Abstract
Outcomes of allogeneic hematopoietic cell transplantation (HCT) for patients with advanced acute leukemia and myelodysplastic syndromes (MDS) remain uncertain. All published series include the important and often not stated selection bias that influences outcome. Performance status, patient age, prompt donor availability, risk phenotype of the leukemia, and tumor burden all influence the decision-making process about HCT with active disease. In addition, patients with MDS do not achieve a true pre-HCT complete remission, and thus much less stringent measures are used to indicate suitability for allografting in that disease. Post-HCT maintenance or investigational approaches for tumor depletion may improve the outcomes.
Learning Objectives
Provide indications for transplantation of leukemia not in remission
Indicate conditional outcomes for leukemia not in remission
Introduction
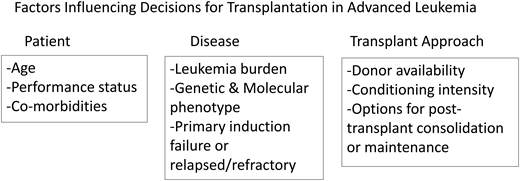
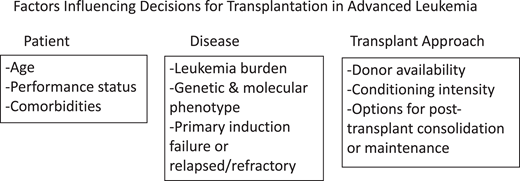
Outcomes of allogeneic hematopoietic cell transplantation (HCT) for patients with advanced acute leukemia remain uncertain. All published series include the important and often not stated selection bias that influences outcome. Therefore, it is often difficult to appreciate and interpret the most important and unreported findings indicating who did not receive a transplant.1-5 Factors influencing the decision about utilization of HCT and then outcome are shown in Figure 1.
Factors influencing decisions for transplantation in advanced leukemia.
Who receives an allogeneic transplant while not in remission
While allografting with persisting leukemia still is chosen as therapy for ~300 (of ~3200 allografts) patients yearly with acute myeloid leukemia (AML), it is much less common in acute lymphoblastic leukemia (ALL) (~30/year of ~1300 allografts).6
Those proceeding to allotransplantation while not in complete remission (CR) most often have certain well- recognized favorable characteristics. These include generally favorable performance status, limited disease burden, no active infections, good organ function, and usually younger age. Those undergoing transplantation in second but incomplete remission generally have had long initial remissions but failed to achieve CR with repeat reinduction. Modern widespread utilization of venetoclax plus decitabine, azacytidine, or cytarabine can yield good tumor reduction but sometimes persisting detectable (and measurable) residual disease (MRD).7 Earlier reports using these novel and highly effective induction regimens most often failed to note or formally present detailed data on MRD for those reported in CR. Depth of remission manifesting as incomplete recovery of peripheral counts may also affect outcomes post-HCT with CR with incomplete recovery and MRD each associated with adverse post-HCT outcomes (Figure 2).8
Independently adverse outcomes with CR with incomplete recovery and/or MRD. Adapted with permission from Percival et al.8
Independently adverse outcomes with CR with incomplete recovery and/or MRD. Adapted with permission from Percival et al.8
In contrast, for myelodysplastic syndromes (MDS), transplant with active disease is the rule, as CR is rarely a goal and rarely achieved prior to HCT. An often stated cutpoint for MDS is therapy to reduce the pre-HCT blast count to <5%—thus, gross residual disease is regularly apparent in HCT patients with MDS. High-risk cytogenetics and blast counts >10% predict poor outcomes for allogeneic HCT in MDS, but little refined data are available to determine a meaningful cutpoint for proceeding to HCT. Revised International Prognostic Scoring System cutpoints show higher post-HCT relapse risk and poorer overall survival for patients who score intermediate, high, or very high, reflecting cytopenias, blast counts, and cytogenetic characteristics independent of other HCT features.8-15 HCT remains a preferred therapy for those with advanced MDS (intermediate, high, and very high Revised International Prognostic Scoring System), although in population-based studies, patients with advanced disease were less often able to receive an allograft.13
Measurable residual disease prior to HCT
Outcomes of allografting with MRD are also difficult to interpret.15-18 Relevant covariates include sensitivity of the MRD testing. Certain mutations, of course, are also more easily detected as always pathogenic (NPM1 [nucleophosmin 1] mutated) vs those quantitative allelic ratio tumor burden measures such as AML with FLT3 ITD (FMS-like tyrosine kinase interval tandem duplication). Assays using flow cytometry for aberrant antigen expression or recognition of the original diagnostic phenotype may be valuable but less sensitive than molecular assays and depend on the breadth of the antigen panel tested and the sensitivity of the flow cytometry analysis. Some highly sensitive MRD measures (sequence-based analysis or digital droplet polymerase chain reaction [PCR]) may identify many more patients with residual disease than flow cytometry or fluorescence in situ hybridization or even conventional PCR.19-24 Use of these different assays, therefore, may influence the number of MRD-negative vs MRD-positive patients, even among those in clinical or cytomorphologic CR. This has been recently evaluated in a formal position paper by the European Leukemia Net and related commentaries.25
Conditioning regimen intensity and outcome
Persisting MRD may be overcome (or not) by the planned pretransplant conditioning intensity and perhaps yield different outcomes using reduced intensity regimens of different composition. The BMT CTN 0901 prospective randomized trial of myeloablative vs reduced intensity conditioning (RIC) HCT demonstrated improved outcome for those in CR.26 However, when recategorized by retesting available samples using targeted next-generation sequencing with focused validation by a highly sensitive droplet digital PCR, the myeloablative conditioning (MAC) regimen was superior only for those with persisting pre-HCT MRD, suggesting some clinical misclassification of negative MRD by the original clinical assays.27 In contrast, these “truly” MRD-negative patients had similar outcomes with either MAC or RIC transplantation.
Only a modest number of patients with active disease proceed to transplantation using RIC.28 However complete remission with detectable MRD happens more frequently. Pre-HCT conditioning intensity is often chosen by the patient's age, performance status, and comorbidity rather than the residual disease burden. Some series have compared melphalan-based RIC (most often fludarabine/melphalan) to alternatives, often with busulfan.29-32
A recent Center for International Blood and Marrow Transplant Research analysis using highly sensitive MRD measures demonstrated a favorable outcome using fludarabine/melphalan for MRD-positive allografts compared with all other RIC regimens. More widely available and less sensitive assays blur the distinction between true MRD-negative and MRD-positive pre-HCT status. Therefore, outcomes reported with less sensitive assays emphasize each MRD assay's internal validity but confound the comparability of these newer data to earlier reports.
How difficult it was to get a transplant or to achieve CR and its influence on HCT outcomes
Importantly, the extent of therapy prior to a decision to proceed to allotransplantation while not in CR may also influence the reported outcomes. Low-level residual disease after only 2 induction cycles is likely more amenable to a promising outcome then gross persisting relapse after 3 to 4 or more cycles of therapy or trials of investigational treatments prior to transplantation. Little data are available to guide this decision-making, which amplifies the selection bias of those reporting HCT with persisting leukemia.
Some specifics can help guide decision-making about transplantation with active leukemia.33 A large series from the Center for International Blood and Marrow Transplant Research published some years ago reported 16% (ALL) and 19% (AML) 3-year survival for allografts during relapse (Figure 3).34 In multivariate analysis, outcomes for AML HCT in relapse were worse if the first CR duration was shorter than 6 months or there were persisting circulating blasts, use of a donor other than a human leukocyte antigen (HLA)-identical sibling, a Karnofsky or Lansky score <90, and poor-risk cytogenetics. ALL outcomes were worse in first refractory or second or greater relapse or when there were ≥25% marrow blasts, a cytomegalovirus-seropositive donor was used, and in recipients aged ≥10 years.
Survival after HCT for advanced AML based on 5 risk factors: first CR duration <6 months, circulating blasts, non-HLA-identical sibling donor, performance score <90%, and poor-risk cytogenetics. CI, confidence interval. Adapted with permission from Duval et al.33
Survival after HCT for advanced AML based on 5 risk factors: first CR duration <6 months, circulating blasts, non-HLA-identical sibling donor, performance score <90%, and poor-risk cytogenetics. CI, confidence interval. Adapted with permission from Duval et al.33
A more recent series addressing pre-HCT therapy and its influence on post-HCT outcome has analyzed the extent of prior treatment and time from diagnosis to transplantation for patients with primary induction failure (PIF).35 After MAC HCT for AML, survival was 1.3-fold better for those in first CR (CR1) after 1 cycle than for those requiring more than 2 cycles to CR1 (P < .01) and 1.47-fold better than with those with ≥3 cycles (P < .01).
This poorer survival in those requiring added therapy to achieve CR1 was a consequence of higher post-HCT relapse risk (1.65-fold greater) in patients needing ≥3 cycles to achieve CR (P < .01). However, after RIC allo-HCT, the number of induction cycles did not influence overall survival (OS), yet compared with CR1 achieved in 1 cycle, relapse risk after RIC HCT was 1.24- to 1.41-fold greater in patients receiving either 2 or ≥3 cycles to CR1.35
Importantly, outcomes for patients with AML undergoing transplantation during PIF yielded only limited survival beyond 2 years. However, allograft outcomes during PIF were still acceptable for some patients. Recognizing the selection influencing which patients proceeded to HCT with persisting leukemia, after MAC HCT in PIF, 3-year probability of OS was 30% (95% confidence interval, 25.1%-35.1%), and after RIC, the 3-year probability of OS was 26.3% (95% confidence interval, 19.1%-34.2%; P = .43). However, transplantation in complete remission even requiring numerous cycles to achieve CR was always favorable to transplantation during PIF. After MAC HCT, 5-year survival was only 25% in PIF patients vs 46% to 59% for those in CR after 1, 2, or 3+ cycles. After RIC, 1-year survival (too few to analyze at 5 years) was 49.8% for PIF vs 57% to 65% for those in CR.
Summary and application to clinical decision-making
Allogeneic transplantation for acute leukemia in relapse still has potential value but only for a restricted population. Younger patients with good performance status and with only a small tumor burden may have acceptable although still unfavorable outcomes using myeloablative conditioning and with a well-matched donor available at the proper time. This must be balanced against the toxicity of further reinduction attempts, which can compromise performance status and associated extended neutropenia yielding difficult infections—either of which might increase non-relapse mortality. Thus, HCT with some reduced but persisting disease burden may be justified, but selectively. RIC has limited value for those in relapse or those with MRD detectable in their pretransplant marrow assessments. Novel and more potent antileukemic tools must be added to the backbone of an RIC HCT for those with persisting disease.
The traditional model implied that conditioning and graft vs leukemia would encompass all the antileukemic benefit of an allograft. However, for patients with persisting morphologic or molecularly measurable disease, augmented induction or conditioning intensity,36 posttransplant consolidation or maintenance therapy,37-42 or immunotherapy42 may improve outcomes. Yet today, we do not have clear data outlining a data-driven clinical algorithm. These options all require careful study and objective comparability of patients' relapse risk to assess the value of these new antileukemic interventions. There may be more to add to an allograft, but new measures need disciplined evaluation to prove their value in reducing relapse risks and expanding the curative potential of transplantation.
Conflict-of-interest disclosure
Daniel Weisdorf has no competing financial interests to declare.
Off-label drug use
Daniel Weisdorf: nothing to disclose.