Abstract
Arterial thrombotic events in younger patients without a readily apparent etiology present significant diagnostic and management challenges. We present a structured approach to diagnosis with consideration of common causes, including atherosclerosis and embolism, as well as uncommon causes, including medications and substances, vascular and anatomic abnormalities, systemic disorders, and thrombophilias. We highlight areas of management that have evolved within the past 5 years, including the use of dual-pathway inhibition in atherosclerotic disease, antithrombotic therapy selection in embolic stroke of undetermined source and left ventricular thrombus, the role of closure of patent foramen ovale for secondary stroke prevention, and the thrombotic potential of coronavirus disease 2019 infection and vaccination. We conclude with a representative case to illustrate the application of the diagnostic framework and discuss the importance of consideration of bleeding risk and patient preference in determining the appropriate management plan.
Learning Objectives
Perform a structured evaluation of patients with unexplained arterial thrombosis, including consideration of 6 categories of causes
Select appropriate antithrombotic therapy and other necessary interventions to prevent recurrent arterial thrombotic events
CLINICAL CASE
A 36-year-old woman with no medical history sought treatment for acute-onset left facial droop and arm weakness. Computed tomography angiography (CTA) revealed occlusion of the right middle cerebral artery at the M2 segment. She underwent endovascular thrombectomy, resulting in complete reperfusion. Given the patient's young age and no apparent risk factors for stroke, hematology was consulted to assist in the workup of stroke etiology and to determine a management plan for secondary stroke prevention.
Introduction
Arterial thrombosis is a leading cause of morbidity and mortality worldwide.1 The most common forms of arterial thrombosis, ischemic heart disease (including acute myocardial infarction), and ischemic stroke are managed by cardiology and neurology, respectively. However, when arterial events in these sites or in uncommon anatomic locations occur without a readily apparent etiology, particularly in the younger patient, hematologists may be called upon to assist in evaluation and management.
Herein, we present a systematic approach to the diagnostic evaluation and management of patients with unexplained arterial thrombosis while also highlighting areas where the literature guiding diagnosis and/or treatment of specific sites of arterial thrombosis has evolved in the past 5 years.
Defining the clot
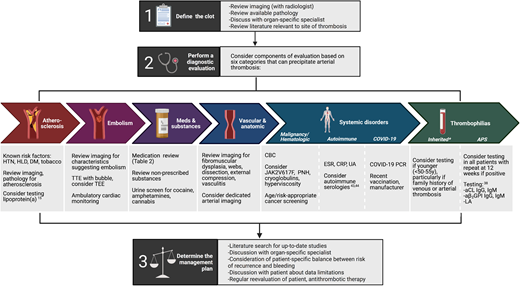
An overview of our approach to patients with unexplained arterial clot is presented in Figure 1. The first step when trying to determine etiology and subsequent treatment of a thrombotic event is to confirm the anatomic vascular location and understand the resultant end-organ damage. Although arterial thrombosis may be clearly evident in some vascular beds, others, such as those supplying visceral organs or central retinal vessels, may be mischaracterized as arterial events when they are, in fact, venous. A dedicated review of the patient's presenting symptoms, as well as the imaging along with a discussion with an expert radiologist or ophthalmologist, can be helpful.
A structured approach to the diagnosis and management of unexplained arterial thrombosis.2-5 *Includes factor V Leiden, prothrombin G20210A mutation, protein C deficiency, protein S deficiency, and antithrombin deficiency. aCL, anticardiolipin; aβ2GPI, anti-β2 glycoprotein 1; CBC, complete blood count; CRP, C-reactive protein; DM, diabetes; ESR, erythrocyte sedimentation rate; HbA1c, hemoglobin A1c; HLD, hyperlipidemia; HTN, hypertension; LA, lupus anticoagulant; PCR, polymerase chain reaction; PNH, paroxysmal nocturnal hemoglobinuria; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram; UA, urinalysis.
A structured approach to the diagnosis and management of unexplained arterial thrombosis.2-5 *Includes factor V Leiden, prothrombin G20210A mutation, protein C deficiency, protein S deficiency, and antithrombin deficiency. aCL, anticardiolipin; aβ2GPI, anti-β2 glycoprotein 1; CBC, complete blood count; CRP, C-reactive protein; DM, diabetes; ESR, erythrocyte sedimentation rate; HbA1c, hemoglobin A1c; HLD, hyperlipidemia; HTN, hypertension; LA, lupus anticoagulant; PCR, polymerase chain reaction; PNH, paroxysmal nocturnal hemoglobinuria; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram; UA, urinalysis.
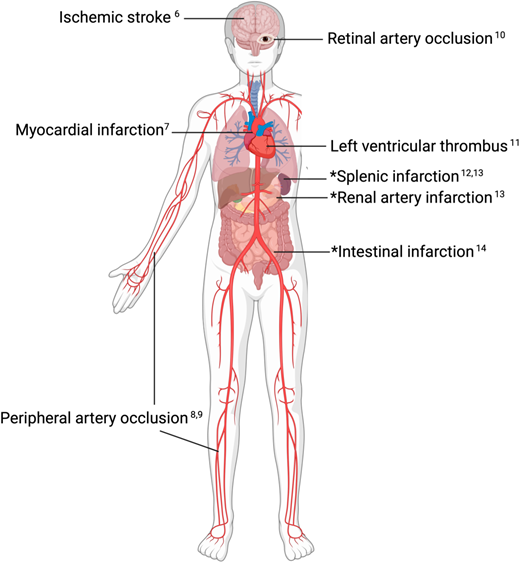
Once an arterial thrombotic event is confirmed, a literature search for up-to-date comprehensive reviews, as well as new developments, relevant to the site of thrombosis may be a good next step, as the approach to arterial events varies significantly by anatomic location and may rapidly evolve. Helpful recent reviews for specific sites of arterial thrombosis are presented in Figure 2.6-14
Anatomic sites of unexplained acute arterial thrombosis with citations for relevant comprehensive reviews.6-14 *Organ infarctions can occur due to arterial thrombosis but also venous thrombosis, low flow states, and other causes. “Defining the clot” (ie, determining if the end-organ infarction is caused by arterial thrombosis) is a first step in determining further workup and best management.
Anatomic sites of unexplained acute arterial thrombosis with citations for relevant comprehensive reviews.6-14 *Organ infarctions can occur due to arterial thrombosis but also venous thrombosis, low flow states, and other causes. “Defining the clot” (ie, determining if the end-organ infarction is caused by arterial thrombosis) is a first step in determining further workup and best management.
Diagnosis and management
Next, the approach to evaluation and management can be guided by consideration of 6 categories that can precipitate arterial thrombosis: (1) atherosclerosis, (2) embolism, (3) systemic disorders, (4) medications/substances, (5) vascular/anatomic abnormalities, and (6) thrombophilias (Figure 1). Most arterial events occur due to atherosclerosis and embolism, so focused evaluation for these causes is recommended prior to proceeding with evaluation for less common causes.
Importantly, in all patients in whom antithrombotic therapy is being considered, risk of recurrent thrombosis must be balanced against risk of bleeding, so we recommend a directed history for bleeding risk assessment. Risk calculators15 may be used to guide questioning and incorporated into the risk conversation with recognition that they are not validated in this rare population.
Atherosclerosis
Diagnosis: The diagnosis of atherosclerotic disease is often based on a combination of imaging findings and the presence of risk factors, as well as pathology specimens if available. In imaging evaluations, identification of luminal narrowing is often used to determine atherosclerotic etiology of a thrombotic event despite the fact that visible narrowing of an artery is a poor predictor of plaque vulnerability.16 This is particularly relevant in the evaluation of ischemic stroke—traditional criteria require >50% stenosis to designate large-artery atherosclerosis, but studies support that nonstenotic plaques may also rupture and cause stroke.17 Therefore, evaluation of images for the presence of nonstenotic plaque and calcification may also be useful.
Given the limitations of imaging, thorough evaluation of atherosclerotic risk factors should be pursued and, if identified, managed aggressively. Lipoprotein(a) is an emerging biomarker of atherosclerotic cardiovascular disease (ASCVD) risk, and guidelines now recommend consideration of testing in patients with premature ASCVD or ischemic stroke (<55 years of age), family history of ASCVD, or recurrent/progressive ASCVD despite optimal lipid-lowering therapy.2 New treatments, including proprotein convertase subtilisin/kexin type 9 inhibitors, show promise in lipoprotein(a) lowering and decreasing the risk of recurrent ischemic events.
Management: Antiplatelet therapy has long been the foundation of treatment and prevention of atherosclerotic events. However, recent studies in stable coronary artery disease18 and peripheral artery disease after revascularization19 have demonstrated the efficacy of dual-pathway inhibition (DPI; not to be confused with dual antiplatelet therapy), incorporating very low-dose anticoagulation (rivaroxaban 2.5 mg twice daily) with antiplatelet therapy.20 Because of similarity in nomenclature between DPI and dual antiplatelet therapy, as well as similarity between very low-dose rivaroxaban 2.5 mg and low-dose apixaban 2.5 mg, careful use of terminology and careful selection of direct oral anticoagulant dose are essential to prevent medication error (Table 1). The role of DPI beyond currently approved indications (stable coronary artery disease, peripheral artery disease after revascularization) has not been studied, but we expect it may be increasingly considered for events in uncommon anatomic locations where limited evidence exists to guide antithrombotic therapy selection.
Terminology for anticoagulation and antiplatelet therapy in arterial thrombosis
| . | Term . | Abbreviation . | Meaning . |
|---|---|---|---|
| Combination therapy | Dual antiplatelet therapy | DAPT | Aspirin 81 mg + P2Y12 inhibitor |
| Dual antithrombotic therapy | DAT | Anticoagulation + one antiplatelet agent (commonly in patients with AF and CAD) | |
| Dual pathway inhibition | DPI | Very low-dose anticoagulation + antiplatelet agent(s) | |
| Triple antithrombotic therapy Triple therapy | TAT TT | Anticoagulation + 2 antiplatelet agents | |
| DOAC dosing | Standard-dose DOAC | — | Apixaban 5 mg BID Dabigatran 150 mg BID Dabigatran 220 mg daily Edoxaban 60 mg daily Rivaroxaban 20 mg daily |
| Reduced-dose DOAC* | — | Apixaban 2.5 mg BID† Rivaroxaban 15 mg daily‡ | |
| Low dose DOAC Prophylactic dose DOAC | — | Apixaban 2.5 mg BID Rivaroxaban 10 mg daily | |
| Very low-dose DOAC | — | Rivaroxaban 2.5 mg BID |
| . | Term . | Abbreviation . | Meaning . |
|---|---|---|---|
| Combination therapy | Dual antiplatelet therapy | DAPT | Aspirin 81 mg + P2Y12 inhibitor |
| Dual antithrombotic therapy | DAT | Anticoagulation + one antiplatelet agent (commonly in patients with AF and CAD) | |
| Dual pathway inhibition | DPI | Very low-dose anticoagulation + antiplatelet agent(s) | |
| Triple antithrombotic therapy Triple therapy | TAT TT | Anticoagulation + 2 antiplatelet agents | |
| DOAC dosing | Standard-dose DOAC | — | Apixaban 5 mg BID Dabigatran 150 mg BID Dabigatran 220 mg daily Edoxaban 60 mg daily Rivaroxaban 20 mg daily |
| Reduced-dose DOAC* | — | Apixaban 2.5 mg BID† Rivaroxaban 15 mg daily‡ | |
| Low dose DOAC Prophylactic dose DOAC | — | Apixaban 2.5 mg BID Rivaroxaban 10 mg daily | |
| Very low-dose DOAC | — | Rivaroxaban 2.5 mg BID |
Dosing regimens for patients with renal impairment, low body weight, and/or advanced age. See apixaban† and rivaroxaban‡ details.
Dosing for patients with AF who meet 2 of the following 3 criteria: creatinine ≥1.5 mg/dL, age ≥80 years, and weight ≤60 kg.
Dosing used in patients with AF with creatinine clearance ≤50 mL/min.
AF, atrial fibrillation; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; BID, twice daily.
Embolism
Diagnosis: Embolic events can arise from the heart, where thrombus forms due to structural or functional abnormalities, but emboli can originate anywhere in the vasculature and be composed of many materials (eg, thromboemboli, atheroemboli, fat emboli, septic emboli). Certain imaging characteristics (eg, different ages of ischemic changes in the same vascular territory) can suggest embolic etiology. In the cerebral vasculature, the finding of multiple infarcts in different vascular territories has classically been considered an indicator of embolism, but a recent investigation revealed that the presence of this pattern is unable to consistently predict, and its absence is unable to consistently exclude, an embolic mechanism.21 In contrast, a lacunar infarct (defined as a single, small [<15 mm in diameter], subcortical infarct in the distribution of a perforating artery)22 may suggest small-vessel disease and can be helpful to exclude embolic causes. Also, infarcts in certain anatomic sites are more commonly caused by embolic phenomenon (eg, renal and splenic infarctions occur due to embolism in 68% and 43%, respectively13 ). Again, a discussion with an expert radiologist and a literature review may be helpful.
Cardiac imaging with transthoracic echocardiogram is often the first step in the evaluation for an embolic event, to identify increased atrial size, valvular disease, and intracardiac material. Initial evaluation for patent foramen ovale (PFO), through which venous material can enter arterial circulation, is performed with the use of agitated saline while the patient is coughing and/or performing a Valsalva maneuver (a “bubble study”). Transesophageal echocardiography is the gold standard for PFO morphologic characterization and valve evaluation and should be considered if initial studies are unreavealing.23 Imaging of surrounding arteries (ie, carotid arteries, aorta) is indicated if there is concern for an atheroembolic etiology. Ambulatory rhythm monitoring is also recommended, although optimal duration and preferred devices are debated. One group of experts favors at least 30 days in ischemic stroke, with consideration of implantable loop recorders in high-risk patients.24 However, existing data have not yet demonstrated that increased arrhythmia detection via extended monitoring reduces risk of recurrent stroke, and the optimal treatment for device-detected brief runs of atrial fibrillation is the subject of ongoing clinical trials (NCT01938248, NCT02618577).
Management: When cardiac thromboembolism associated with atrial fibrillation is identified, management is straightforward, and evidence-based guidelines exist. The management of patients with presumed cardioembolism without identifiable arrhythmia and without a documented intracardiac thromboembolic source has been explored in ischemic stroke with the creation of the category of embolic stroke of undetermined source (ESUS).25 It was hypothesized that ESUS was commonly caused by covert atrial fibrillation, and therefore, anticoagulation would be superior to antiplatelet therapy for secondary prevention. However, 2 large randomized trials in patients with ESUS revealed that the use of anticoagulation (rivaroxaban 15 mg daily, dabigatran 150 mg or 100 mg twice daily) compared with aspirin 100 mg daily did not decrease recurrence yet was associated with increased bleeding. Therefore, antiplatelet agents remain the preferred antithrombotic agent in patients with ESUS.26 Consequently, outside of clinic trials, there is no current clinical usefulness of defining a stroke as ESUS. The applicability of these findings to other sites of unexplained arterial thrombosis is unknown, and the decision to use antiplatelet therapy vs anticoagulation is often empiric.
Management of left ventricular thrombus is an area of controversy and care evolution, where despite existing guidelines recommending warfarin,27-29 off-label use of direct oral anticoagulants (DOACs) is common. A recent retrospective cohort study (RED VELVT, Retrospective Evaluation of DOACs and Vascular Endpoints of Left Ventricular Thrombi) introduced further uncertainty, as patients taking DOACs had an increased risk of stroke or systemic embolism compared with warfarin.30 There are many limitations to these data, including retrospective and observational design, unknown DOAC dosing regimens, and frequent crossover, and a subsequent small, unblinded randomized trial (Comparative Study of Oral Anticoagulation in Left Ventricular Thrombi, No-LVT) demonstrated rivaroxaban as being noninferior to warfarin.31 Pending additional data, we favor warfarin for left ventricular thrombus but consider patient-specific factors and have a detailed discussion of data limitations with all patients.
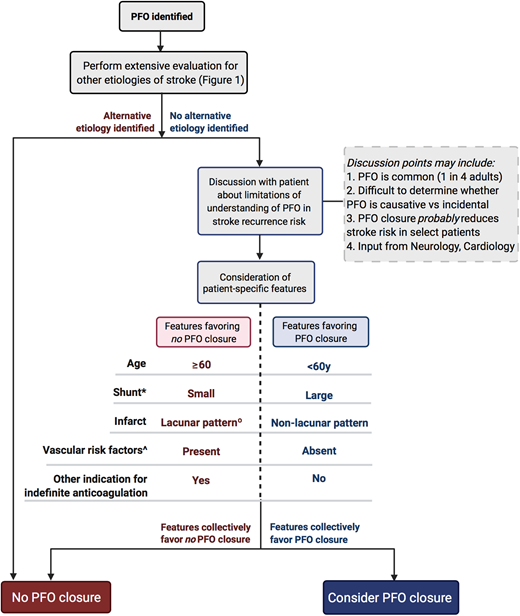
The role of PFO closure in the prevention of recurrent embolic events is also debated and has been extensively reviewed elsewhere.23 PFO is present in 25% of the adult population,32 so the challenge for clinicians is to determine when a PFO is coincidental, therefore not warranting closure, vs causative, therefore warranting closure. Updated guidelines from the American Academy of Neurology outline factors to consider when determining the value of PFO closure.22 An algorithm based on these guidelines is presented in Figure 3. Importantly, no single factor is sufficient to justify intervention, so a comprehensive, patient-specific evaluation in close collaboration with neurology and cardiology is essential, with significant consideration of patient preference.
Algorithm for consideration of PFO closure in secondary stroke prevention based on the 2020 Practice Advisory Update Summary from the American Academy of Neurology.22 *Shunt descriptor refers to degree of right-to-left shunting, not anatomic size. ^Includes hypertension, diabetes, hyperlipidemia, or smoking. °Single, small (<15 mm), subcortical, in the distribution of single penetrating artery.
Algorithm for consideration of PFO closure in secondary stroke prevention based on the 2020 Practice Advisory Update Summary from the American Academy of Neurology.22 *Shunt descriptor refers to degree of right-to-left shunting, not anatomic size. ^Includes hypertension, diabetes, hyperlipidemia, or smoking. °Single, small (<15 mm), subcortical, in the distribution of single penetrating artery.
Medications and substances
After extensive evaluation for atherosclerosis and embolism, we then performed a structured investigation of less common causes (Figure 1). Certain medications are known to increase thrombotic risk, although associations with venous events are more common than with arterial ones. Furthermore, risk is more consistently demonstrated with cardiovascular events and ischemic stroke, as thromboses in other sites are rare, and it is therefore difficult to determine causality. Example medications and substances, along with their proposed pathophysiologic mechanism, are presented in Table 2.
Medications associated with increased risk of arterial thrombosis33-41
| Medication . | Sites . | Proposed mechanism(s) . | Notes . |
|---|---|---|---|
| Estrogens33 | Stroke, MI | Increased platelet activation and procoagulant factors, decreased anticoagulant and fibrinolysis factors | Variable risk reported, likely low absolute risk with commonly used lower estrogen doses. Higher risk in patients with HTN, smoking |
| Androgenic anabolic steroids34 | Stroke, MI | Accelerated atherosclerosis, coagulation abnormalities, elevated hematocrit | General term for performance-enhancing drugs with promyogenic and androgenic effects |
| Heparin35 | PA > stroke, MI | Autoimmune response to heparin-PF4 complex causing heparin-induced thrombocytopenia | Well-documented arterial thrombotic risk, but venous thrombosis more common |
| Intravenous immunoglobulin36 | Stroke, MI | Many proposed: hyperviscosity, platelet activation, vasospasm, factor XI content | Risk factors: older age, hypertension, hypercholesterolemia. Consider lower dose, slower infusion rate |
| Cocaine37 | Stroke, MI, PA | Platelet activation, vasoconstriction, accelerated atherosclerosis | Contributes to acute thrombosis risk and chronic arterial remodeling |
| Tobacco, cannabis38 | PA | Thromboangiitis obliterans (Buerger disease) | Inflammatory vascular disease of the small- and medium-sized vessels of the extremities |
| Erythropoiesis-stimulating agents39 | Stroke, MI | Platelet activation, hyperviscosity, hypertension, increased procoagulant factors | Risk most clearly documented in patients with renal disease treated to higher hemoglobin targets |
| Anticancer therapies40 | |||
| VEGF inhibitors* | Stroke, MI | Endothelial dysfunction, increased procoagulant factors, accelerated atherosclerosis, HTN | Also associated with hemorrhage |
| Tamoxifen | Stroke | Unknown | Initial trials suggested increased stroke risk but decreased cardiac mortality, but variable in later trials |
| Fluorouracil | MI | Vasospasm, endothelial damage, increased procoagulant factors | Cardiotoxicity incidence 4% to 19%, but role of thrombosis vs other mechanisms unclear |
| Immune checkpoint inhibitors41 | Stroke, MI, PA | Increased procoagulant factors, platelets activation, impaired fibrinolysis, accelerated atherosclerosis | Venous thrombosis more common than arterial (reported 13% vs 2%) |
| Medication . | Sites . | Proposed mechanism(s) . | Notes . |
|---|---|---|---|
| Estrogens33 | Stroke, MI | Increased platelet activation and procoagulant factors, decreased anticoagulant and fibrinolysis factors | Variable risk reported, likely low absolute risk with commonly used lower estrogen doses. Higher risk in patients with HTN, smoking |
| Androgenic anabolic steroids34 | Stroke, MI | Accelerated atherosclerosis, coagulation abnormalities, elevated hematocrit | General term for performance-enhancing drugs with promyogenic and androgenic effects |
| Heparin35 | PA > stroke, MI | Autoimmune response to heparin-PF4 complex causing heparin-induced thrombocytopenia | Well-documented arterial thrombotic risk, but venous thrombosis more common |
| Intravenous immunoglobulin36 | Stroke, MI | Many proposed: hyperviscosity, platelet activation, vasospasm, factor XI content | Risk factors: older age, hypertension, hypercholesterolemia. Consider lower dose, slower infusion rate |
| Cocaine37 | Stroke, MI, PA | Platelet activation, vasoconstriction, accelerated atherosclerosis | Contributes to acute thrombosis risk and chronic arterial remodeling |
| Tobacco, cannabis38 | PA | Thromboangiitis obliterans (Buerger disease) | Inflammatory vascular disease of the small- and medium-sized vessels of the extremities |
| Erythropoiesis-stimulating agents39 | Stroke, MI | Platelet activation, hyperviscosity, hypertension, increased procoagulant factors | Risk most clearly documented in patients with renal disease treated to higher hemoglobin targets |
| Anticancer therapies40 | |||
| VEGF inhibitors* | Stroke, MI | Endothelial dysfunction, increased procoagulant factors, accelerated atherosclerosis, HTN | Also associated with hemorrhage |
| Tamoxifen | Stroke | Unknown | Initial trials suggested increased stroke risk but decreased cardiac mortality, but variable in later trials |
| Fluorouracil | MI | Vasospasm, endothelial damage, increased procoagulant factors | Cardiotoxicity incidence 4% to 19%, but role of thrombosis vs other mechanisms unclear |
| Immune checkpoint inhibitors41 | Stroke, MI, PA | Increased procoagulant factors, platelets activation, impaired fibrinolysis, accelerated atherosclerosis | Venous thrombosis more common than arterial (reported 13% vs 2%) |
Includes bevacizumab, tyrosine kinase inhibitors (eg, sorafenib), aflibercept.
HTN, hypertension; MI, myocardial infarction; PA, peripheral artery; PF4, platelet factor 4; VEGF, vascular endothelial growth factor.
Systemic diseases
Patients with malignancy are at increased risk for arterial thrombosis in the 5 months prior to diagnosis, with highest risk 1 month prior.42 A thorough history and physical examination for signs of malignancy should be performed, with cancer screening based on age (eg, colonoscopy, mammogram) and risk factors (eg, low-dose computed tomography of the chest in patients with tobacco use). The benefit of more extensive imaging in patients with arterial thromboembolism has not been investigated. However, given the rarity of arterial events due to cancer, supported by the lack of benefit even in patients with venous thromboembolism,43 it is unlikely to be beneficial, and we therefore do not recommend it.
Additional categories of systemic disorders to consider include autoimmune disorders (eg, rheumatoid arthritis, lupus, vasculitis)44 and hematologic disorders (eg, myeloproliferative neoplasms, paroxysmal nocturnal hemoglobulinuria [PNH], sickle cell disease, plasma cell disorders, cryoglobulinemia), with proposed evaluation outlined in Figure 1.
Emerging data indicate infection with severe acute respiratory syndrome coronavirus 2 is associated with an increased thrombotic risk, with reported occurrences in multiple arterial sites.45 The COVID-19 vaccines manufactured by AstraZeneca and Johnson & Johnson have also been shown to cause a prothrombotic syndrome that mimics heparin-induced thrombocytopenia, named thrombosis with thrombocytopenia syndrome (TTS) or vaccine-induced immune thrombotic thrombocytopenia (VITT). TTS/VITT presents with thrombocytopenia and primarily central venous sinus thrombosis but has also been reported to cause arterial thrombosis.46 A careful history of potential COVID-19 exposures, vaccination timing and manufacturer, COVID-19 testing, and platelet count (if concern for TTS/VITT) should be considered in all patients during the pandemic.
Vascular and anatomic disorders
Many vascular abnormalities with potential to precipitate thrombus formation have been reviewed in detail.47 Such disorders may affect the artery wall (eg, dissection, aneurysm, fibromuscular dysplasia, vasculitis) and/or caliber (eg, external compression, vasospasm). Therefore, existing imaging should be reviewed closely for vessel abnormalities. Additional imaging with CTA, magnetic resonance angiography, or catheter-directed angiogram may be required to better visualize areas of concern, with unique considerations for each vascular bed.48
Thrombophilias
The association between inherited thrombotic disorders and arterial thrombosis is not well characterized. We have previously reviewed the existing literature,49 which is limited to case series and retrospective analyses. Given the rarity of the stronger inherited thrombophilias and their questionable role in arterial thrombosis formation, we only consider testing after extensive evaluation for other causes in younger patients (younger than 50-55 years), particularly if there is a family history of venous or arterial thrombosis. If testing is pursued, we consider evaluation for protein C deficiency, protein S deficiency, antithrombin deficiency, factor V Leiden mutations, and prothrombin 20210 mutations. Testing for factor V Leiden and prothrombin 20210 is performed to identify homozygous or compound heterozygous patients only, as isolated heterozygosity does not have a clinically significant association with arterial thrombotic risk. Importantly, some assays are inaccurate during an acute thrombotic episode and while on anticoagulation, so it is essential to understand assay limitations to ensure appropriate timing of collection and correct interpretation. Antithrombotic agent selection in patients with an identified inherited thrombophilia is again without data for guidance. We consider the use of anticoagulation, with or without aspirin 81 mg daily, in patients with low bleeding risk but with regular evaluation of risk/benefit and consideration of newly published data.
Antiphospholipid syndrome (APS) is an acquired thrombophilia with a well-documented risk of arterial thrombosis, so we consider testing based on the updated Sapporo (also known as Sydney) criteria5 in all patients without an identifiable etiology. Patients with arterial thrombosis and antiphospholipid antibodies (APLAs) who do not fulfill Sydney criteria for APS (eg, low titer APLA, positive assay on only 1 occasion) should be managed in the same manner as APLA-negative patients with a similar thrombotic event.50 The optimal antithrombotic strategy for patients with confirmed APS is debated; anticoagulation, antiplatelet therapy, or a combination of both can be considered.50 We favor combination therapy in patients with a low bleeding risk based on a small randomized trial demonstrating lower incidence of stroke recurrence and similar bleeding incidence with warfarin (target international normalized ratio [INR] 2-3) plus aspirin 100 mg daily vs aspirin alone.51 When anticoagulation is used, warfarin is preferred, given 2 recent trials demonstrating an increased risk of arterial thrombosis with rivaroxaban vs warfarin.52,53 However, debate remains if warfarin is required for all patients with APS vs only those with high thrombosis risk features (triple positive), given the 2 aforementioned trials primarily included triple-positive patients and had important methodologic limitations.54 Although we generally favor warfarin for anticoagulation in patients with APS, we consider DOACs in patients who are single or double positive and who (1) have tolerated a DOAC for more than a year, (2) struggle with the limitations of warfarin (eg, fluctuation in INR, inability to comply with regular monitoring), or (3) have an invalid INR due to the presence of a lupus anticoagulant. However, given the limitations of existing data, an informed discussion with the patient and the incorporation of patient preference are important components of anticoagulation selection.
CLINICAL CASE (Continued)
Additional evaluation was performed based on consideration of the 6 potential causes of unexplained arterial thrombosis outlined in Figure 1.
Atherosclerosis: The patient confirmed no diagnosis of hypertension or diabetes, and she did not use tobacco products. She was not obese and had no known family history of premature stroke or cardiovascular disease. Intracranial vascular imaging was reviewed with radiology and did not demonstrate findings concerning for atherosclerosis. Bilateral carotid ultrasonography was performed and there was no significant stenosis. Lipid panel, hemoglobin A1c, and lipoprotein(a) were sent and without abnormality.
Embolism: Telemetry during hospitalization did not demonstrate arrhythmia, so arrangements were made at discharge for 30-day ambulatory cardiac monitoring. Transthoracic echocardiogram with normal saline bubble study was without valvular abnormality, intracardiac material, and increased atrial size but did demonstrate an intracardiac shunt. Transesophageal echocardiography revealed a PFO with a mild bidirectional shunt. Upper and lower extremity ultrasounds were negative for deep vein thrombosis. Per the algorithm in Figure 3, continued evaluation for alternative etiologies was pursued before determining the role of PFO closure.
Medications and substances: The patient was taking no medications. She had a progesterone intrauterine device for contraception. She denied use of nonprescribed substances, and a urine drug screen was negative.
Vascular and anatomic: CTA of the head and neck was obtained and did not reveal evidence of stenosis or compression, dissection, or vessel wall thickening.
Systemic disorders: The patient denied recent weight loss, fever, rash, joint pain, or systemic symptoms. Physical examination was without adenopathy, organomegaly, or breast masses. She had a normal Pap smear 1 year prior and denied any family history of malignancy. A complete blood count was without abnormality, and there was no evidence of hemolysis. JAK2V617F mutation testing and flow for PNH were considered but not sent pending additional evaluation given low clinical suspicion. In the absence of systemic symptoms concerning for autoimmune disorders, serologic testing was also deferred. She had received the COVID-19 vaccine manufactured by Pfizer-BioNTech 2 months prior.
Thrombophilias: The patient denied a family history of venous and arterial thrombosis in first- or second-degree relatives. She had had 2 pregnancies without complications and no miscarriages. In the absence of a family or personal history, the decision was first made to test for APS, an acquired disorder, prior to consideration of testing for inherited disorders. Lupus anticoagulant returned clearly positive, IgG anticardiolipin was elevated at 85 microgram of IgG antibody (GPL) (normal, 0-14 GPL), and IgG anti-β2 glycoprotein I was elevated at 95 GPL (normal, 0-20 GPL).
In summary, the diagnostic workup identified a PFO and clearly positive APLA (“triple positive”), likely representing APS pending repeat testing at 12 weeks. After a multidisciplinary discussion between hematology, cardiology, and neurology and discussion with the patient, the decision was made to initiate anticoagulation for secondary stroke prevention for likely APS. Warfarin was recommended given concern for increased arterial thrombotic risk with rivaroxaban. The patient had no significant bleeding risk factors and was very concerned about the potential for repeat stroke, so the joint decision was made to add aspirin 81 mg daily with close follow-up for regular reassessment of bleeding risk. PFO closure was not recommended given the small degree of right-to-left shunting observed and the fact that the patient would be continued on indefinite anticoagulation for APS regardless of plan for PFO closure.
Conclusion
The diagnosis and management of unexplained arterial thrombosis are a complex, multidisciplinary effort in which hematology plays an integral role. A systematic approach to evaluation is warranted and includes consideration of common etiologies (atherosclerosis and thromboembolism) as well as uncommon etiologies (medications and substances, vascular and anatomic abnormalities, systemic disorders, and thrombophilias). Management requires review of available literature for specific vascular territories or conditions and relies heavily on patient-specific thrombotic and bleeding risk and patient preference. Continued close follow-up with regular reassessment of the management plan is also essential, as new data or new symptoms may present over time, and new bleeding and thrombotic risk factors may develop.
Acknowledgment
Figures were created with BioRender.com.
Conflict-of-interest disclosure
Jori E. May: no competing financial interests to declare.
Stephan Moll: no competing financial interests to declare.
Off-label drug use
Jori E. May: off-label use of DOACs for left ventricular thrombus is discussed.
Stephan Moll: off-label use of DOACs for left ventricular thrombus is discussed.