Abstract
Anti-CD20 monoclonal antibodies (mAbs) have revolutionized the treatment of chronic lymphocytic leukemia (CLL) by improving survival of patients with CLL in conjunction with chemotherapy. However, the novel targeted agents such as Bruton tyrosine kinase inhibitors (BTKis) and venetoclax have now mostly replaced chemotherapy in frontline treatment of CLL. Several clinical trials have been conducted to examine the role of anti-CD20 mAbs in combination with BTK inhibitors and venetoclax. Addition of rituximab to ibrutinib does not improve progression-free survival (PFS) of treatment-naive patients with CLL, possibly related to ibrutinib's antagonistic effect on anti-CD20 antibodies. Alternatively, addition of a glycoengineered anti-CD20 mAb obinutuzumab to a more selective BTKi acalabrutinib may improve PFS but does not improve overall survival of patients with CLL in the frontline setting, pending long-term follow-up. Thus, we suggest that the addition of an anti-CD20 mAb to a BTKi is of most benefit to patients with autoimmune cytopenia or rapidly progressive disease. In contrast to BTKis, combination of fixed-duration venetoclax and anti-CD20 mAb can induce deep remission with high rates of undetectable minimal residual disease, correlating with improved survival of patients with CLL in both frontline and relapsed/refractory settings. In this review, we discuss clinical trials of BTKis and venetoclax that have investigated the role of anti-CD20 mAbs in frontline and relapsed settings of CLL treatment. We also provide an algorithm suggesting how anti-CD20 mAbs may be incorporated in the treatment of patients with CLL, including specific scenarios.
Learning Objectives
Anti-CD20 monoclonal antibody plus BTK inhibitor may provide clinical benefit in patients with active autoimmune cytopenia or rapidly progressive disease.
Adding anti-CD20 antibody to venetoclax induces deep remissions, allowing for fixed-duration treatment.
CLINICAL CASE
Mr. Smith, a 67-year-old man, was diagnosed with chronic lymphocytic leukemia (CLL) 3 years ago. He was asymptomatic, without cytopenias, and was observed. Three months ago, he had purpura and thrombocytopenia. Evaluation revealed immune thrombocytopenic purpura (ITP). Over the course of 2 months, his ITP was refractory to treatment with steroids and intravenous immunoglobulin. Today, Mr. Smith reports drenching night sweats, fatigue, and early satiety. Laboratory tests show anemia and thrombocytopenia. He has refractory ITP and meets criteria for CLL treatment.
Introduction
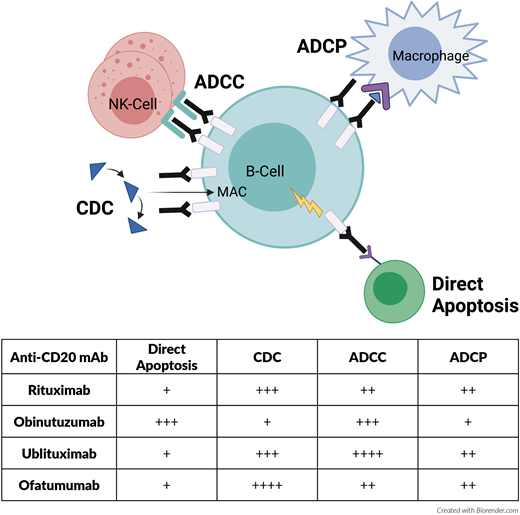
Anti-CD20 monoclonal antibodies (mAbs) have been integrated in treatment of CLL in combination with chemotherapy for longer than a decade. Rituximab is a chimeric type I antibody that eradicates CLL cells primarily by means of complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) after binding to CD20 (Figure 1).1 Addition of rituximab to the standard chemotherapy agents fludarabine and cyclophosphamide (FCR) improved overall survival (OS) and progression-free survival (PFS) compared with fludarabine and cyclophophamide in the frontline treatment of patients with CLL. This combination became a standard option for fit patients.2 Although bendamustine (another standard chemotherapy agent) with or without rituximab was not directly compared in a head-to-head clinical trial, bendamustine plus rituximab (BR) showed favorable overall response rates (ORRs) and PFS compared with bendamustine alone in treatment-naive patients with CLL in an imprecise cross-trial comparison.3,4 The direct comparison of FCR to BR for treatment-naive fit patients with CLL concluded, though, that BR was better tolerated in patients older than 65 years and is considered an option for this population.5 Obinutuzumab is a potent humanized, glycoengineered type II antibody that also targets CD20 on the cell surface but with enhanced ADCC, antibody-dependent phagocytosis, and direct cell death effects compared with rituximab (Figure 1).6,7 In treatment-naive patients with CLL with preexisting conditions, rituximab or obinutuzumab in combination with chlorambucil was compared head-to-head.8 The combination of chlorambucil and obinutuzumab (Chl-O) resulted in higher complete remission (CR) rates, PFS, and OS compared with the rituximab combination, providing evidence to support the superiority of obinutuzumab compared with rituximab.8,9 Based on these pivotal studies, the use of anti-CD20 mAbs has a firmly established role in the frontline treatment of CLL when used in combination with standard chemotherapy agents. Over the past few years, the standard of care has shifted from standard chemotherapy agents to using targeted kinase inhibitors, such as Bruton tyrosine kinase inhibitors (BTKis) or B-cell lymphoma 2 (BCL2) inhibitors. It is not clear whether anti-CD20 mAbs are required for efficacy when used in combination with these targeted kinase inhibitors. In this study, we review the available data surrounding anti-CD20 mAb combinations in CLL treatment.
Mechanism of action of select anti-CD20 monoclonal antibodies used to treat CLL. More “+” sign indicates stronger mechanism of action. ADCP, antibody-dependent cellular phagocytosis; CDC, complement dependent cytotoxicity; MAC, membrane attack complex.
Mechanism of action of select anti-CD20 monoclonal antibodies used to treat CLL. More “+” sign indicates stronger mechanism of action. ADCP, antibody-dependent cellular phagocytosis; CDC, complement dependent cytotoxicity; MAC, membrane attack complex.
BTK Inhibitors
BTK is a kinase within the TEC family that leads to downstream activation of AKT, extracellular signal–regulated kinase, and nuclear factor κ light-chain enhancer of activated B cells, pathways important for malignant B cells' growth and survival.10-12 Ibrutinib is an irreversible BTKi that covalently binds to the cysteine 481 amino acid of the BTK enzyme. It has shown significant activity in patients with CLL in both frontline and relapsed/refractory (R/R) settings.13,14 Because of the durability of responses with BTKis as monotherapy, there is a question of whether there is a need for an anti-CD20 mAb in conjunction to improve clinical efficacy.
Ibrutinib ± rituximab or obinutuzumab
The E1912 trial compared FCR to ibrutinib plus rituximab (IR) in treatment-naive patients younger than 70 years (Table 1).15 This study demonstrated prolonged PFS and OS of patients receiving IR compared with FCR. Results of this trial led to approval of the IR combination in frontline treatment of CLL, but this study did not answer whether addition of rituximab to ibrutinib is necessary to obtain similar clinical benefit.
Frontline CLL studies with BTKi ± anti-CD20 monoclonal antibody
| Study . | Targeted regimen . | Comparator . | N (targeted regimen) . | ORR, % . | CRR, % . | uMRD, % . | PFS . | OS . | Notable AEs . | Median follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| E191215 | IR | FCR | IR = 354 | 95 | 17 | 8 (*n = 276) | NR (3-y 89%) | NR (3-y 98%) | Grade ≥3 infections: FCR = 20.3% vs IR = 10.5%; grade ≥3 HTN: FCR = 8.2% vs IR = 18.8% | 33.6 |
| A04120216 | I ± R | BR | I = 182 IR = 182 | I = 93 IR = 94 | I = 7 IR = 12 | I = 1 IR = 4 | I = NR (2-y 87%) IR = NR (2-y 88%) | I = NR (2-y 90%) IR = NR (2-y 94%) | Grade ≥3 neutropenia: IR = 21% vs I = 15% | 38 |
| NCT0200704417 | IR | Ibrutinib | I = 104 IR = 104 TN = 27 RR = 181 | I = 92.3 IR = 92.3 | I = 20.2 IR = 26 | NA | I = NR (3-y 86%) IR = NR (3-y 86.9%) | I = NR (3-y 89%) IR = NR (3-y 92%) | No significant difference in toxicity profile | 36 |
| iLLUMINATE18 | IO | Chl-O | IO = 113 | 88 | 19 | 35 (*n = 113) | NR (30-mo 79%) | NR (30-mo 86%) | SAEs in 58% of IO and 35% of Chl-O; obinutuzumab-related SAEs (febrile neutropenia, thrombocytopenia) in 15% of the IO arm | 31.3 |
| ELEVATE TN19 | A ± O | Chl-O | A = 179 AO = 179 | A = 86 AO = 94 | A = 1 AO = 13 | A = 7% of those in CR/CRi (*n = 14) AO = 56% of those in CR/CRi (*n = 43) | A = NR (2-y 87%) AO = NR (2-y 93%) | A = NR (2-y 95%) AO = NR (2-y 95%) | Grade ≥3 neutropenia: AO = 30% vs A = 10%; grade ≥3 infections: AO = 21% vs A = 14% | 28.3 |
| Study . | Targeted regimen . | Comparator . | N (targeted regimen) . | ORR, % . | CRR, % . | uMRD, % . | PFS . | OS . | Notable AEs . | Median follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| E191215 | IR | FCR | IR = 354 | 95 | 17 | 8 (*n = 276) | NR (3-y 89%) | NR (3-y 98%) | Grade ≥3 infections: FCR = 20.3% vs IR = 10.5%; grade ≥3 HTN: FCR = 8.2% vs IR = 18.8% | 33.6 |
| A04120216 | I ± R | BR | I = 182 IR = 182 | I = 93 IR = 94 | I = 7 IR = 12 | I = 1 IR = 4 | I = NR (2-y 87%) IR = NR (2-y 88%) | I = NR (2-y 90%) IR = NR (2-y 94%) | Grade ≥3 neutropenia: IR = 21% vs I = 15% | 38 |
| NCT0200704417 | IR | Ibrutinib | I = 104 IR = 104 TN = 27 RR = 181 | I = 92.3 IR = 92.3 | I = 20.2 IR = 26 | NA | I = NR (3-y 86%) IR = NR (3-y 86.9%) | I = NR (3-y 89%) IR = NR (3-y 92%) | No significant difference in toxicity profile | 36 |
| iLLUMINATE18 | IO | Chl-O | IO = 113 | 88 | 19 | 35 (*n = 113) | NR (30-mo 79%) | NR (30-mo 86%) | SAEs in 58% of IO and 35% of Chl-O; obinutuzumab-related SAEs (febrile neutropenia, thrombocytopenia) in 15% of the IO arm | 31.3 |
| ELEVATE TN19 | A ± O | Chl-O | A = 179 AO = 179 | A = 86 AO = 94 | A = 1 AO = 13 | A = 7% of those in CR/CRi (*n = 14) AO = 56% of those in CR/CRi (*n = 43) | A = NR (2-y 87%) AO = NR (2-y 93%) | A = NR (2-y 95%) AO = NR (2-y 95%) | Grade ≥3 neutropenia: AO = 30% vs A = 10%; grade ≥3 infections: AO = 21% vs A = 14% | 28.3 |
*Of those evaluable for uMRD in the bone marrow/peripheral blood.
A, acalabrutinib; AE, adverse event; CRi, complete response with incomplete count recovery; CRR, complete response rate; HTN, hypertension; I, ibrutinib; IO, ibrutinib and obinutuzumab; NA, not available; NR, not reached; RR, relapsed/refractory; SAE, serious adverse event; TN, treatment-naive.
The A041202 study evaluated frontline therapy with ibrutinib vs IR vs BR in patients with CLL 65 years or older.16 This study demonstrated prolonged PFS with ibrutinib-based treatments compared with BR. However, there was no difference in PFS and OS benefits between ibrutinib alone and the IR combination. Rates of CR (12% vs 7%) and undetectable minimal residual disease (uMRD) (4% vs 1%) were only marginally higher with the IR combination. The safety profile of both ibrutinib alone and the IR combination was similar except for slightly higher grade 3 or more neutropenia with IR. Another study (NCT02007044) comparing ibrutinib vs IR in treatment-naive (13% of total) and R/R (87% of total) settings also failed to detect improvement in PFS with IR but concluded that time to CR was faster (11.5 vs 22.2 months), and patients had lower levels of residual disease as assessed by flow cytometry with the IR combination.17 Considering the existing data and continuous application of BTKis, the significance of uMRD and its relation to long-term disease eradication has not been well established. Based on these 2 studies, addition of rituximab to ibrutinib does not seem to provide any clinically meaningful survival benefit to most patients with treatment-naive CLL. In a preclinical study, ibrutinib's off-target inhibition of interleukin 2 inducible tyrosine kinase led to downregulation of natural killer (NK) cell-mediated ADCC and impaired antibody-dependent phagocytosis, both of which are known anti-CD20 mAb mechanisms of action (Figure 1).20,21 This finding has been hypothesized as one of the explanations for the lack of significant survival benefit with the addition of rituximab to ibrutinib.
The iLLUMINATE study compared frontline CLL therapy of ibrutinib plus obinutuzumab vs Chl-O.18 This study demonstrated prolonged PFS with the ibrutinib-based regimen in treatment-naive patients with CLL. Adding obinutuzumab to ibrutinib deepened responses (35% with uMRD), but serious adverse events were more common in this arm. This study led to Food and Drug Administration (FDA) approval for marketing of the ibrutinib and obinutuzumab combination in the frontline setting. However, no prospective head-to-head comparisons of ibrutinib vs ibrutinib plus obinutuzumab have been done, leaving the question of the clinical significance of obinutuzumab's addition to ibrutinib unanswered.
Acalabrutinib ± obinutuzumab
Acalabrutinib is a highly selective BTKi with less off-target inhibition, including inducible tyrosine kinase, compared with ibrutinib.22,23 In preclinical studies, acalabrutinib did not inhibit anti-CD20 mAb-dependent NK-cell mediated cytotoxicity.22 The phase 3 ELEVATE treatment-naive (TN) study was designed to answer whether acalabrutinib is superior to chemoimmunotherapy (Chl-O) in terms of PFS in the frontline treatment of CLL. It also evaluated whether there is a benefit of adding a more potent anti-CD20 mAb obinutuzumab to acalabrutinib (AO), although the study was not powered to detect this difference.19 As expected, patients who received acalabrutinib ± obinutuzumab had improved PFS compared with Chl-O. After 28 months of follow-up, the 2-year PFS rates were not clinically or statistically different between the AO and acalabrutinib (93% vs 87%) groups. More patients with AO had CR (13% vs 1%) and uMRD (56% vs 7% of those evaluated) compared with acalabrutinib alone. AO resulted in higher rates of grade 3 or higher neutropenia, infections, and infusion reactions compared with acalabrutinib alone. Findings from this study ultimately led to FDA approval for marketing of acalabrutinib ± obinutuzumab for frontline treatment of patients with CLL.
As we await the long-term results of the ELEVATE TN study, the mature data from the original phase 2 study of single-agent acalabrutinib in treatment-naive CLL are encouraging.24 After a median follow-up of 53 months, median event-free survival and duration of response are not reached with a very high ORR of 97% (7% CR, 90% partial response). In addition, 86% of patients are still receiving treatment, and only 6% have discontinued due to adverse events. The first data describing a head-to-head comparison of acalabrutinib and ibrutinib in patients with R/R CLL were presented at the American Society of Clinical Oncology meeting in 2021 (ELEVATE Relapsed/Refractory (RR) Study).25 This study found that acalabrutinib was noninferior to ibrutinib in terms of PFS and had a better toxicity profile—importantly, a lower incidence of atrial fibrillation and hypertension.25 This improved toxicity profile makes acalabrutinib a very appealing treatment option for treatment-naive patients with CLL, especially those who are elderly or have multiple medical comorbidities.
Summary
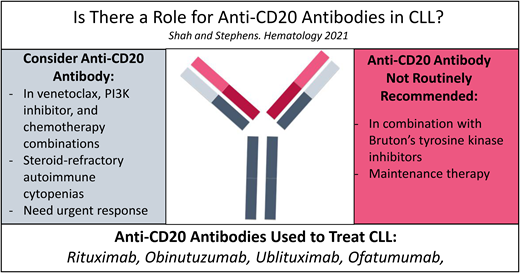
Of the described trials that compared a BTKi plus anti-CD20 mAb vs BTKi alone, none demonstrated a clinically meaningful superiority of the anti-CD20 mAb combination in terms of improved PFS or OS. The depth of response (CR and uMRD rates) and the speed of response (time to CR) are marginally improved with the addition of an anti-CD20 mAb. As such, there are 2 scenarios in which addition of an anti-CD20 mAb to a BTKi may be clinically meaningful. The first scenario is in the presence of an active and steroid-refractory autoimmune cytopenia such as ITP or autoimmune hemolytic anemia. Both rituximab and BTKi have been effective as monotherapy in control or resolution of autoimmune cytopenia related to CLL.26-31 Thus, a combination might lead to deeper and more rapid control of autoimmune cytopenia, especially in the steroid-refractory patients. The second scenario, in which the upfront addition of a mAb to BTKi could be helpful, is in patients needing rapid control of disease (eg, lymphadenopathy endangering a critical organ). From a practical standpoint, BTKis can take time to be authorized by insurance and are not often included on inpatient hospital formularies secondary to the high cost of these therapies. Anti-CD20 mAbs are often readily available and can expedite treatment for patients who need a rapid response. For all other patients, we recommend the use of ibrutinib or acalabrutinib alone in frontline treatment of CLL.
CLINICAL CASE (continued)
Mr. Smith received 6 cycles of obinutuzumab and continuous oral acalabrutinib and achieved clinical remission. Three years later, he sought treatment from the clinic with a progressive return of drenching night sweats and decline of hemoglobin. No evidence of hemolysis was detected. A next-generation sequencing panel showed a mutation of cysteine to serine at the 481 moiety of BTK (C481S).
Venetoclax + rituximab
Venetoclax is an oral, highly selective BCL2 homology domain 3-mimetic that binds to BCL2, resulting in initiation of apoptosis mediated by BCL2-associated protein X and BCL2-antagonist/killer in primed cells.32 Single-agent venetoclax showed promising activity in patients with R/R CLL who had previously progressed on ibrutinib or idelalisib or had 17p deletion.33-35 However, the combination of venetoclax with rituximab was found to be able to overcome microenvironment-induced resistance to venetoclax in preclinical studies, leading to its further investigation in patients with R/R CLL.36 In the phase 1 study of venetoclax plus rituximab (V-R) in R/R CLL, no significant additional toxicities were found with addition of rituximab. The combination resulted in a 2-year PFS of 82% with a 51% CR rate and 57% of the patients achieving uMRD.37 The depth and durability of the response observed with V-R popularized the concept of a time-limited course in the treatment of patients with CLL, a treatment strategy to avoid costs and toxicity from continuous drug exposure.
The phase 3 randomized MURANO trial compared the time-limited 2-year course of V-R with BR in patients with R/R CLL (Table 2).38 Rates of 4-year PFS (57% vs 5%) and OS (85% vs 67%) were higher with V-R compared with BR.39 At the end of combination therapy, 62.4% of patients with V-R had uMRD compared with 13.3% of those with BR, and this end point was associated with superior PFS in both groups. Based on the results of the MURANO study, the combination of fixed-duration V-R has been approved for treatment in patients with R/R CLL. No randomized study comparing venetoclax vs venetoclax plus rituximab has been reported. Due to the time-limited nature of this regimen, the deep responses are more desirable, and as such, the V-R combination has become standard of care when used in the R/R setting.
Select pivotal CLL studies with venetoclax + anti-CD20 monoclonal antibody
| Study . | Population . | Targeted regimen . | Comparator . | N (targeted regimen) . | ORR, % . | CRR, % . | uMRD, % . | PFS . | OS . | Notable AEs . | Median follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MURANO37-43 | R/R | V-R | BR | 194 | 92 | 27 | 62 (*n = 194) Peripheral blood | 53.6 mo | NR (5-y 82.1%) | More grade ≥3 neutropenia with V-R; more grade ≥3 febrile neutropenia and infections with BR | 59.2 |
| CLL1439-44 | Treatment naive | V-O | Chl-O | 216 | 85 | 50 | 76 (*n = 216) Peripheral blood | NR (4-y 74%) | NR (4-y 85%) | Grade ≥3 neutropenia (53%) and thrombocytopenia (13%) of V-O | 52.4 |
| Study . | Population . | Targeted regimen . | Comparator . | N (targeted regimen) . | ORR, % . | CRR, % . | uMRD, % . | PFS . | OS . | Notable AEs . | Median follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MURANO37-43 | R/R | V-R | BR | 194 | 92 | 27 | 62 (*n = 194) Peripheral blood | 53.6 mo | NR (5-y 82.1%) | More grade ≥3 neutropenia with V-R; more grade ≥3 febrile neutropenia and infections with BR | 59.2 |
| CLL1439-44 | Treatment naive | V-O | Chl-O | 216 | 85 | 50 | 76 (*n = 216) Peripheral blood | NR (4-y 74%) | NR (4-y 85%) | Grade ≥3 neutropenia (53%) and thrombocytopenia (13%) of V-O | 52.4 |
Of those evaluable for uMRD in the bone marrow/peripheral blood.
CRR, complete response rate; NR, not reached.
Venetoclax + obinutuzumab
Alternatively, in the frontline treatment of CLL, venetoclax has been standardly paired with obinutuzumab (V-O). The phase 3 CLL14 study compared frontline CLL therapy with a fixed 1-year duration of V-O vs Chl-O.40 Patients who received V-O had significantly longer PFS than patients receiving Chl-O (not reached vs 35.6 months).41 Of the patients who received V-O, 76% achieved uMRD compared with 35% for the Chl-O group 3 months after completion of therapy. Based on this study, V-O has been approved by the FDA for marketing as a fixed-duration therapy for treatment-naive patients with CLL.
Summary
Although the evidence from a direct randomized study is lacking, combination of an anti-CD20 mAb and venetoclax in CLL allows for time-limited therapy by achieving clinically relevant improvement in PFS, OS, and uMRD rates. Thus, we recommend a fixed course of V-R for 2 years in the R/R setting and a fixed course of V-O in the frontline setting.
Umbralisib + ublituximab
Ublituximab is a type I glycoengineered anti-CD20 mAb with enhanced ADCC that targets a unique epitope on CD20 (Figure 1). Ublituximab has been recently combined with umbralisib, a dual inhibitor of phosphoinositide 3-kinase δ (PI3Kδ) and casein kinase 1ε. In the phase 3 UNITY CLL trial, this combination (U2 ) yielded improved 2-year PFS of 76.6% compared with 52.1% with Chl-O in treatment-naive patients and 2-year PFS of 41.3% with U2 compared with 24.8% with Chl-O in patients with R/R CLL.42 The safety profile of umbralisib was favorable compared with historical data with other PI3K inhibitors. As such, the U2 combination will likely be submitted for evaluation of FDA approval within the next few years.
CLINICAL CASE (continued)
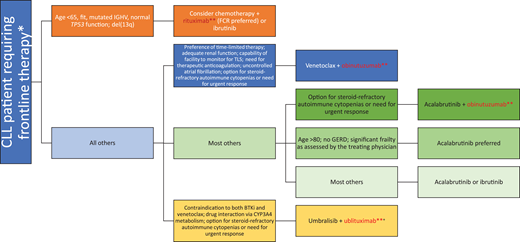
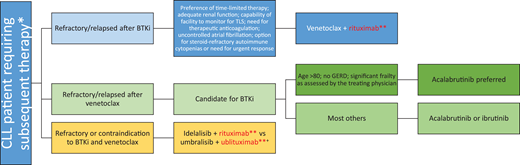
Mr. Smith received V-R therapy, which led to another clinical remission. There is some question whether maintenance with anti-CD20 mAbs can improve clinical outcomes. Ofatumumab, a type I humanized anti-CD20 mAb with a better complement- dependent cytotoxicity profile compared with rituximab (Figure 1), is approved for maintenance therapy after chemoimmunotherapy in patients with R/R CLL but has not been widely accepted into clinical practice due to the emergence of novel agents; we do not routinely recommend its utilization.43 Our algorithm on incorporation of anti-CD20 mAbs for both frontline and R/R CLL is summarized in Figures 2 and 3. Given the multiple choices for therapy, a clinical trial is always preferable for patients who qualify. However, we have limited our algorithms to currently available therapies. If using chemotherapy for a young patient (aged <65 years) with good prognostic factors, FCR can be considered. If a patient has steroid-refractory autoimmune cytopenia or a need for an urgent response, a combination of a BTKi and an anti-CD20 mAb can be considered. Due to ibrutinib's suspected antagonism of an anti-CD20 mAb mechanism, we would favor an acalabrutinib backbone with an anti-CD20 mAb. A fixed 1-year course of V-O would also be acceptable in this scenario. In all other patients, we recommend monotherapy with a BTKi based on the patient's comorbidities (eg, acalabrutinib is preferred for frail/elderly patients due to a favorable toxicity profile and should be avoided in patients with significant gastroesophageal reflux disease requiring proton pump inhibitors due to drug interaction) or V-O combination in frontline treatment. In the R/R setting for patients with CLL with previous intolerance or progression on a BTKi, we would favor 2-year fixed treatment with a V-R combination. For those patients with venetoclax-refractory disease, we would use monotherapy with a BTKi. Idelalisib plus rituximab is also approved in the R/R CLL setting, but utilization of this PI3K inhibitor requires careful management of toxicities. The combination of umbralisib and ublituximab is an emerging option in patients who are not able to use BTKis or venetoclax in both frontline and R/R settings, but FDA approval for marketing is pending.
How to incorporate anti-CD20 monoclonal antibody into frontline treatment for patients with CLL. *All eligible patients should be considered for participation in clinical trials if available. **Anti-C20 monoclonal antibody. +Pending Food and Drug Administration approval for marketing for CLL at time of submission. GERD, gastroesophageal reflux disease; IGHV, immunoglobulin variable heavy chain; TLS, tumor lysis syndrome.
How to incorporate anti-CD20 monoclonal antibody into frontline treatment for patients with CLL. *All eligible patients should be considered for participation in clinical trials if available. **Anti-C20 monoclonal antibody. +Pending Food and Drug Administration approval for marketing for CLL at time of submission. GERD, gastroesophageal reflux disease; IGHV, immunoglobulin variable heavy chain; TLS, tumor lysis syndrome.
How to incorporate anti-CD20 monoclonal antibody into subsequent lines of treatment for patients with CLL. *All eligible patients should be considered for participation in clinical trials if available. **Anti-C20 monoclonal antibody. +Pending Food and Drug Administration approval for marketing for CLL at time of submission. GERD, gastroesophageal reflux disease; IGHV, immunoglobulin variable heavy chain; TLS, tumor lysis syndrome.
How to incorporate anti-CD20 monoclonal antibody into subsequent lines of treatment for patients with CLL. *All eligible patients should be considered for participation in clinical trials if available. **Anti-C20 monoclonal antibody. +Pending Food and Drug Administration approval for marketing for CLL at time of submission. GERD, gastroesophageal reflux disease; IGHV, immunoglobulin variable heavy chain; TLS, tumor lysis syndrome.
Conclusion
Addition of an anti-CD20 mAb to chemotherapy is considered standard in the treatment of CLL. However, with the emergence of potent targeted agents such as BTKis and venetoclax, the question of the clinical impact of mAbs has been raised. Several phase 3 trials have shown that addition of an anti-CD20 mAb to ibrutinib does not improve the PFS or OS of patients with CLL, possibly related to ibrutinib's off-target effects. Long-term follow-up of the acalabrutinib plus obinutuzumab combination is awaited. On the contrary, combination of venetoclax and an anti-CD20 mAb leads to deep remissions with uMRD, allowing for fixed-duration therapy and ultimately correlating with improved survival. The role of anti-CD20 mAbs remains important for patients with CLL who have active autoimmune cytopenia or need urgent response regardless of a combination targeted therapy being used. Novel anti-CD20 mAbs such as ublituximab are further being investigated in combination with novel agents. As a whole, treating physicians have a strong armamentarium to combat CLL.
Acknowledgment
D.S. is supported by NIH/NCI Grant K23 CA212271.
Conflict-of-interest disclosure
Harsh R. Shah has received research funding from Epizyme.
Deborah M. Stephens has received research funding from Acerta, Gilead, Karyopharm, Verastem, JUNO, Arqule, and Mingsight. She has served on advisory boards for Innate, Jannsen/Pharmacyclics, Epizyme, Karyopharm, Beigene, Adaptive, AstraZeneca, and TG Therapeutics.
Off-label drug use
Harsh R. Shah: Ublituximab and umbralisib are discussed and neither drug is yet FDA approved.
Deborah M. Stephens: Ublituximab and umbralisib are discussed and neither drug is yet FDA approved.