Abstract
Over the last decade, the advent of Bruton tyrosine kinase inhibitors (BTKi) has profoundly modified the therapeutic strategy in chronic lymphocytic leukemia (CLL), introducing the concept of treatment until progression. Initially, the bcl-2 inhibitor venetoclax (VEN) was used as a single agent and then was rapidly combined in VEN-based regimens associated with either anti-CD20 or with BTKi. These regimens yielded a high rate of complete remission, leading to their use as a fixed duration treatment. The decision between continuous treatment with BTKi and VEN-based combinations relies mostly on comorbidities, comedications, and patient/physician preferences. Notably, with BTKi, cardiovascular comorbidities, hypertension, and potential pharmacological interactions should be carefully evaluated. On the other hand, the risk of tumor lysis syndrome with VEN should be monitored at treatment initiation. TP53 alteration and IGHV mutational status should also be assessed, as they remain important for therapeutic decisions. Fit patients with a TP53 wild type and IGHV-mutated CLL may still benefit from fludarabine-cyclophosphamide-rituximab chemoimmunotherapy (CIT), as it may result in a very long remission duration. VEN-based treatments are well tolerated, and no additional toxicity has been observed when combined with anti-CD20 or BTKi. The 1-year fixed-duration association of VEN plus obinutuzumab was evaluated in frontline for older adult patients. Nonetheless, considering the favorable outcome, an extension of indication for fit younger patients is expected. The association of VEN and BTKi is promising, even if the follow-up is still short. It is currently being tested against CIT, BTKi continuous treatment, and VEN plus anti-CD20.
Learning Objectives
Recognize the important biological pretherapeutic parameters for allocating CLL patients to optimal frontline treatment
Review the indications of time-limited treatment for the various categories of treatment-naive CLL patients
Review the various fixed-duration combination therapies and the major trials testing the time-limited strategies
Be aware of future developments that will allow a more personalized therapeutic approach
Introduction
The concept of time-limited treatment is obviously not new in chronic lymphocytic leukemia (CLL) since up until the arrival of BCR inhibitors, treatment relied mainly on chemotherapy and was therefore of limited duration. In recent years, Bruton tyrosine kinase inhibitors (BTKi) have been shown to be superior overall to chemoimmunotherapy (CIT) in the first-line treatment of CLL, but they must be given continuously until progression as they have only a suspensive effect on the disease.1,2 This has led to new concerns about compliance, quality of life, and cost. The BCL2 inhibitor venetoclax (VEN), either alone or combined with other agents, is likely to yield high rates of both complete response and undetectable minimal residual disease (uMRD), and it can be discontinued safely after optimal response is achieved.3
CLINICAL CASE 1
Mr. V., who is 58, is fit and works as an engineer. He has a history of coronary thrombosis dating from 5 years ago, is on acetylsalicylic acid, and takes amlodipine for high blood pressure. At diagnosis of CLL, his blood count showed 23 × 109/L lymphocytes and no cytopenia. An immunophenotype revealed clonal B cells with low CD20 expression harboring positive CD5 expression, CD23 and CD200hi, low kappa light chain, and CD79b. CD38 was not expressed on CLL cells. Clinical examination found centimetric cervical and axillary lymph nodes and no splenomegaly. Five years later in 2010, Mr. V. was still fit, but his CLL had progressed with lymphadenopathy from 3 to 5 cm (cervical, axillary, and inguinal) and a splenomegaly 6 cm below the costal margin. He had lost 3 kg and experienced night sweats. His blood counts were as follows: lymphocytes 120 × 109/L; hemoglobin 135 g/L; platelets 176 × 109/L. Biological parameters were as follows: estimated glomerular filtration rate (eGFR), 70 mL/min; del13q deletion and no 17p deletion; mutated IGHV status with VH 3-30 with 95% homology; and hypogammaglobulinemia at 5 g/L. In 2010 he was started on 6 courses of an oral fludarabine-cyclophosphamide-rituximab (FCR) regimen.
CLINICAL CASE 2
Mr. F. is 72 years old, retired, and a previous bank employee. He is a heavy smoker with type 2 diabetes and dyslipidemia and takes biguanide and atorvastatin. He was diagnosed with CLL in 2009 with a characteristic phenotype. He rapidly progressed into active disease, with multiple lymphadenopathy, splenomegaly, and night sweats. His lymphocyte doubling time was 6 months, and blood counts showed hemoglobin 110 × 109/L; lymphocytes: 130 × 109/L; and platelets: 90 × 109/L. He had VH1-69 IGHV with 100% homology and a normal karyotype and fluorescence in situ hybridization (FISH) analysis. His eGFR was 55 mL/min, and the direct antibody test was negative. He was started in 2010 on 6 courses of bendamustine-rituximab (BR).
What if we had to do it again today?
Indication for treatment
It is important to keep in mind that the therapeutic options discussed here are intended for bona fide CLL and that adequate phenotyping (Table 1) should be performed at diagnosis.
Recommended markers for the diagnosis of CLL (European Research Initiative on CLL recommendations)
| Minimally recommended . | Other important markers . |
|---|---|
| CD19 CD5 Ig light chains kappa and lambda (membrane staining) CD23 CD79b and/or CD22 CD20 FMC7 CD200 | Intracellular Ig light chains kappa and lambda (if absence of membrane staining) CD81 CD43 ROR1 CD38 CD10 |
| Minimally recommended . | Other important markers . |
|---|---|
| CD19 CD5 Ig light chains kappa and lambda (membrane staining) CD23 CD79b and/or CD22 CD20 FMC7 CD200 | Intracellular Ig light chains kappa and lambda (if absence of membrane staining) CD81 CD43 ROR1 CD38 CD10 |
Ig, immunoglobulin.
Indications for treatment initiation according to the International Workshop on Chronic Lymphocytic Leukemia have not dramatically changed over the years. As exemplified in these 2 cases, specific treatment is indicated when patients show signs of CLL progression. The progression criteria have been very clearly defined by the International Workshop on Chronic Lymphocytic Leukemia and are summarized in Table 2.4
Criteria for treatment initiation
| • Progressive BM failure with the development or aggravation of anemia and/or thrombocytopenia. |
| • Massive or progressive or symptomatic splenomegaly. |
| • Significantly enlarged or symptomatic or progressive lymphadenopathies. |
| • Progressive lymphocytosis, with an increase of more than 50% over a period of 2 months or an LDT of less than 6 months. In patients with an initial lymphocyte count <30 × 109/L, LDT alone should not be used as a single parameter to decide treatment initiation and should be interpreted in the overall clinical context. |
| • Autoimmune anemia and/or thrombocytopenia that is poorly responsive to corticosteroids or other standard therapies. |
| • The presence of constitutive symptoms as defined by 1 or more of the following signs or symptoms related to the disease: |
| Unintentional weight loss of 10% or more in the previous 6 months, significant fatigue (ECOG PS 2 or worse; inability to perform usual activities), fever over 38.0 °C for 2 weeks or more without signs of infection, and night sweats lasting more than a month with no sign of infection. |
| • Progressive BM failure with the development or aggravation of anemia and/or thrombocytopenia. |
| • Massive or progressive or symptomatic splenomegaly. |
| • Significantly enlarged or symptomatic or progressive lymphadenopathies. |
| • Progressive lymphocytosis, with an increase of more than 50% over a period of 2 months or an LDT of less than 6 months. In patients with an initial lymphocyte count <30 × 109/L, LDT alone should not be used as a single parameter to decide treatment initiation and should be interpreted in the overall clinical context. |
| • Autoimmune anemia and/or thrombocytopenia that is poorly responsive to corticosteroids or other standard therapies. |
| • The presence of constitutive symptoms as defined by 1 or more of the following signs or symptoms related to the disease: |
| Unintentional weight loss of 10% or more in the previous 6 months, significant fatigue (ECOG PS 2 or worse; inability to perform usual activities), fever over 38.0 °C for 2 weeks or more without signs of infection, and night sweats lasting more than a month with no sign of infection. |
At least one of the criteria must be fulfilled.
Pretreatment assessment and preventive measures
International and national societies have published common recommendations for pretreatment assessments, including history, physical examination, and standard biological tests (Table 3).4-6 The current pretherapeutic workup includes the previous recommendations of the CIT era, but some new assessments have been added.
Pretreatment evaluation
| . | Routine practice . | Clinical trials specific . |
|---|---|---|
| Clinical evaluation | ||
| ECOG PS | Always | |
| Constitutive symptoms | Always | |
| Clinical examination | Always | |
| Cardiovascular comorbidities | Always | |
| Comedications | Always | |
| Biological standard tests | ||
| Complete blood count and reticulocyte count | Always | |
| LDH, haptoglobin and direct antiglobulin test | Always | |
| Serum creatinine level and glomerular filtration rate | Always | |
| Transaminases, bilirubin, GGT | Always | |
| LDH and beta-2 microglobulin | Always | |
| Serum protein electrophoresis | Always | |
| HIV, B and C hepatitis serology | Always | |
| Cryopreservation of blood cells (tumor library) | Desirable | Always |
| Genetics | ||
| Karyotype | Desirable | Always |
| FISH | ||
| 17p deletion | Always | |
| 11q deletion | Desirable | Always |
| 13q deletion | Desirable | Always |
| Trisomy 12 | Desirable | Always |
| IGHVmutational status | Always | |
| TP53mutations by NGS | Always | |
| NGS of recurrent mutations | NGI | Desirable |
| Imaging | ||
| CT scan of chest, abdomen, and pelvis | Desirable | Recommended |
| Preventive measures | ||
| Vaccination against pneumococcus | Always | |
| Vaccination against influenza (yearly) | Always | |
| Vaccination against SARS-CoV-2 | Always | |
| . | Routine practice . | Clinical trials specific . |
|---|---|---|
| Clinical evaluation | ||
| ECOG PS | Always | |
| Constitutive symptoms | Always | |
| Clinical examination | Always | |
| Cardiovascular comorbidities | Always | |
| Comedications | Always | |
| Biological standard tests | ||
| Complete blood count and reticulocyte count | Always | |
| LDH, haptoglobin and direct antiglobulin test | Always | |
| Serum creatinine level and glomerular filtration rate | Always | |
| Transaminases, bilirubin, GGT | Always | |
| LDH and beta-2 microglobulin | Always | |
| Serum protein electrophoresis | Always | |
| HIV, B and C hepatitis serology | Always | |
| Cryopreservation of blood cells (tumor library) | Desirable | Always |
| Genetics | ||
| Karyotype | Desirable | Always |
| FISH | ||
| 17p deletion | Always | |
| 11q deletion | Desirable | Always |
| 13q deletion | Desirable | Always |
| Trisomy 12 | Desirable | Always |
| IGHVmutational status | Always | |
| TP53mutations by NGS | Always | |
| NGS of recurrent mutations | NGI | Desirable |
| Imaging | ||
| CT scan of chest, abdomen, and pelvis | Desirable | Recommended |
| Preventive measures | ||
| Vaccination against pneumococcus | Always | |
| Vaccination against influenza (yearly) | Always | |
| Vaccination against SARS-CoV-2 | Always | |
CT, computed tomography; GGT, gamma-glutamyl transferase; LDH, lactate dehydrogenase; NGI, not generally indicated.
Whenever targeted agents are considered, notably BTKi, cardiovascular comorbidities, hypertension, and comedications should be carefully assessed.7 The prevention of tumor lysis syndrome is well established and should be applied to any VEN-based strategy.8
Patients with CLL are at risk of infection with targeted agents as well, and all possible preventive measures should be undertaken before starting therapy, notably vaccination (pneumococcus and annually for influenza).9 SARS-CoV-2 vaccination should be performed, preferably before starting therapy, particularly if the patient is to receive anti-CD20 or BTKi.10
In general, a body computed tomography scan may be helpful to assess the tumor load and risk of tumor lysis syndrome, mainly before treatment with the BCL2 inhibitor VEN.5
The following tests have become important for decision-making.
TP53
Alteration of TP53 function can be mediated by either 17p deletion, TP53 mutation, or both. These alterations are associated with resistance to CIT and remain an unfavorable prognostic factor with targeted agents.2,3,11,12 It is therefore mandatory to test for both these alterations.
In the 2 cases illustrated here, in 2010 only 17p FISH was available. It should be stressed that FISH alone detects only half of TP53 alterations, as in approximately 50% of cases only a TP53 mutation is present and therefore not identified by FISH analysis.13 Nowadays, sequencing analysis of TP53 is required before the initiation of therapy.4,14 Next-generation sequencing (NGS) is rapidly replacing Sanger sequencing, as it allows the detection of small subclones. In our observations, both patients were tested retrospectively for TP53 mutations and were negative.
IGHV
The variable region of immunoglobulin heavy chain gene (IGHV) mutational status, which until recently was only indicated in clinical trials, has become an important decision-making parameter and must be performed before frontline therapy.4 IGHV mutational status has been considered in the last 20 years to be the most powerful predictive indicator. Patients with unmutated IGHV genes (UM-IGHV CLL, ie, ≥98% homology with germ line sequence) have inferior outcomes after any CIT regimen.11,15,16 Targeted agents have demonstrated a clear benefit over CIT for these patients.17
Even for FCR ineligible patients, determination of IGHV mutational status is of interest. The genetic complexity appears different: UM-IGHV patients accumulate significantly more genetic alterations than M-IGHV CLL.18 The negative impact of this increased mutation burden has been reduced in UM-IGHV CLL by targeted agents, but there are different dominant mutagenic mechanisms in M-IGHV and UM-IGHV, underlying a very likely different clonal evolution at relapse.19 Therefore, determination of IGHV mutational status has been recommended for all patients.4,5
Whether a karyotype should be performed in routine practice remains open. Complex karyotype is a known detrimental factor in clinical trials, both for CIT and VEN.20 It is of interest when it can be easily obtained, but the results still do not clearly influence treatment decision. Similarly, several recurrent gene mutations are observed in CLL, such as NOTCH1, SF3B1, and ATM,21 and some of them have been associated with exponential growth.22 This expanding knowledge about growth dynamics in individual patients will be helpful for predicting the course of the disease and informing therapy in the future but is not yet incorporated into a treatment decision algorithm.
Treatment options today
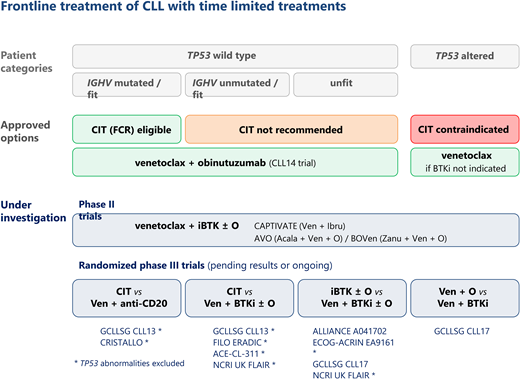
Outside of clinical trials, as of today, 2 different strategies may be considered: either continuous therapy until progression with BTKi alone or combined with obinutuzumab (O), or time-limited treatment with either CIT or VEN-based regimens. In this section, we will consider only the time-limited therapies.
CIT is the prototype of time-limited treatment and may still be of benefit for some patients. The therapeutic regimens have been modulated based on fitness and age, from chlorambucil (CLB) and anti-CD20 to BR/FCR.11,15,16 Targeted-agent regimens have been compared to CIT in frontline (Table 4; Figure 1).
Summary of time-limited frontline trials
| Reference . | Trial . | Patients . | Primary end point . | Results . |
|---|---|---|---|---|
| Randomized phase 3 trials | ||||
| Shanafelt et al17 , ECOG1912 | FCR vs I-R | Age <70 no del 17p N = 529 | PFS FU: 33.6 mo | I - R > FCR (89.4% vs 72.9% at 3 y; HR: 0.35; 95% CI, 0.22-0.56; P < .001) OS: I - R > FCR (98.8% vs 91.5% at 3 y) HR: 0.17; 95% CI, 0.05-0.54; P < .001 |
| Al-Sawaf et al3 , CLL14 | CLB-O vs VEN-O | CIRS >6 and/or Cl. creat 30-69 mL/min N = 432 | PFS FU: 39.6 mo | VEN-O > CLB-O (NE vs 35.5 mo) HR: 0.31, 95% CI, 0.22–0.44; P < .0001 |
| Woyach et al1 , ALLIANCE | BR/I-R/I | Age >65 N = 447 | PFS FU: 38 mo | I-R > BR. HR: 0.38; 95% CI, 0.25-0.59; P < .001 I > BR. HR: 0.39; 95% CI, 0.26-0.58; P < .001 |
| Kater et al23 , GLOW | VEN-I/CLB-O | Age >65 or CIRS >6 or Cl. creat <70 mL/min N = 211 | PFS FU: 27.7 mo | VEN-I > CLB-O HR: 0.216 (95% CI, 0.131-0.357); p < .0001) |
| Phase 2 trials | ||||
| Michallet et al24 , ICLL07 | I-O+/−4 courses FCO-I | Fit patients, no del 17p or p53 N = 135 | CR + BM MRD <0.01% FU: 26.3 | CR + MRD <0.01%: 62% (95% CI 55% to 69%) |
| Davids et al25 | FCR 6 courses-I (stop 2 y if BM uMRD) | Age <65 N = 85 | CR + BM uMRD 2 mo post FCR FU: 16.5 mo | CR + BM uMRD: 33%, (95% CI 23-44%) |
| Jain et al27 | VEN-I | Del17P and/or TP53 mut and/or del11q and/or U-IGHV and/or age >65 N = 80 | CR + CRi FU: 14.8 mo | CR + CRi: 88%, CR + uMRD: 61% |
| Wierda et al28 , CAPTIVATE | VEN-I uMRD : I vs placebo MRD+ : I vs V-I | Age <70 N = 164 | 1 y DFS FU: NA | I vs placebo: NS (95% vs 100%) 30-mo PFS ∼95% in all arms |
| Reference . | Trial . | Patients . | Primary end point . | Results . |
|---|---|---|---|---|
| Randomized phase 3 trials | ||||
| Shanafelt et al17 , ECOG1912 | FCR vs I-R | Age <70 no del 17p N = 529 | PFS FU: 33.6 mo | I - R > FCR (89.4% vs 72.9% at 3 y; HR: 0.35; 95% CI, 0.22-0.56; P < .001) OS: I - R > FCR (98.8% vs 91.5% at 3 y) HR: 0.17; 95% CI, 0.05-0.54; P < .001 |
| Al-Sawaf et al3 , CLL14 | CLB-O vs VEN-O | CIRS >6 and/or Cl. creat 30-69 mL/min N = 432 | PFS FU: 39.6 mo | VEN-O > CLB-O (NE vs 35.5 mo) HR: 0.31, 95% CI, 0.22–0.44; P < .0001 |
| Woyach et al1 , ALLIANCE | BR/I-R/I | Age >65 N = 447 | PFS FU: 38 mo | I-R > BR. HR: 0.38; 95% CI, 0.25-0.59; P < .001 I > BR. HR: 0.39; 95% CI, 0.26-0.58; P < .001 |
| Kater et al23 , GLOW | VEN-I/CLB-O | Age >65 or CIRS >6 or Cl. creat <70 mL/min N = 211 | PFS FU: 27.7 mo | VEN-I > CLB-O HR: 0.216 (95% CI, 0.131-0.357); p < .0001) |
| Phase 2 trials | ||||
| Michallet et al24 , ICLL07 | I-O+/−4 courses FCO-I | Fit patients, no del 17p or p53 N = 135 | CR + BM MRD <0.01% FU: 26.3 | CR + MRD <0.01%: 62% (95% CI 55% to 69%) |
| Davids et al25 | FCR 6 courses-I (stop 2 y if BM uMRD) | Age <65 N = 85 | CR + BM uMRD 2 mo post FCR FU: 16.5 mo | CR + BM uMRD: 33%, (95% CI 23-44%) |
| Jain et al27 | VEN-I | Del17P and/or TP53 mut and/or del11q and/or U-IGHV and/or age >65 N = 80 | CR + CRi FU: 14.8 mo | CR + CRi: 88%, CR + uMRD: 61% |
| Wierda et al28 , CAPTIVATE | VEN-I uMRD : I vs placebo MRD+ : I vs V-I | Age <70 N = 164 | 1 y DFS FU: NA | I vs placebo: NS (95% vs 100%) 30-mo PFS ∼95% in all arms |
A, acalabrutinib; B, bendamustine; C, Cyclophosphamide; Cl. creat, clearing of creatine; CR, complete remission; DFS, disease-free survival; F, Fludarabine; FU, median follow-up; I, ibrutinib; NA, not applicable; NE, not evaluable; NS, not significant; O, obinutuzumab; OS, overall survival; PFS, progression-free survival; R, rituximab; VEN, venetoclax.
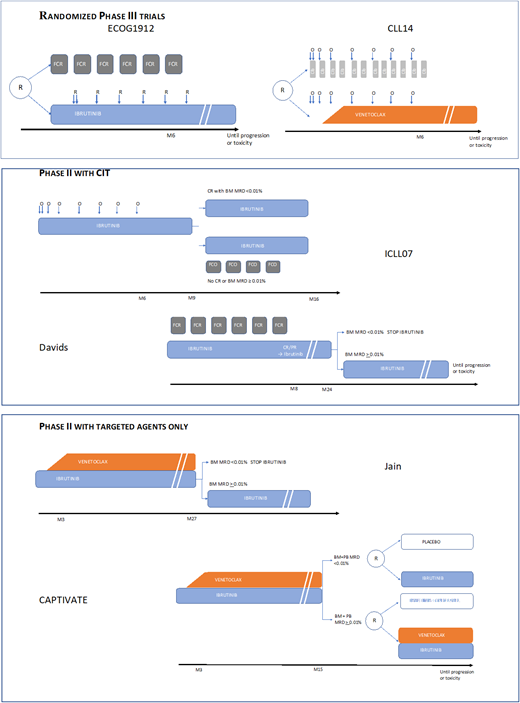
Outline of phase 3 and phase 2 time-limited frontline trials.
Outline of phase 3 and phase 2 time-limited frontline trials.
Ibrutinib-rituximab (I-R) until progression has been compared to FCR in a large randomized (2:1) phase 3 trial including 529 patients younger than 70 with no 17p deletion.17 I-R is clearly superior in terms of progression-free survival (PFS) and even OS. This is related to the large benefit of I-R over FCR for UM-IGHV patients. It is noteworthy that in M-IGHV cases, PFS after I-R and FCR remains similar at a follow-up of 33.6 months despite the fact that FCR is a 6-month treatment. The incidence of grade >3 adverse events (AEs) is similar in both arms, with fewer grade 3 or more infectious complications in the I-R arm than in the FCR (10.5% vs 20.3%).
The ALLIANCE trial compared 183 patients receiving bendamustine plus rituximab BR to 182 patients receiving ibrutinib plus rituximab I-R and 182 receiving ibrutinib (I) alone in a phase 3 randomized (1:1:1) trial including older adult patients (≥65 years of age).1 The median follow-up was 38 months. PFS was superior with the ibrutinib regimen vs the addition of B + R, with no difference between I and I-R. There was no difference in overall survival (OS). Patients receiving BR had a higher number of grade ≥3 hematologic AEs compared to ibrutinib-based treatments (61% vs 41% and 29%) and a lower number of grade ≥3 nonhematologic AEs (63% vs 74% each).
The CLL14 phase 3 trial comparing the venetoclax-obinutuzumab (VEN-O) combination with chlorambucil-obinutuzumab (CLB-O) randomized (1:1) 432 frail patients as defined by a cumulative illnesses rating scale (CIRS) >6 and/or eGFR between 30 and 69 mL/min.3 With a follow-up of 39.6 months, PFS and time to next treatment were significantly longer with VEN-O in both M- and UM-IGHV CLL, but no survival benefit was observed. Serious AEs occurred in 54% and 44% of the patients, respectively. The most common grade ≥3 was neutropenia in both groups (48% and 54%, respectively). Neutropenia occurred mainly during the course of obinutuzumab treatment.
The GLOW trial is a randomized (1:1) phase 3 trial stratified on IGHV mutational status and del(11q) comparing ibrutinib and VEN to CLB-O in patients older than 65 or younger with a CIRS >6 or with a creatinine clearance <70 mL/min.23 Patients with a del(17p) or TP53 mutation were not eligible. Two hundred and eleven patients were randomized (median age, 71 years). After a median follow-up of 27.7 months, VEN-I was significantly superior to CLB + O in terms of PFS (hazard ratio [HR], 0.216), complete remission (CR) rate, uMRD in bone marrow (BM), and time to next treatment. The most frequent grade >3 events for patients treated by I + V were neutropenia (34.9%), diarrhea (10.4%), and hypertension (7.5%).
In addition to these phase 3 trials, several phase 2 trials have explored the combination of ibrutinib with either CIT or VEN in frontline (Table 4; Figure 1).
In the ICLL07FILO trial,24 135 fit TP53 wild-type unmutated IGHV CLL patients received the combination of obinutuzumab and 15-month fixed-duration ibrutinib. At month 9, the 120 patients (92%) who were not in BM CR with MRD <0.01% received 4 courses of FC-O. At the final evaluation at month 15, a CR with BM uMRD rate of 62% was obtained with an acceptable toxicity. At a median follow-up at 36.7 months, OS and PFS at 3 years were 95.7% and 98%, respectively.
In a phase 2 trial,25 85 patients aged 65 or younger received I + FCR (6 cycles). Responders continued on ibrutinib maintenance for up to 2 years, and patients with BM uMRD were able to discontinue treatment. Thirty-three percent of patients achieved a CR with BM uMRD 2 months after the last course of FCR. The most common all-grade toxic effects were hematological. At a median follow-up of 16.5 months, 1 death was observed but no progression. The M. D. Anderson group tested 3 cycles of the I-FC-O combination in 45 M-IGHV fit patients, with similar excellent results.26
The venetoclax-ibrutinib combination (VEN-I) was tested in 80 “high-risk” patients defined by at least one of the following: 17p or 11q deletion, TP53 mutation, UM-IGHV, or an age of 65 years or older.27 In this heterogeneous population, the CR + CRi (complete remission with incomplete recovery) rate was 88%, and CR with uMRD was 61%. There was no additional toxicity in the combination of the 2 agents. Considering the short follow-up (14.8 months), no survival data are available.
Finally, in the CAPTIVATE MRD trial,28 VEN-I was also tested in 164 “high-risk” patients defined by at least one of the following: del(17p), del(11q) TP53 mutation, complex karyotype, or UM-IGHV. There was an MRD-guided randomization after 12 cycles of combined treatment, and uMRD patients were randomly assigned to placebo or ibrutinib, the others being randomized to receive ibrutinib or continued VEN-I. PFS rates were >95% across all randomized arms at 30 months.
In summary, all phase 3 trials have shown the superiority of targeted agents over CIT when considering the whole cohort. This difference is related to the large benefit of targeted agents in UM-IGHV cases. When considering M-IGHV cases, only CLB-O was inferior to a targeted-agent regimen whether compared to ibrutinib or I-O (ILLUMINATE) or VEN-O (CLL14).3,12 Conversely, in the ECOG trial and ALLIANCE trial no benefit of BTKi over CIT treatment (FCR or BR) was observed for M-IGHV cases whether alone or in combination with rituximab.1,17
Phase 2 trials have explored the addition of ibrutinib to an FC-anti-CD20 backbone with impressive results, but unfortunately this strategy was not compared to chemo-free therapies. Recent trials have tested time-limited targeted-agent combinations with very good results. The VEN-I combination MRD data are very encouraging, but follow-up is still short for survival analysis.27,28
It is noteworthy that targeted agents show a significant activity in TP53-altered cases. Any chemo-free regimen was superior to what was previously observed with CIT, as TP53-altered cases are resistant to CIT. Available data are the result of unplanned subgroup analysis in trials and tend to favor the use of BTKi or VEN-I over VEN +/− anti-CD20, despite the absence of a current head-to-head comparison.
In the absence of comorbidities preventing the use a certain targeted agent, usually BTKi, the decision of which targeted agent is preferable is always a matter of debate among physicians and of discussion with the patient. Besides the case of TP53 alterations, some clinical situations may tip the balance in favor of one or the other targeted agent. For example, in the case of a progressive CLL with a very short doubling time but no bulky lymph nodes we would tend to favor the use of VEN + anti-CD20, whereas in the case of a bulky disease, BTKi might be preferred.
Several trials are also ongoing that are testing mainly time-limited strategies (Table 6). They explore combinations with other BTKi and/or the triple combination of VEN with BTKi and anti-CD20. The results will be available in the near future.
Major ongoing trials evaluating fixed-duration targeted therapies against CIT or continuous targeted therapies
| Trial . | N pts . | Trial population . | Compared arms . | Primary end point . | |
|---|---|---|---|---|---|
| CLL13- GAIA GCLLSG NCT02950051 | 926 | ≥18 y fit/TP53 abn excluded | vs vs vs | CIT: FCR/BR × 6 cycles RVe: VEN-R (12 mo) GVe: VEN-O (12 mo) GIVe: VEN-O + I (up to 36 mo; VEN 12 mo) | MRD at mo 15 (CIT vs GVe) PFS (CIT vs GIVe) |
| ACE-CL-311 AstraZeneca NCT03836261 | 780 | ≥18 y fit/TP53 abn excluded | vs vs | CIT: FCR/BR × 6 cycles AV: VEN-O + acalabrutinib (15 mo; VEN 12 mo) AVG: VEN-O + acalabrutinib (15 mo; VEN 12 mo) | PFS (CIT vs AV) |
| CRISTALLO Hoffmann La Roche NCT04285567 | 165 | ≥18 y fit/TP53 abn excluded | vs | CIT: FCR or BR (if ≥65 y) × 6 cycles VG: VEN-O (12 mo) | BM MRD at mo 15 |
| FILO ERADIC NCT04010668 | 120 | ≥18 y fit/TP53 abn excluded | vs | CIT: FCR × 6 cycles VI: VEN-I (15 or 27 mo according to MRD) | BM MRD at mo 27 |
| NCRI UK CLL10 FLAIR CRUK/12/037 | 1516 | ≤75 y fit/eGFR >30 mL/min del(17p) <20% | vs vs | CIT: FCR × 6 cycles IR → I*: I + ritux → I (until progression) VI: VEN-I (flexible duration according to MRD) * arm IR replaced by I monotherapy in 2018 | PFS |
| ALLIANCE A041702 NCT03737981 | 454 | ≥70 y | vs | IO: I + O (until progression) IVO: VEN-O + I (15 mo; VEN 12 mo) | PFS |
| ECOG-ACRIN EA9161 NCT03701282 | 720 | 18-69 y del(17p) excluded | vs | IO: I + O (until progression) IVO: VEN-O + I (19 mo; VEN 12 mo) | PFS |
| GCLLSG CLL17 NCT04608318 | 897 | ≥18 y fit and unfit w/or w/o TP53 abn | vs vs | I: I (until progression) VG: VEN-O (12 mo) VI: VEN-I (15 mo; VEN 12 mo) | PFS |
| Trial . | N pts . | Trial population . | Compared arms . | Primary end point . | |
|---|---|---|---|---|---|
| CLL13- GAIA GCLLSG NCT02950051 | 926 | ≥18 y fit/TP53 abn excluded | vs vs vs | CIT: FCR/BR × 6 cycles RVe: VEN-R (12 mo) GVe: VEN-O (12 mo) GIVe: VEN-O + I (up to 36 mo; VEN 12 mo) | MRD at mo 15 (CIT vs GVe) PFS (CIT vs GIVe) |
| ACE-CL-311 AstraZeneca NCT03836261 | 780 | ≥18 y fit/TP53 abn excluded | vs vs | CIT: FCR/BR × 6 cycles AV: VEN-O + acalabrutinib (15 mo; VEN 12 mo) AVG: VEN-O + acalabrutinib (15 mo; VEN 12 mo) | PFS (CIT vs AV) |
| CRISTALLO Hoffmann La Roche NCT04285567 | 165 | ≥18 y fit/TP53 abn excluded | vs | CIT: FCR or BR (if ≥65 y) × 6 cycles VG: VEN-O (12 mo) | BM MRD at mo 15 |
| FILO ERADIC NCT04010668 | 120 | ≥18 y fit/TP53 abn excluded | vs | CIT: FCR × 6 cycles VI: VEN-I (15 or 27 mo according to MRD) | BM MRD at mo 27 |
| NCRI UK CLL10 FLAIR CRUK/12/037 | 1516 | ≤75 y fit/eGFR >30 mL/min del(17p) <20% | vs vs | CIT: FCR × 6 cycles IR → I*: I + ritux → I (until progression) VI: VEN-I (flexible duration according to MRD) * arm IR replaced by I monotherapy in 2018 | PFS |
| ALLIANCE A041702 NCT03737981 | 454 | ≥70 y | vs | IO: I + O (until progression) IVO: VEN-O + I (15 mo; VEN 12 mo) | PFS |
| ECOG-ACRIN EA9161 NCT03701282 | 720 | 18-69 y del(17p) excluded | vs | IO: I + O (until progression) IVO: VEN-O + I (19 mo; VEN 12 mo) | PFS |
| GCLLSG CLL17 NCT04608318 | 897 | ≥18 y fit and unfit w/or w/o TP53 abn | vs vs | I: I (until progression) VG: VEN-O (12 mo) VI: VEN-I (15 mo; VEN 12 mo) | PFS |
abn, abnormalities; A, acalabrutinib; I, ibrutinib; O, obinutuzumab; R, rituximab; VEN, venetoclax.
CLINICAL CASE 1 (continued)
How would you treat the first patient today? In 2021, 11 years after receiving FCR, this patient is still in clinical CR. These very long remissions were highlighted by the M. D. Anderson group, showing that a significant percentage of M-IGHV patients experience very prolonged remissions (>10 years) after FCR.29
Ten years later, this patient would still be eligible for treatment with FCR since he has M-IGHV CLL and no TP53 alteration. In 2021, continuous BTKi can also be considered but continued until progression. His comorbidities (high blood pressure, a history of coronary thrombosis on acetylsalicylic acid) do not restrain FCR but on the contrary, ibrutinib should be used with caution. Concerning the 1-year VEN-O combination, the results from CLL14 might be extrapolated to younger patients, in which the data show the superiority of VEN-O over CLB-O even in M-IGHV patients. As of yet, however, no data are available estimating the magnitude of the effect—if any—compared to FCR.
CLINICAL CASE 2 (continued)
How would you treat the second patient today? The outcome was, unfortunately, poorer in this patient, who progressed 30 months after BR with altered performance status and rapidly growing superficial polyadenopathy and splenomegaly. Despite adequate supportive care with infection prophylaxis, he died of sepsis during the reintroduction of BR.
All trials show the superiority of targeted agents in UM-IGHV CLL. Time-limited treatments are VEN-based combinations, either with an anti-CD20 or BTKi. Data from the CLL14 trial show the clear superiority of a 1-year treatment of VEN-O over CLB-O.
Moreover, in the CLL14 trial it was recently reported that the VEN-O combination could reduce the growth dynamics, as a lower growth rate was observed at reprogression after VEN-O.30
Approval of the targeted agents was obtained through randomization against a referenced therapeutic regimen, ie, age-adapted CIT. Data from a head-to-head comparison of chemo-free regimens are not available yet, and for patients eligible for either continuous BTKi or time-limited VEN-based combinations, a treatment decision relies mostly on comorbidities, comedications, and patient preferences. It is most likely that the life expectancy of this patient would have been increased with first-line targeted agents. This patient today would receive a targeted agent, at least in relapse.
What would be helpful for adjusting the duration of time-limited treatment?
Most of the data available to date in frontline have been generated by treatment strategies of an arbitrarily fixed duration, such as the CLL14 trial with a 1-year fixed duration of VEN-O.
The value of MRD status as a surrogate of PFS has been widely proven in the context of CIT.31 The demonstration of the MRD value through large trials led to approval by the European Medicines Agency of the use of uMRD as an intermediate end point in randomized clinical trials for drug approval.32 It appeared to be a worthy predictor in VEN-based treatment.3
Through the analysis of a large number of patients who received FCR in 3 clinical trials, it was demonstrated that high-sensitivity (0.0007%) MRD assessment in blood yielded additional prognostic information beyond the current standard sensitivity (0.01%).33 Currently, the duration of time-limited treatment is not guided by MRD status at the end of treatment. In the future, the use of high-sensitivity MRD assessment could be useful for determining response at a deep level in potentially curative time-limited regimens. Moreover, MRD kinetics will be needed to better understand the impact of the various novel agents and to be able to decide when to discontinue therapy in patients who do or do not achieve undetectable disease.34
Conclusion
Numerous ongoing trials demonstrate both physician and patient aspirations for time-limited therapies. Combining targeted agents is very promising, but it is important to keep in mind that follow-up is still short and that information on the efficacy of subsequent lines of therapy is lacking. Sensitive MRD should help tailor the optimal duration of the various strategies.
Today, a decision between continuous treatment with BCR inhibitors and VEN-based combinations relies mostly on comorbidities, comedications, and patient/physician preferences. The rapidly increasing understanding of genetic background and clonal evolution is not yet integrated into therapeutic decisions, but in the coming years it could supplant the “one-size-fits-all” tendency and allow more personalized approaches.
Conflict-of-interest disclosure
Vincent Lévy: honoraria: Abbvie, Astra-Zeneca, CSL Behring, Janssen.
Alain Delmer: honoraria: Janssen, Abbvie, AstraZeneca, Roche.
Florence Cymbalista: honoraria: Janssen, Abbvie, AstraZeneca, Roche.
Off-label drug use
Vincent Lévy: nothing to disclose.
Alain Delmer: nothing to disclose.
Florence Cymbalista: nothing to disclose.