evidence.9
Summary of meta-analyses comparing direct oral anticoagulants with vitamin K antagonists in the obese population with VTE
| Study . | Obesity definition . | n (studies) . | N (patients) . | Recurrent VTEOR, RR, or IRR (95% CI) . | Major bleedingOR, RR, or IRR (95% CI) . | Conclusion . |
|---|---|---|---|---|---|---|
| Elshafei et al6 (2020) | Weight >120 kg or BMI >40 kg/m2 | 5 | 6642 | OR 1.07 (0.93-1.23) | OR 0.80 (0.54-1.17) | Use of DOACs in patients with weight >120 kg or BMI >40 kg/m2 is effective and safe |
| Mai et al7 (2020) | BMI ≥30 kg/m2 | 11 | 14 890 | RR 1.03 (0.93-1.15) | RR 0.57 (0.34-0.94) | Comparable outcomes with DOACs vs VKA/LMWH in those with BMI ≥30 kg/m2 and ≥40 kg/m2 |
| BMI ≥40 kg/m2 | 4 | 6304 | RR 1.06 (0.94-1.19) | RR 0.71 (0.50-1.00) | ||
| Wang et al8 (2021) | Weight ≥120 kg or BMI ≥40 kg/m2 | 9 | 5148 | IRR 0.58 (0.44-0.77) | IRR 0.68 (0.41-1.11) | Compared to VKAs, DOACs were associated with lower rates of recurrent VTE and similar rates of major bleeding |
| Study . | Obesity definition . | n (studies) . | N (patients) . | Recurrent VTEOR, RR, or IRR (95% CI) . | Major bleedingOR, RR, or IRR (95% CI) . | Conclusion . |
|---|---|---|---|---|---|---|
| Elshafei et al6 (2020) | Weight >120 kg or BMI >40 kg/m2 | 5 | 6642 | OR 1.07 (0.93-1.23) | OR 0.80 (0.54-1.17) | Use of DOACs in patients with weight >120 kg or BMI >40 kg/m2 is effective and safe |
| Mai et al7 (2020) | BMI ≥30 kg/m2 | 11 | 14 890 | RR 1.03 (0.93-1.15) | RR 0.57 (0.34-0.94) | Comparable outcomes with DOACs vs VKA/LMWH in those with BMI ≥30 kg/m2 and ≥40 kg/m2 |
| BMI ≥40 kg/m2 | 4 | 6304 | RR 1.06 (0.94-1.19) | RR 0.71 (0.50-1.00) | ||
| Wang et al8 (2021) | Weight ≥120 kg or BMI ≥40 kg/m2 | 9 | 5148 | IRR 0.58 (0.44-0.77) | IRR 0.68 (0.41-1.11) | Compared to VKAs, DOACs were associated with lower rates of recurrent VTE and similar rates of major bleeding |
IRR, incidence rate ratio; OR, odds ratio.
Although the recent ISTH guidance statement did not recommend routine anti-Xa monitoring, several studies have evaluated the use of anti-Xa levels in the obese population and provided some insights. They showed that trough levels were more likely to be within the “in-therapy” range (~95% of cases) compared with peak levels (55%-70% of cases),10,11 which might explain the maintained efficacy and potentially improved safety with DOACs in patients with obesity, although this is purely speculative, and more prospective studies are needed.
In our practice, we use standard-dose rivaroxaban or apixaban for VTE treatment in patients with weight >120 kg or BMI >40 kg/m2. While we do not routinely monitor anti-Xa levels, we do consider it in patients with extreme weight (such as weight >150 kg or BMI >50 kg/m2), as clinical data in this specific population are very limited. An “in-range” anti-Xa trough level can be reassuring, although this practice remains investigational.
CLINICAL CASE 1 (Continued)
After an informed discussion, standard-dose rivaroxaban was started. He tolerated rivaroxaban well for 2 months without bleeding or thrombotic complications but unfortunately developed septic shock from bacteremia and acute renal failure. After resuscitation, he was stabilized, but his renal function remained significantly compromised with creatinine clearance (CrCl) of 25 mL/min. How should one manage his anticoagulation?
Renal impairment
Renal impairment is not uncommon in patients with VTE, as shown in the SWIss Venous ThromboEmbolism Registry (SWIVTER). Among 2062 patients with acute VTE, 12% had CrCl <30 mL/min.12 Patients with chronic renal disease had a 1.5- to 3-fold increased risk of VTE.13 In those who had a VTE, renal impairment was also associated with an increased risk of mortality, recurrent VTE, and/or bleeding events.12,14,15
However, renal impairment (especially CrCl <30 mL/min) creates a significant challenge for anticoagulant choice. Since all LMWH, fondaparinux, and DOACs are metabolized through the kidney to some degree, a CrCl cutoff of >30 mL/min is typically desired for their safe use. In this regard, VKAs, which do not rely on renal metabolism, remain a commonly used anticoagulant in patients with CrCl <30 mL/min, including those on dialysis. However, the need for frequent laboratory monitoring, common food and drug interactions, increased risk of bleeding, and so on all make VKAs suboptimal. Unfractionated heparin is also commonly used in this population but can mostly be used as a continuous drip in the hospital, which severely limits its applicability. Tinzaparin is an LMWH with the highest average molecular weight and is more independent of renal clearance. It is available in Canada and Europe but not in the United States. Meta-analysis showed that tinzaparin is safe in patients with CrCl >20 mL/min with no bioaccumulation.16 It provides an alternative for patients with CrCl 20 to 30 mL/min and can be useful as a bridge to warfarin.
All RCTs involving DOACs excluded patients with CrCl <30 mL/min (CrCl <25 mL/min for apixaban trials). Table 2 summarizes the precautions and dose recommendations in product labeling from different regulatory agencies. For patients with end-stage renal disease, while the US Food and Drug Administration approved the use of apixaban without dose adjustment based on a single-dose pharmacokinetic (PK) study,17 Health Canada and the European Medicines Agency did not recommend apixaban in this population (Table 2). A recent large US database study in patients with end-stage renal disease and VTE showed that apixaban was associated with a significantly lower risk of major bleeding (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.62-0.98) and recurrent VTE (HR, 0.58; 95% CI, 0.43-0.77) compared with warfarin.18 Residual confounding remains a concern in this nonrandomized retrospective cohort study. Additional limitations include the lack of data on apixaban doses, time in therapeutic range with warfarin, and milder but clinically relevant bleeding events. Several systematic reviews compared DOACs with warfarin for the treatment of VTE in patients with renal impairment.19,20 Overall, compared with LMWH/VKA, DOACs were associated with a similar risk of recurrent VTE, and the risk of major bleeding was either comparable or favorable with DOACs. While these results were promising, they remained extremely limited and observational, especially in patients with CrCl <30 mL/min and/or on dialysis. The appropriate dose of apixaban, if used, is also unclear. Results from the ongoing RCT such as the VERDICT trial (NCT02664155) are awaited. Therefore, major guidelines continue to recommend against routine use of DOACs in patients with CrCl <15 to 30 mL/min.21-23
Dosing recommendations of oral factor Xa inhibitors for VTE from different regulatory agencies
| Drug . | Renal clearance, % . | Standard dose for VTE . | FDA . | Health Canada . | EMA . |
|---|---|---|---|---|---|
| Rivaroxaban | 33 | 15 mg twice daily for 21 days, then 20 mg daily | • Avoid use if CrCl <15 mL/min | • Avoid use if CrCl <15 mL/min | • Avoid use if CrCl <15 mL/min • Use with caution for CrCl 15-29 mL/min • For CrCl <50 mL/min, a reduction from 20 mg to 15 mg daily can be considered if risk of bleeding outweighs recurrent VTE |
| Apixaban | 27 | 10 mg twice daily for 7 days, then 5 mg twice daily | • No dose adjustment for any CrCl | • Avoid use if CrCl <15 mL/min • Use with caution for CrCl 15-29 mL/min | • Avoid use if CrCl <15 mL/min • Use with caution for CrCl 15-29 mL/min |
| Edoxaban | 50 | LWMH for ≥5 days, then 60 mg daily | • Not recommended if CrCl <15 mL/min • CrCl 15-50 mL/min: 30 mg daily | • Avoid use if CrCl <30 mL/min • Weight ≤60 kg, CrCl 30-50 mL/min, or coadministration with P-gp inhibitors: 30 mg daily | • Avoid use if CrCl <15 mL/min • Weight ≤60 kg, CrCl 15-50 mL/min, or coadministration with P-gp inhibitors: 30 mg daily |
| Drug . | Renal clearance, % . | Standard dose for VTE . | FDA . | Health Canada . | EMA . |
|---|---|---|---|---|---|
| Rivaroxaban | 33 | 15 mg twice daily for 21 days, then 20 mg daily | • Avoid use if CrCl <15 mL/min | • Avoid use if CrCl <15 mL/min | • Avoid use if CrCl <15 mL/min • Use with caution for CrCl 15-29 mL/min • For CrCl <50 mL/min, a reduction from 20 mg to 15 mg daily can be considered if risk of bleeding outweighs recurrent VTE |
| Apixaban | 27 | 10 mg twice daily for 7 days, then 5 mg twice daily | • No dose adjustment for any CrCl | • Avoid use if CrCl <15 mL/min • Use with caution for CrCl 15-29 mL/min | • Avoid use if CrCl <15 mL/min • Use with caution for CrCl 15-29 mL/min |
| Edoxaban | 50 | LWMH for ≥5 days, then 60 mg daily | • Not recommended if CrCl <15 mL/min • CrCl 15-50 mL/min: 30 mg daily | • Avoid use if CrCl <30 mL/min • Weight ≤60 kg, CrCl 30-50 mL/min, or coadministration with P-gp inhibitors: 30 mg daily | • Avoid use if CrCl <15 mL/min • Weight ≤60 kg, CrCl 15-50 mL/min, or coadministration with P-gp inhibitors: 30 mg daily |
EMA, European Medicines Agency; FDA, Food and Drug Administration.
In our practice, we take cautions in using DOACs in patients with CrCl <15 to 30 mL/min, especially given that apixaban is not approved in patients with CrCl <15 mL/min in Canada. However, in special situations where no safer alternative anticoagulants are available, apixaban had been tried, commonly with close monitoring for bleeding symptoms along with anti-Xa levels whenever possible. Tinzaparin is another consideration in patients with CrCl >20 mL/min.
CLINICAL CASE 2
A 62-year-old woman presented with fatigue, paler, and left leg swelling and redness. She was found to have iron-deficiency anemia and a deep vein thrombosis (DVT) in the left lower extremity. Further workup revealed a gastric mass, with biopsy confirming adenocarcinoma. She is referred to discuss anticoagulation options.
Gastrointestinal cancer
The management of VTE is a frequent and important clinical issue in patients with cancer. The 6-month risk of VTE risk is 12-fold higher among patients with cancer compared with the general population and as much as 23-fold higher in patients receiving chemotherapy or targeted therapy.24 The management of anticoagulant therapy for cancer-associated VTE is complex due to an increased risk of both recurrent VTE and major bleeding in this patient population.25,26 Hence, selection of anticoagulant therapy needs to be individualized based on patient characteristics, type of cancer, and anticancer treatments.27,28
A meta-analysis of the results of all RCTs comparing LMWH with VKA for the management of cancer-associated thrombosis reported a 44% reduction in the risk of recurrent VTE (relative risk [RR], 0.56; 95% CI, 0.43-0.7), without a significant increase in the risk of major bleeding (RR, 1.07; 95% CI, 0.66-1.79) in patients treated with LMWH.29 A similar meta-analysis including all RCTs comparing DOACs to LMWH for the treatment of acute cancer-associated VTE reported a significantly lower risk of recurrent VTE (RR, 0.67; 95% CI, 0.52-0.85) without a significant increase in major bleeding (RR, 1.17; 95% CI, 0.82-1.67) with DOACs as compared with LMWH.30 The risk of major bleeding was higher with DOACs compared with LMWH in both the Hokusai-VTE Cancer and Select-D trials but similar in the Caravaggio, ADAM-VTE, CASTA-DIVA, and CANVAS trials.31-36 The risk of clinically relevant nonmajor bleeding was higher among patients with cancer-associated VTE receiving DOACs (RR, 1.66; 95% CI, 1.31-2.09).30 Hence, identifying patients at higher risk of bleeding complications might be helpful to tailor anticoagulation in patients with cancer- associated VTE.
In the Hokusai-VTE Cancer trial, the excess risk in major bleeding complications with DOACs (over LMWH) was mainly attributable to patients with GI cancers (HR, 4.0; 95% CI, 1.5-10.6) (Table 3).37 Furthermore, in these patients, the upper GI tract was the most common site of major bleeding in those receiving edoxaban (16 of 21 cases), as compared with 1 in 5 cases in those receiving dalteparin.37 Similarly, in the Select-D trial, 45.5% of all major bleeding episodes in patients receiving rivaroxaban occurred in the GI tract.35 Moreover, a recent observational study reported that apixaban had a higher rate of major bleeding complications in patients with luminal GI cancers compared to those with non-GI cancers (15.6 vs 3.7 per 100 person-years, P = .004) and compared to enoxaparin in patients with luminal GI cancer (15.6 vs 3.2, P = .04).38
Bleeding outcomes in the gastrointestinal cancer subgroup in randomized controlled trials comparing DOACs with dalteparin for acute cancer-associated VTE
| Trials . | Hokusai-VTE Cancer34 . | Select-D35 . | ADAM-VTE32 . | Caravaggio31 . | CASTA-DIVA33 . |
|---|---|---|---|---|---|
| Total N | 1046 | 406 | 300 | 1155 | 158 |
| DOACs | Edoxaban | Rivaroxaban | Apixaban | Apixaban | Rivaroxaban |
| GI cancer | 305 (29.2) | 177 (43.6) | 105 (35) | 375 (32.5) | 46 (29.1) |
| Upper GI cancer | 54 (5.2) | 41 (10.1) | 11 (3.7) | 54 (4.7) | 3 (1.9) |
| Major bleeding (DOAC vs dalteparin) | 21/165 (12.7) vs 5/140 (3.6) HR 4.0 (95% CI, 1.5-10.6) | 8/91 (8.8) vs 5/86 (5.8) | 0/48 (0) vs 0/57 (0) | 9/188 (4.8) vs 9/187 (4.8) | NR |
| CRNMB (DOAC vs dalteparin) | NR | 7/91 (7.7) vs 1/86 (1.2) | NR | 19/188 (10.1) vs 7/187 (3.7) | NR |
| Trials . | Hokusai-VTE Cancer34 . | Select-D35 . | ADAM-VTE32 . | Caravaggio31 . | CASTA-DIVA33 . |
|---|---|---|---|---|---|
| Total N | 1046 | 406 | 300 | 1155 | 158 |
| DOACs | Edoxaban | Rivaroxaban | Apixaban | Apixaban | Rivaroxaban |
| GI cancer | 305 (29.2) | 177 (43.6) | 105 (35) | 375 (32.5) | 46 (29.1) |
| Upper GI cancer | 54 (5.2) | 41 (10.1) | 11 (3.7) | 54 (4.7) | 3 (1.9) |
| Major bleeding (DOAC vs dalteparin) | 21/165 (12.7) vs 5/140 (3.6) HR 4.0 (95% CI, 1.5-10.6) | 8/91 (8.8) vs 5/86 (5.8) | 0/48 (0) vs 0/57 (0) | 9/188 (4.8) vs 9/187 (4.8) | NR |
| CRNMB (DOAC vs dalteparin) | NR | 7/91 (7.7) vs 1/86 (1.2) | NR | 19/188 (10.1) vs 7/187 (3.7) | NR |
Values are presented as number (%) unless otherwise indicated.
CRNMB, clinically relevant nonmajor bleeding; NR, not reported.
In our practice, we follow the American Society of Hematology 2021 guidelines for the management of VTE in patients with cancer, which suggest using DOAC over LMWH for the management of cancer-associated thrombosis.28 However, the use of DOACs should be avoided in patients with unresected luminal GI cancers due to the higher risk of GI bleeding.
CLINICAL CASE 2 (Continued)
Her oncologist recommended intravenous chemotherapy and a peripherally inserted central catheter (PICC) to facilitate delivery of chemotherapy. The patient is apprehensive about the risks of thrombosis associated with a central venous catheter (CVC).
Catheter-related thrombosis
The use of a CVC, either peripherally inserted central catheter or infusion ports (eg, Port-a-Cath), has significantly enhanced the management of patients requiring parenteral treatment.39,40 Central venous catheters facilitate delivery of chemotherapy, transfusions, antibiotics, and parenteral nutrition. Additionally, they allow readily available venous access for laboratory testing and have been shown to improve patients' quality of life and reduce health care costs by allowing patients to receive parenteral therapy at home.
Catheter-related thrombosis is a common complication of CVC. A recent systematic review including 3000 patients with cancer and CVCs reported an overall rate of symptomatic VTE of 6.8% (95% CI, 5.5-8.3%).41 Vessel injury caused by the catheter insertion, venous stasis caused by the catheter, ongoing movements of the catheter within the vein, and cancer-related hypercoagulability all contribute to the development of VTE.42,43 As a consequence, the mean duration from CVC insertion to VTE is 10 days, and most VTEs occur within the initial 100 days following insertion.44,45
Several studies have assessed the role of thromboprophylaxis using unfractionated heparin, LMWH, or VKA for the prevention of VTE in patients with cancer and CVC.41 A recent systematic review comparing thromboprophylaxis (mostly LMWH or VKA) to observation found that thromboprophylaxis significantly reduced the risk of total VTE (odds ratio, 0.51; 95% CI, 0.32-0.82).46 However, these findings were not translated into clinical practice, and routine use of thromboprophylaxis in patients with cancer and CVCs is not recommended by guidelines due to the need for daily subcutaneous injections, high costs associated with LMWH, and the difficulty managing VKA in patients with cancer who are receiving chemotherapy due to drug interactions and unpredictable oral intake.28 An RCT assessing the efficacy and safety of rivaroxaban for the primary prevention of VTE in patients with cancer and CVC is ongoing (NCT05029063).
Although catheter-related thrombosis is common in patients with cancer and CVC, there is limited evidence to guide its management as these patients were excluded from all RCTs except for the ADAM-VTE trial.32 Two studies of patients with cancer and catheter-related thrombosis suggested that LMWH and VKA were safe and effective.47,48 No recurrent VTE events were reported in either study, and major bleeding event rates were 4% and 2% at 3 months. However, a prospective cohort study evaluating rivaroxaban in patients with cancer and catheter-related thrombosis reported a low rate of recurrent VTE (1.4%) but a high rate of bleeding complications (12.9%) at 3 months.49 More recently, another prospective cohort study of patients with upper extremity DVT managed with DOACs (29% with active cancer and 33% with catheter-related thrombosis) reported a risk of recurrent VTE and major bleeding complications of 0.9 and 1.7 per 100 patient-years, respectively.50
In our practice, tailoring of anticoagulant for the management of catheter-related thrombosis in patients with cancer is individualized in a similar way as for proximal lower extremity DVT and pulmonary embolism based on patient characteristics, type of cancer, and anticancer treatments.27
CLINICAL CASE 2 (Continued)
Her cancer progressed, and her oncologist recommended changing therapy to vascular endothelial growth factor (VEGF) inhibitors. The oncology pharmacist is concerned about potential drug-drug interactions with her anticoagulant.
Drug-drug interactions
Drug-drug interactions (DDIs) account for 20% to 30% of adverse drug reactions.51 Patients with cancer are at increased risks of DDIs given older age, increased comorbidities, and polypharmacy. Two main types of DDIs concerning concurrent anticoagulant use include PK (shared metabolism pathways) and pharmacodynamic (1 drug potentiating the effects of the other) interactions.51 While LMWH is traditionally thought to have the least potential PK interactions, recent data revealed that there could be relevant pharmacodynamic interactions.52 A retrospective study showed that the combination of VEGF tyrosine kinase inhibitors (TKIs) and FXa inhibitors (LMWH and DOAC) was associated with a significantly increased risk of bleeding episodes compared with FXa inhibitors alone (HR, 2.45; 95% CI, 1.28-4.69).53 Likewise, other studies also showed that combination of VEGF TKIs and anticoagulants (including DOACs, LMWH, and warfarin) was associated with an increased risk of bleeding events compared with VEGF TKIs alone.54,55
As regards to DOACs, a secondary analysis of the Caravaggio trial showed that concurrent anticancer therapies during the study period did not affect the rates of recurrent VTE or major bleeding on apixaban or dalteparin.56 However, heterogeneous types and durations of anticancer therapies were administrated, making it difficult to determine outcomes of specific combinations. Recently, the TacDOAC study evaluated the clinical outcomes of 202 patients with cancer taking concurrent selected anticancer TKIs and DOACs.57 In this study, the overall 6-month cumulative incidence of major bleeding was 4% (95% CI, 2%-8%), comparable to the findings from RCTs.58 However, there was a significant variation in the risks of bleeding depending on the type of concomitant anticancer therapies. Specifically, Bruton tyrosine kinase (BTK) inhibitors and VEGF inhibitors were associated with the highest risk of bleeding (6-month incidence of major bleeding with BTK inhibitors + DOACs: 10% [95% CI, 3%-21%] and VEGF inhibitors + DOACs: 7% [95% CI, 1%-21%]). Pharmacodynamic interactions, such as the off-target antiplatelet effects from BTK inhibitors, were thought to play a role. The study highlighted the importance of DDI considerations in selected high-risk combinations.
Major guidelines mainly focus on PK interactions and caution against the concurrent use of DOACs with dual strong cytochrome (CYP) and/or P-glycoprotein (P-gp) inhibitors or inducers.28,59 However, these recommendations are based on in vitro PK data, and their correlations with clinical outcomes remain largely unknown. As an example, tamoxifen is reported as an inhibitor of both CYP3A4 and P-gp pathways, and concurrent use of DOACs had been cautioned.60,61 However, a recent large population-based database analysis showed that in DOAC users with breast cancer, combination with tamoxifen was not associated with an elevated risk of bleeding complications compared to combination with an aromatase inhibitor (minimal involvement in the CYP/P-gp pathways), providing reassurance for concurrent use of tamoxifen and DOACs.62 More studies of specific anticancer therapies focusing on clinical outcomes are needed.
In our practice, we consider potential DDIs when an anticoagulant is prescribed by utilizing resources such as Lexicomp, Micromedex, University of Liverpool Drug Interaction Checker,63 package inserts, and so on. Collaboration with experienced pharmacists is highly valuable. As patients' clinical situations and cancer therapies evolve over time, we monitor and adjust plans accordingly.
Conclusion
In this article, we discussed several commonly asked questions regarding the use of FXa inhibitors, including obesity, renal impairment, GI cancer, catheter-related thrombosis, and drug-drug interactions. We aimed to summarize relevant literature and provide practical guidance for clinicians (Table 4). More research is welcome in many of these areas.
Summary of practice suggestions for the 5 frequently asked questions
| Issues/questions . | Our practice . |
|---|---|
| Obesity | • We use standard-dose rivaroxaban or apixaban for VTE treatment in patients with weight >120 kg or BMI >40 kg/m2. While we do not routinely monitor anti-Xa levels, we do consider it in patients with weight >150 kg or BMI >50 kg/m2 (when available), although this practice remains investigational. |
| Renal impairment | • We avoid using DOACs in patients with CrCl <15-30 mL/min. However, apixaban might be used with close monitoring in situations where no safer alternative anticoagulants are available. • Tinzaparin can be used (when available) in patients with CrCl >20 mL/min. |
| Gastrointestinal malignancy | • We avoid DOACs in patients with unresected luminal GI cancers due to the higher risk of GI bleeding. |
| Catheter-related thrombosis | • We tailor anticoagulant for catheter-related thrombosis in patients with cancer similarly as for proximal lower extremity DVT and PE based on patient characteristics, type of cancer, and anticancer treatments. |
| Drug-drug interactions | • We consider DDIs whenever an anticoagulant is prescribed by utilizing resources such as Lexicomp, Micromedex, package inserts, and pharmacists. |
| Issues/questions . | Our practice . |
|---|---|
| Obesity | • We use standard-dose rivaroxaban or apixaban for VTE treatment in patients with weight >120 kg or BMI >40 kg/m2. While we do not routinely monitor anti-Xa levels, we do consider it in patients with weight >150 kg or BMI >50 kg/m2 (when available), although this practice remains investigational. |
| Renal impairment | • We avoid using DOACs in patients with CrCl <15-30 mL/min. However, apixaban might be used with close monitoring in situations where no safer alternative anticoagulants are available. • Tinzaparin can be used (when available) in patients with CrCl >20 mL/min. |
| Gastrointestinal malignancy | • We avoid DOACs in patients with unresected luminal GI cancers due to the higher risk of GI bleeding. |
| Catheter-related thrombosis | • We tailor anticoagulant for catheter-related thrombosis in patients with cancer similarly as for proximal lower extremity DVT and PE based on patient characteristics, type of cancer, and anticancer treatments. |
| Drug-drug interactions | • We consider DDIs whenever an anticoagulant is prescribed by utilizing resources such as Lexicomp, Micromedex, package inserts, and pharmacists. |
Acknowledgments
Marc Carrier is the recipient of a Tier 1 Research Chair in Cancer and Thrombosis from the Department and Faculty of Medicine at the University of Ottawa.
Figures were created with BioRender.com.
Conflict-of-interest disclosure
Tzu-Fei Wang reports advisory board honoraria from Servier and Valeo and research funding to the institution from Leo Pharma.
Marc Carrier has received research funding from BMS, Pfizer, and Leo Pharma and honoraria from Bayer, Pfizer, BMS, Servier, and Leo Pharma.
Off-label drug use
Tzu-Fei Wang: nothing to disclose.
Marc Carrier: nothing to disclose.