Abstract
COVID-19 is frequently associated with abnormalities on coagulation testing and a coagulopathy driven by inflammation, intravascular coagulation activation, and microvascular thrombosis. Elevated D-dimer is the most common finding and is a predictor of adverse outcomes including thrombosis, critical illness, and death. Although COVID-19-associated coagulopathy has some similarities to disseminated intravascular coagulation, the platelet count is usually preserved, coagulation times are usually normal or minimally prolonged, and thrombosis is more common than bleeding, at least in noncritically ill patients. Bleeding is uncommon but may be a significant problem in critically ill patients, including those who may develop a consumptive coagulopathy with frank disseminated intravascular coagulation and those on extracorporeal membrane oxygenation. Blood product support to correct coagulopathy is reserved for bleeding patients or those requiring invasive procedures. Current recommendations suggest that all hospitalized patients should receive at least a prophylactic dose of anticoagulation. Results from a multiplatform randomized clinical trial suggest that therapeutically dosed anticoagulation may improve outcomes, including the need for organ support and mortality in moderately ill patients but not in those requiring critical care. The results of ongoing trials evaluating the impact of different antithrombotic strategies (therapeutic agents and intensity) on COVID-19 outcomes are eagerly awaited and are expected to have important implications for patient management. We also discuss COVID-19 vaccine-associated cytopenias and bleeding as well as vaccine-induced thrombotic thrombocytopenia, in which thrombosis is associated with thrombocytopenia, elevated D-dimer, and, frequently, hypofibrinogenemia.
Learning Objectives
Recognize the coagulation derangements commonly encountered in COVID-19 associated with outcomes including thrombosis, need for critical care support, and mortality
Appropriately monitor and manage coagulation abnormalities in hospitalized patients with COVID-19
Recognize and manage the rare complications of COVID-19 vaccination-associated thrombocytopenia with bleeding and vaccine-induced thrombotic thrombocytopenia (VITT)
Introduction
The COVID-19 pandemic has contributed to significant mortality and morbidity across the world. Despite unprecedented public health efforts including the development of multiple effective vaccines, the pandemic continues to rage in many parts of the world, with novel viral variants leading to recurrent outbreaks. While COVID-19 is a predominantly respiratory illness, hemostatic and thrombotic complications are common.1,2 Early in the pandemic, a high prevalence of thromboembolic complications was observed in patients with COVID-19.3,4 Many patients exhibit laboratory evidence of a coagulopathy reminiscent of, but not identical to, disseminated intravascular coagulation (DIC), which is associated with thrombosis risk, critical illness, and all-cause mortality.5,6 In critically ill patients, this coagulopathy may evolve into frank DIC. While less common than laboratory coagulopathy or thrombosis, bleeding occurs in up to 5% of hospitalized patients and has important implications for management.3 In this review we discuss our approach to COVID-19-associated coagulopathy (CAC) and bleeding.
CLINICAL CASE
A 51-year-old woman with hypertension and asthma presented with fever, cough, and dyspnea; polymerase chain reaction for SARS-CoV-2 was positive, and she was hospitalized due to the need for supplemental oxygen. Laboratory testing on admission revealed elevated dimerized plasmin fragment D (D-dimer; 1.4 mg/L [0.00-0.49]) and fibrinogen (345 mg/dL). Platelet count (238 × 109/L) and prothrombin time (PT; 10.8 seconds) were normal. Activated partial thromboplastin time (aPTT) was prolonged (43 seconds [23.1-30.9]).
CAC
Laboratory findings in CAC
Coagulation derangements suggesting hypercoagulability are common in COVID-19. Markedly elevated D-dimer is the most common finding and is associated with adverse outcomes, including thrombosis, the need for critical care support, and overall mortality.3,5,7 Elevated D-dimer levels correlate with levels of inflammatory markers, including C-reactive protein, sedimentation rate, procalcitonin, and ferritin, supporting an association with the inflammatory state of COVID-19.3 However, fibrinogen levels are usually elevated/normal, a platelet count <100 × 109/L is uncommon,3,8 and the PT is normal at presentation in the majority of patients. Thus, in most cases CAC is distinct from the prototypical consumptive coagulopathies, such as sepsis-induced coagulopathy (SIC) and DIC (Table 1).9,10 Moreover, CAC is less likely to be associated with bleeding than thrombosis. These differences are probably due to the fact that intravascular coagulation activation and microvascular thrombosis are relatively localized to the pulmonary circulation in CAC versus the more systemic activation of coagulation in DIC. Additionally, fibrinolysis is suppressed in CAC, which may contribute to the prothrombotic phenotype.11,12 Iba et al have proposed diagnostic criteria for CAC (Table 2).13
Similarities and differences between CAC and DIC/SIC
| . | CAC . | DIC . | SIC . |
|---|---|---|---|
| Platelet count | ↑↓ | ↓↓ | ↓ |
| D-dimer | ↑ | ↑↑ | ↑ |
| PT/aPTT | ↔↑ | ↑↑ | ↑ |
| Fibrinogen | ↑ | ↓↓ | ↓ |
| Antithrombin | ↔ | ↓ | ↓ |
| VWF | ↑ | ↑ | ↑ |
| FVIII | ↑ | ↑↓ | ↑ |
| LA/aPL | + | - | - |
| Thrombosis | ↑↑ | ↑ | ↑ |
| Bleeding | ↔↑ | ↑↑ | ↑ |
| . | CAC . | DIC . | SIC . |
|---|---|---|---|
| Platelet count | ↑↓ | ↓↓ | ↓ |
| D-dimer | ↑ | ↑↑ | ↑ |
| PT/aPTT | ↔↑ | ↑↑ | ↑ |
| Fibrinogen | ↑ | ↓↓ | ↓ |
| Antithrombin | ↔ | ↓ | ↓ |
| VWF | ↑ | ↑ | ↑ |
| FVIII | ↑ | ↑↓ | ↑ |
| LA/aPL | + | - | - |
| Thrombosis | ↑↑ | ↑ | ↑ |
| Bleeding | ↔↑ | ↑↑ | ↑ |
Proposed criteria for CAC compared with ISTH criteria for SIC and DIC
| Proposed CAC criteria13 . | ISTH SIC criteria10 . | ISTH DIC criteria9 . |
|---|---|---|
| ≥2 of the following: | ≥4 points: | ≥5 points: |
| 1) Platelet count <150 × 109/L 2) D-dimer elevation >2 × ULN 3) PT prolonged >1s or INR >1.2 4) Presence of micro and/or macrovascular thrombosis **Risk factors for the development of CAC include elevated fibrinogen and VWF and presence of lupus anticoagulant or antiphospholipid antibodies | 1) Platelet count, × 109/L <150 but ≥100 = 1 <100 = 2 2) INR >1.2 but ≤1.4 = 1 >1.4 = 2 3) SOFA score 1 = 1 ≥2 = 2 | 1) Platelet count, × 109/L <100 = 1 <50 = 2 2) D-dimer increased <5 × ULN = 2 ≥5 × ULN = 3 3) PT prolonged ≥3 s but <6 s = 1 prolonged ≥6 s = 2 4) Fibrinogen ≤1.0 g/L = 1 |
| Proposed CAC criteria13 . | ISTH SIC criteria10 . | ISTH DIC criteria9 . |
|---|---|---|
| ≥2 of the following: | ≥4 points: | ≥5 points: |
| 1) Platelet count <150 × 109/L 2) D-dimer elevation >2 × ULN 3) PT prolonged >1s or INR >1.2 4) Presence of micro and/or macrovascular thrombosis **Risk factors for the development of CAC include elevated fibrinogen and VWF and presence of lupus anticoagulant or antiphospholipid antibodies | 1) Platelet count, × 109/L <150 but ≥100 = 1 <100 = 2 2) INR >1.2 but ≤1.4 = 1 >1.4 = 2 3) SOFA score 1 = 1 ≥2 = 2 | 1) Platelet count, × 109/L <100 = 1 <50 = 2 2) D-dimer increased <5 × ULN = 2 ≥5 × ULN = 3 3) PT prolonged ≥3 s but <6 s = 1 prolonged ≥6 s = 2 4) Fibrinogen ≤1.0 g/L = 1 |
INR, international normalized ratio; SOFA, Sequential Organ Failure Assessment; ULN, upper limit of normal.
Thrombocytopenia and PT prolongation are less common in COVID-19 but predict poor clinical outcomes. In an analysis of 201 hospitalized patients from China, prolonged PT on admission was associated with the development of acute respiratory distress syndrome (ARDS; hazard ratio, 1.56 [1.32-1.83]; P < .001).14 Interestingly, thrombocytopenia was present in only 18.8% of these patients compared to >50% in patients with ARDS from other infectious causes. Compared to non-COVID ARDS, COVID-19 is associated with higher PT, antithrombin, fibrinogen, and platelet count and lower aPTT and D-dimer levels.15 In a meta-analysis of 9 studies including 1779 COVID-19 patients, the platelet count was significantly lower in patients with severe disease and patients who died.16 Similarly, a marked decrease in plasma fibrinogen has been reported shortly before death in a number of patients.5 An early study by Tang et al noted a higher prevalence of DIC by International Society on Thrombosis and Haemostasis (ISTH) criteria on admission in nonsurvivors vs survivors (71.4% vs 0.6%). However, subsequent studies report a much lower incidence of DIC at initial presentation. For example, none of the 150 consecutive patients with COVID-19 and ARDS and only 2.2% of 388 consecutive patients hospitalized with COVID-19 in Milan met the criteria for DIC at presentation.15,17 Thus, overt DIC is uncommon in COVID-19 but may develop in critically ill patients.3
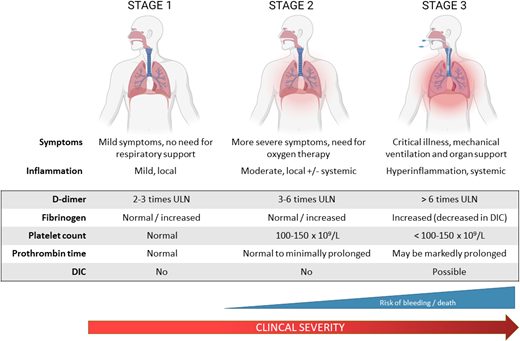
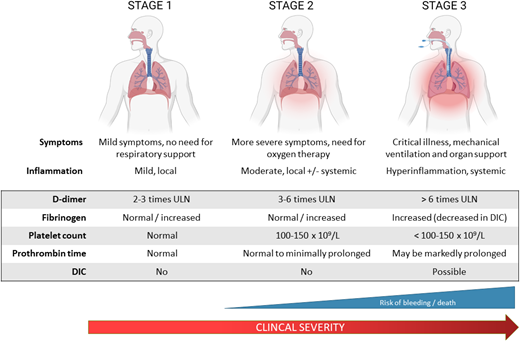
Based on the spectrum of coagulation abnormalities in COVID-19 that parallels disease severity, three stages of COVID-19 coagulopathy have been proposed (Figure 1).18 In stage 1, patients exhibit mild symptoms and mild localized inflammation and elevated D- dimer. Stage 2 is characterized by more severe symptoms requiring supplemental oxygen, with pulmonary inflammation and intravascular coagulation activation (elevated D-dimer, fibrinogen). Patients who progress to stage 3 require critical care support and demonstrate severe systemic inflammation and coagulopathy with markedly elevated D-dimer and fibrinogen, prolonged PT, and thrombocytopenia. A subset of these patients develops overt DIC that may be due to COVID-19 or bacterial superinfection and is associated with a high risk of bleeding and death.
Stages of CAC. The spectrum of coagulation abnormalities in COVID-19 parallels disease severity. In stage 1, patients exhibit mild symptoms and evidence of mild localized inflammation and coagulopathy with elevations in D-dimer approximately 2 to 3 times the upper limit of normal. Stage 2 is characterized by more severe symptoms requiring supplemental oxygen with pulmonary inflammation and intravascular coagulation activation in the lungs as well as some systemic involvement. This is reflected in further D-dimer elevation to 3 to 6 times the upper limit of normal. Patients progressing to stage 3 require critical care with respiratory and other organ support and demonstrate severe systemic inflammation and coagulopathy with markedly elevated D-dimer and fibrinogen, prolonged PT, thrombocytopenia, and high incidence of thromboembolism. A proportion of these patients will develop overt DIC as a result of their COVID-19 illness or other superimposed infections.18
Stages of CAC. The spectrum of coagulation abnormalities in COVID-19 parallels disease severity. In stage 1, patients exhibit mild symptoms and evidence of mild localized inflammation and coagulopathy with elevations in D-dimer approximately 2 to 3 times the upper limit of normal. Stage 2 is characterized by more severe symptoms requiring supplemental oxygen with pulmonary inflammation and intravascular coagulation activation in the lungs as well as some systemic involvement. This is reflected in further D-dimer elevation to 3 to 6 times the upper limit of normal. Patients progressing to stage 3 require critical care with respiratory and other organ support and demonstrate severe systemic inflammation and coagulopathy with markedly elevated D-dimer and fibrinogen, prolonged PT, thrombocytopenia, and high incidence of thromboembolism. A proportion of these patients will develop overt DIC as a result of their COVID-19 illness or other superimposed infections.18
Antiphospholipid antibodies in COVID-19
The aPTT is prolonged in some patients with COVID-19. A likely explanation is the presence of a lupus anticoagulant (LA) or antiphospholipid antibodies (aPL). In a series of 56 hospitalized patients with COVID-19, LA was detected in 44.6%; 8.9% also had either anticardiolipin (aCL) or anti-beta-2-glycoprotein antibodies.19 Both traditional aPL (such as immunoglobulin [Ig] G and IgM aCL) and various “noncriteria” aPL (including anti-PS/PT as well as IgA isotypes of aCL and anti-beta-2-glycoprotein) have been detected in COVID-19. Whether these are transient aPL, as reported in other viral infections,20 or persistent aPL, more likely to be associated with long-term thrombotic risk, is unknown. Moreover, LA assays must be interpreted with caution in critically ill patients due to potential confounding by anticoagulant therapy. High levels of acute phase reactants such as C-reactive protein can also cause false positives by prolonging in vitro clotting times. Prolonged aPTT due to LA may necessitate the use of the anti-factor Xa assay for monitoring heparin therapy.
Other coagulation parameters
In addition to the commonly evaluated laboratory parameters discussed above, elevated coagulation factor VIII (FVIII) and von Willebrand factor (VWF) are frequent findings in hospitalized COVID-19 patients and correlate with elevations in inflammatory markers.15,21-23 Nonsurvivors have lower antithrombin levels than survivors (84% vs 91%), but levels generally do not drop below 80%.5,15,22 In a study comparing 48 intensive care unit (ICU) to 30 general ward patients, fibrinolytic pathway parameters, including plasminogen activator inhibitor, tissue plasminogen activator, and thrombin activatable fibrinolysis inhibitor, were significantly higher in the ICU patients.22 Viscoelastic testing (thromboelastography, rotational thromboelastometry) reveals increased clot strength with contributions of both elevated fibrinogen and platelets.21,24 Fibrinolysis is also suppressed.11,12 Thrombin generation is normal to increased even in patients receiving prophylactic anticoagulation.25 However, the clinical utility of these assays is uncertain, and they are not recommended outside of a research setting.
Pathogenesis of CAC
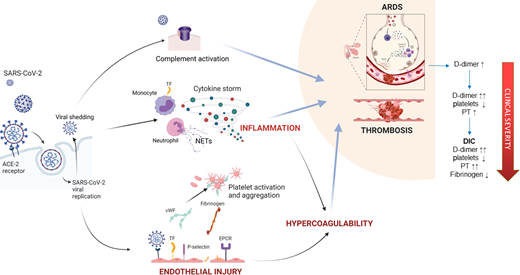
Thromboinflammation is central to the pathophysiology of CAC, which is driven by a combination of direct effects of viral infection on pulmonary vascular endothelium, a local and systemic inflammatory response, and cross talk between the inflammatory and coagulation pathways (Figure 2). Pathogenic mechanisms contributing to CAC have been reviewed in detail by Iba et al13 : (1) direct viral effects and inflammation: SARS-CoV-2 enters respiratory epithelium and endothelium via the angiotensin-converting enzyme 2 receptor. Direct endothelial effects include procoagulant changes such as surface adhesion molecule expression and the release of VWF and FVIII. Viral replication and shedding trigger an inflammatory response, or “cytokine storm.” Proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor upregulate procoagulant molecules, including tissue factor (TF), P-selectin, fibrinogen, FVIII, and VWF; (2) neutrophil extracellular traps: neutrophil activation and release of neutrophil extracellular traps promote platelet activation, phosphatidylserine exposure, activation of factor V and factor XI, and thrombin generation26 ; (3) complement activation: SARS-CoV-2 spike proteins directly activate complement,27 and complement deposition and microthrombosis are observed in the lungs, skin, kidneys, and hearts of patients with severe disease28 ; (4) effects on coagulation and fibrinolytic systems: hypoxia and vasoconstriction induce the expression of TF and plasminogen activator inhibitor disrupting the balance between coagulation and fibrinolysis.29 Patients with COVID-19 have increased thrombin and plasmin generation potential and an increase in fibrin formation,25 consistent with other reports of decreased fibrinolysis.11,12 Perturbation of the balance between thrombin generation (fibrin clot formation) and plasmin generation (fibrinolytic pathways) is critical to the pathophysiology of COVID-19.11,21 In severe COVID-19, the lag time to thrombin, plasmin, and fibrin formation is increased, suggesting a loss in coagulation-activating mechanisms in severe disease.25 While coagulation activation is initially restricted to the lungs and manifests as an increase in D-dimer, severe disease is characterized by systemic inflammation and disseminated microthrombosis causing a consumptive coagulopathy.
Pathogenesis of CAC. SARS-CoV-2 binds the angiotensin-converting enzyme 2 receptor on respiratory epithelium and endothelial cells. Viral replication and shedding lead to pneumocyte and endothelial cell apoptosis, triggering an inflammatory response, which in a portion of patients leads to pathogenic cytokine storm. These cytokines and endothelial injury result in procoagulant changes, including the release of VWF and FVIII and the upregulation of TF, P-selectin, and fibrinogen. Anticoagulant proteins such as endothelial protein C receptor, antithrombin, and thrombomodulin are downregulated. Fibrinolytic impairment also contributes to hypercoagulability. Neutrophil activation and the release of neutrophil extracellular traps further stimulate thrombosis, and platelets in COVID-19 may exhibit marked hyperreactivity. SARS-CoV-2 spike proteins may directly amplify complement, and contribute to vascular injury and microthrombosis. The coagulation derangements observed in COVID-19 are a direct result of microthrombosis, which is initially localized to the lungs, causing elevations in D-dimer and fibrinogen. As the disease progresses, more systemic inflammation and the activation of coagulation lead to thrombocytopenia and prolongation of clotting times. In some patients this may progress to a frank consumptive coagulopathy.
Pathogenesis of CAC. SARS-CoV-2 binds the angiotensin-converting enzyme 2 receptor on respiratory epithelium and endothelial cells. Viral replication and shedding lead to pneumocyte and endothelial cell apoptosis, triggering an inflammatory response, which in a portion of patients leads to pathogenic cytokine storm. These cytokines and endothelial injury result in procoagulant changes, including the release of VWF and FVIII and the upregulation of TF, P-selectin, and fibrinogen. Anticoagulant proteins such as endothelial protein C receptor, antithrombin, and thrombomodulin are downregulated. Fibrinolytic impairment also contributes to hypercoagulability. Neutrophil activation and the release of neutrophil extracellular traps further stimulate thrombosis, and platelets in COVID-19 may exhibit marked hyperreactivity. SARS-CoV-2 spike proteins may directly amplify complement, and contribute to vascular injury and microthrombosis. The coagulation derangements observed in COVID-19 are a direct result of microthrombosis, which is initially localized to the lungs, causing elevations in D-dimer and fibrinogen. As the disease progresses, more systemic inflammation and the activation of coagulation lead to thrombocytopenia and prolongation of clotting times. In some patients this may progress to a frank consumptive coagulopathy.
Bleeding in COVID-19
Bleeding is infrequent in the setting of CAC, which has a predominantly prothrombotic phenotype. Helms et al reported bleeding complications in 2.7% of 150 patients with COVID-related ARDS.15 Among 2773 hospitalized COVID-19 patients from New York, major bleeding was reported in 1.9% not on therapeutic anticoagulation and in 3% of those on therapeutic anticoagulation.30 However, in an analysis of 400 patients hospitalized with COVID-19 in Boston, Al-Samkari et al found a higher overall bleeding rate of 4.8%; major bleeding occurred in 2.3%, including 5.6% of critically ill patients, and was associated with a platelet count <150 × 109/L at admission (Odds Ratio 2.90 [1.05-7.99]).3 Preliminary data from a multiplatform, randomized controlled trial found a 0.9% and 1.9% rate of major bleeding in noncritically ill participants on prophylactic or therapeutic anticoagulation, respectively.31 Among critically ill patients, however, major bleeding occurred in 3.1% on therapeutic anticoagulation and 2.4% on thromboprophylaxis.32
Among patients with COVID-19 ARDS treated with extracorporeal membrane oxygenation (ECMO), major bleeding occurs in >40%, including intracranial bleeding in up to 12%, which is significantly higher than the 2% to 6% bleeding rate reported in non-COVID-19 ECMO cohorts.33,34 DIC, shear stress-induced acquired von Willebrand syndrome, COVID-19-associated endothelitis, and intensive anticoagulation are implicated in severe bleeding on ECMO.34 The anticoagulation required for ECMO and the activation of coagulation from artificial surfaces may confound interpretation of coagulation studies. Careful interpretation is required to balance anticoagulation, bleeding, and thrombosis in patients receiving ECMO.
Management of CAC
Which laboratory parameters should be evaluated in CAC?
In outpatients with COVID-19, there is currently insufficient evidence to recommend monitoring of coagulation markers, including D-dimer, platelet count, PT, and fibrinogen, since these are unlikely to have an impact on management. For hospitalized patients, we agree with the interim recommendations from the ISTH and the American Society of Hematology to serially monitor D-dimer levels, platelet count, PT, and fibrinogen since these help with risk stratification.35 Worsening of these parameters may warrant more aggressive critical care support and consideration of investigational therapies. D-dimer elevation is nonspecific and need not prompt imaging for venous thromboembolism (VTE) in the absence of clinical symptoms or signs of VTE.36 However, increasing D-dimer in the setting of decreasing C-reactive protein may warrant screening for VTE.
Role of blood product transfusion
There is no evidence that blood component therapy to correct abnormal laboratory parameters improves outcomes in the absence of bleeding. In patients with bleeding or those needing invasive procedures, we suggest transfusion of platelets to maintain a platelet count ≥50 × 109/L, cryoprecipitate if fibrinogen is <150 mg/dL, and plasma for an international normalized ratio ≥1.8 after correcting fibrinogen. Four factor prothrombin complex concentrate (25 units/kg) may be used instead of plasma in patients with severe coagulopathy and bleeding to avoid volume overload that can worsen respiratory status.
Anticoagulation
As with other coagulopathies, treatment of the underlying condition is the only definitive solution. Based on the association of D-dimer and other coagulation markers with severe disease, mortality, and thrombosis, at least prophylactic-dose anticoagulation is recommended in hospitalized patients with COVID-19.1,36,37 Notably, this recommendation is identical to that for other acutely ill inpatients. In the absence of significant renal impairment, we encourage the use of low-molecular-weight heparin (LMWH) over unfractionated heparin (UFH) to avoid possible heparin resistance with UFH. If UFH is used, we suggest monitoring anti-factor Xa levels due to potential confounding of the aPTT due to elevated FVIII and fibrinogen or LAs. Further, anticoagulation recommendations may need to be modified for patients with severe thrombocytopenia, bleeding risk, and extremes of body weight. Early in the pandemic, there were reports of thrombosis despite standard thromboprophylaxis. Based on these reports and extreme laboratory derangements, clinicians frequently escalated anticoagulation to intermediate or even therapeutic intensity. However, whether a higher intensity of anticoagulation reduces morbidity or mortality without an unacceptable increase in bleeding risk remains uncertain and is being evaluated in multiple trials.
Preliminary data from a multiplatform, randomized controlled trial comprising 3 global studies (REMAP-CAP, ATTACC, and ACTIV-4A) comparing the effects of standard-dose vs full-dose heparin on the primary outcome, a composite of 21-day “organ support-free” days and in-hospital mortality, were recently made available as preprints.31,32 The enrollment of moderately ill patients was stopped early for superiority; therapeutic anticoagulation reduced the requirement for organ support and mortality (OR 1.29 [1.04-1.61]) irrespective of baseline D-dimer.31 Meanwhile, the enrollment of critically ill patients was halted due to futility, with no decrease in mortality and a higher rate of major bleeding with therapeutic anticoagulation.32 These apparently discrepant results suggest that perhaps therapeutic heparin works best if started early. The INSPIRATION study reported that in critically ill patients with COVID-19, intermediate- vs prophylactic-dose LMWH did not result in a decrease in the composite end point of thrombosis, extracorporeal membrane oxygenation, or mortality within 30 days.38 The multinational pragmatic RAPID COVID COAG trial (NCT04362085) is evaluating therapeutic vs prophylactic doses of LMWH or UFH in hospitalized, noncritically ill COVID-19 patients with elevated D-dimer on the composite outcome of ICU admission, noninvasive positive pressure ventilation, mechanical ventilation, and death at 28 days. The ACTION trial, a pragmatic trial at 31 sites in Brazil, compared in-hospital therapeutic anticoagulation with rivaroxaban (92%) or enoxaparin followed by rivaroxaban until day 30 vs prophylactic-dose enoxaparin or UFH in hospitalized patients with elevated D-dimer. There was no difference in the primary efficacy outcome of time to death and duration of hospitalization and supplemental oxygen use through 30 days but significantly more bleeding in the rivaroxaban group.39 Pending the final results of these and other studies, the American Society of Hematology guideline panel suggests prophylactic over intermediate or therapeutic dosing in moderately and severely ill patients with COVID-19 without confirmed or suspected VTE. Higher-intensity anticoagulation may be preferred in moderately ill (non-ICU, stage 2) patients deemed at high thrombotic and low bleeding risk. Therapeutically dosed anticoagulation is appropriate for patients with preexisting indications such as atrial fibrillation and mechanical heart valves and, if needed, to maintain extracorporeal circuits and vascular access devices.
COVID-19 vaccines: cytopenias, bleeding, and thrombosis
The COVID-19 vaccines have proven to be highly safe and effective and remain the most promising tool in containing the COVID-19 pandemic. Cases of immune thrombocytopenia and bleeding, including life-threatening hemorrhage, have been diagnosed after exposure to the mRNA-based vaccines produced by Moderna (mRNA-1273) and Pfizer-BioNTech (BNT162b2).40 Fortunately, the majority of these respond to immune thrombocytopenia-directed treatments such as intravenous Ig and corticosteroids.40 It is unclear whether this represents a causal relationship or a coincidental association; however, a recent analysis from the Vaccine Adverse Event Reporting System reported that the incidence of thrombocytopenia after either mRNA vaccine was 0.8 per million doses, which is no higher than the background rate of immune thrombocytopenia (3.3 per 100 000 adults).41
More recently, rare cases of a vaccine-induced thrombotic thrombocytopenia (VITT) were reported after administration of the adenoviral vector vaccines (ChAdOx1 nCoV-19 and Ad26.COV2.S).42 The estimated incidence is 1 case per 100,000 exposures with a slight female preponderance, and it occurs more commonly in younger (<55-60 years) individuals, although this could reflect the demographics of individuals vaccinated early. Occurring 4 to 30 days postvaccination, VITT is associated thrombocytopenia and thrombosis, particularly at unusual sites such as the cerebral or splanchnic circulations, along with high levels of platelet-activating IgG antibodies that recognize platelet factor 4 (PF4) in the absence of heparin. Anti-PF4/heparin antibodies are detectable by enzyme-linked immunosorbent assays, but rapid immunoassay may yield false-negative results. Functional assays should be performed in the presence and absence of PF4. The standard serotonin release assay may yield false-negative results as VITT antibodies exhibit PF4-dependent activity and may demonstrate decreased reactivity in the presence of heparin. Patients with VITT frequently have a consumptive coagulopathy with elevated D-dimer and hypofibrinogenemia. VITT is associated with high mortality, with cerebral hemorrhage as the leading cause of death. Treatment with a nonheparin anticoagulant and intravenous Ig is recommended, with platelet transfusions reserved for bleeding. While much is still to be learned about the incidence, pathogenesis, and optimal management of VITT, given the millions of individuals vaccinated worldwide, these hematologic complications are vanishingly rare and should not hinder vaccination efforts.
CLINICAL CASE (continued)
Our patient had findings typical of CAC with an elevated d-dimer and fibrinogen with normal platelets and PT. An LA was detected, accounting for her prolonged aPTT. She received a prophylactic dose of LMWH in addition to dexamethasone. D-dimer trended up to a peak of 4.1 mg/dL, and platelet count dropped to 118 × 109/L on day 5 of hospitalization. PT remained normal. Oxygen requirements peaked at 4 L by nasal cannula on day 9 and gradually improved over the following week. No thrombotic events occurred. She was discharged home without outpatient thromboprophylaxis on day 15. Repeat testing to determine whether her LA is persistent was pending at discharge.
Summary
Inflammation, intravascular coagulation activation, and microvascular thrombosis cause coagulation derangements in COVID-19. Elevated D-dimer and fibrinogen levels are the most common finding and are predictive of adverse outcomes, including the need for critical care and mortality. In contrast to DIC, thrombocytopenia is rare, and the PT/aPTT are usually normal or minimally prolonged. Some patients exhibit aPTT prolongation due to aPL. Bleeding, while rare, may occur in critically ill patients that develop a consumptive coagulopathy and those on ECMO. Blood-product support to correct the coagulopathy is reserved for bleeding patients or those requiring invasive procedures. The results of ongoing trials evaluating the impact of higher-intensity anticoagulation on COVID-19 outcomes are eagerly awaited. Until then, all hospitalized patients should receive standard thromboprophylaxis.
Acknowledgments
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grant K99HL150594 (to S.C) and T32HL007525 (to G.F.G.) and an American Society of Hematology Scholar Award (to S.C.).
Conflict-of-interest disclosure
Gloria F. Gerber: no competing financial interests to declare.
Shruti Chaturvedi: no competing financial interests to declare.
Off-label drug use
Gloria F. Gerber: the off-label use of prothrombin complex concentrate is discussed.
Shruti Chaturvedi: the off-label use of prothrombin complex concentrate is discussed.