Abstract
Early in the pandemic, COVID-19-related increases in rates of venous and arterial thromboembolism were seen. Many observational studies suggested a benefit of prophylactic anticoagulation for hospitalized patients using various dosing strategies. Randomized trials were initiated to compare the efficacy of these different options in acutely ill and critically ill inpatients as the concept of immune-mediated inflammatory microthrombosis emerged. We present a case-based review of how we approach thromboembolic prophylaxis in COVID-19 and briefly discuss the epidemiology, the pathophysiology, and the rare occurrence of vaccine-induced thrombotic thrombocytopenia.
Learning Objectives
Appreciate the incidence, risk factors, and pathophysiology of thrombosis in COVID-19 infection
Accurately prescribe anticoagulation prophylaxis before, during, and after hospitalization
Be able to recognize and diagnose vaccine-induced immune thrombotic thrombocytopenia
CLINICAL CASE
A 62-year-old physician had been rounding on the COVID-19 ward early in the pandemic (April 2020) and contracted the disease. After 1 week, he became hypoxic and was admitted to the general medical floor, requiring 3L nasal oxygen. He had no major comorbidities and received prophylactic-dose low-molecular-weight heparin (LMWH).
Introduction
COVID-19 infection clearly increases the risk of thrombotic events for hospitalized patients, but rates of reported incidence have varied. A meta-analysis of retrospective studies involving 64 503 patients showed that deep vein thrombosis (DVT) had an overall prevalence of 11.2% and pulmonary embolism (PE) of 7.8% in those needing hospitalization.1 Pooled rates of venous thromboembolism (VTE) were higher in the intensive care unit (ICU) setting (27.9% vs 7.1% in the ward).2 Studies screening patients for VTE reported a prevalence rate of 25.2% compared to a rate of 12.7% in those testing only symptomatic patients.1 When more than 95% of the hospitalized patients received pharmacologic VTE prophylaxis, rates were lower, at 3.1% for non-ICU patients and 7.6% for ICU patients.3 Venous or arterial thrombosis was independently associated with higher mortality risk (hazard ratio [HR], 1.82; 95% CI, 1.54-2.15; P < .001).4 Increased arterial thrombotic events were also reported during hospitalization, with a prevalence of 3.9% for coronary artery events and 1.6% for stroke.1 Acute limb ischemia was reported in 0.3% to 1% of hospitalized patients, predominantly affecting men, with 18% of patients suffering limb loss.5
Rates of postdischarge VTE in COVID-19 patients, however, were not as high. In a cohort from California, VTE rates in COVID-19 patients were no higher than patients testing negative for COVID-19 (1.8% vs 2.2%; P = .16).6 A pre-/postpandemic comparison of postdischarge VTE did not show a statistically significant risk of VTE in COVID-19 patients (odds ratio, 1.6; 95% CI, 0.77-3.1).7
Pathophysiology of thrombosis in COVID-19 infection
The coagulopathy of COVID-19 infection is complex, involving an interplay between endothelial cell injury, inflammation, and coagulation. Infection of pulmonary alveolar cells causes severe endothelial injury and is marked by a local inflammatory infiltrate and microthrombi.8 Microthrombi in alveolar capillaries and vascular congestion were present in nearly 45% of patients who died from acute respiratory complications due to COVID-19.9 Alveolar capillary microthrombi were 9 times more prevalent in those who died from COVID-19 infection compared to similar severe H1N1 infections, and increased thrombotic complications were mostly noted in those with longer hospital stays.8 In severe infection, this immune response can be exaggerated and cause systemic hyperinflammation, marked by high levels of proinflammatory cytokines.10,11 The downstream effects of endothelial cell activation and a systemic inflammatory response include a hypercoagulable state and both micro- and macrovascular thrombosis, with multiple interrelated mechanisms.
Activation of endothelial cells, platelets, and leukocytes
Direct infection of vascular endothelial cells with resultant apoptosis has been described in an autopsy series of COVID-19 patients.12 This, along with complement activation,13 contributes to a local inflammatory reaction and further endothelial cell activation, with the expression of tissue factor, release of von Willebrand factor (VWF), and decreased synthesis of nitric oxide and prostacyclin,14 all of which promote coagulation. An increase in VWF coupled with a relative imbalance in ADAMTS-13 has been demonstrated in patients with COVID-19 of varying severity,15 and elevated VWF antigen and low ADAMTS-13 activity seem to correlate with the development of VTE.16 Platelet activation increases in severe COVID-19 infection, triggering increased tissue factor expression and coagulation.14,17,18 Similarly, neutrophil activation increases, with the release of neutrophil extracellular traps that promote thrombosis in these patients.19
Coagulation and fibrinolysis
The increase in tissue factor expression on a variety of cells induces extrinsic coagulation pathway activation, while the release of factor VIII from damaged endothelium, NETosis, and platelet and complement activation all contribute to intrinsic pathway activation. These events result in thrombin generation and the formation of a fibrin clot. COVID-19 infection is also marked by high levels of fibrinogen,10,11 an acute phase reactant, and the inhibition of fibrinolytic pathways. Patients with severe infection and shutdown of fibrinolysis—as measured by a lack of clot lysis on viscoelastic testing—were found to have greater dimerized plasmin fragment D (D-dimer) and fibrinogen levels and a significantly greater incidence of clinical VTE (Table 1).20
| COVID-19 infection . | COVID-19 (adenovirus) vector vaccines . |
|---|---|
| Older age (>75) | Middle age (18-49) |
| Male | Female |
| Obesity | Platelet count <150 |
| Hypertension | Splanchnic vein and cerebral vein thrombosis reported in higher rates |
| Prior cardiac disease | |
| Active cancer or recent anticancer treatment | |
| Elevated D-dimer, FDP, LA | |
| VTE in lower-extremity deep veins and PE commonly seen |
| COVID-19 infection . | COVID-19 (adenovirus) vector vaccines . |
|---|---|
| Older age (>75) | Middle age (18-49) |
| Male | Female |
| Obesity | Platelet count <150 |
| Hypertension | Splanchnic vein and cerebral vein thrombosis reported in higher rates |
| Prior cardiac disease | |
| Active cancer or recent anticancer treatment | |
| Elevated D-dimer, FDP, LA | |
| VTE in lower-extremity deep veins and PE commonly seen |
FDP, fibrin degradation products.
Heparin-induced thrombocytopenia
Given the propensity for thrombosis, patients admitted with COVID-19 are frequently managed with anticoagulation prophylaxis. This, combined with the systemic inflammatory response, increases the likelihood of development of heparin-induced thrombocytopenia (HIT), another potential etiology of VTE in these patients. Rates of HIT in severe COVID-19 infection are variable across studies, with rates ranging from 0.16% in all admitted patients to 8.1% in an ICU population. Anti-platelet factor 4 (PF4) antibodies are frequently detected even in the absence of clinical HIT,21,22 and 1 group has demonstrated non-heparin-dependent platelet-activating immune complexes that may contribute to thrombosis.23
Antiphospholipid antibodies
A final potential mechanism for thrombosis in patients with COVID-19 is the development of antiphospholipid antibodies, an appealing theory given the hyperinflammatory response and potential for antibody production. As many as 90% of critically ill patients have been found to be positive for a lupus anticoagulant (LA).24,25 The clinical significance of this is unclear, as an association with thrombosis has not been consistently demonstrated,25 assays may be susceptible to interference by C-reactive protein and unfractionated heparin, and rates of anticardiolipin and anti-β2-glycoprotein seem to be significantly lower.24-26 However, 1 study at a tertiary care center in the US found a significant association between LA positivity and venous or arterial thrombosis.26
CLINICAL CASE (continued)
Our patient was a real one and in fact is the senior author of this article. He received prophylactic-dose enoxaparin while on the medical floor.
Anticoagulation prophylaxis
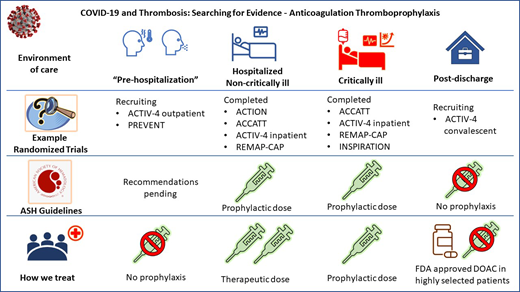
When choosing a prophylactic approach, the risk of bleeding must be considered, particularly if the platelet count is <50 000, and mechanical prophylaxis is likely the best approach until the bleeding risk abates. The dose and choice of anticoagulant also depend on renal function, and it may be reasonable to give “obesity-adjusted” doses—eg, enoxaparin 40 mg twice daily or a daily total dose of 0.5 mg/kg based on limited evidence.27 Of note, obesity-adjusted prophylactic dosing differs from intermediate dosing.
Noncritically ill patients
The American Society of Hematology (ASH) draft guidelines (updated 8 February 2021) suggest prophylactic over intermediate- or therapeutic-dose anticoagulation for patients with acute (not critical) illness and acknowledge pending results of the combined analysis of the REMAP-CAP, ACTIV-4, and ATTAC multiplatform randomized controlled trials (mpRCT).28 These 3 mpRCTs harmonized their protocols to accelerate the battle against COVID-19 infection and reported their pooled results in separate papers for critically ill and noncritically ill patients.29,30
The first patient was randomized on 21 April 2020, and these trials were stopped on 22 January 2021. The preprint report appeared on 17 May 2021 when the prespecified superiority stopping rule threshold was attained.30 The primary analysis population had 2219 participants with confirmed COVID-19 who did not require ICU-level organ support, which was defined as high-flow oxygen, mechanical ventilation (invasive or noninvasive), vasopressors, or inotropes. They were randomized in an open-label manner to therapeutic-dose heparin (94.7% received LMWH, mostly enoxaparin) or usual care thromboprophylaxis (71.7% low dose and 26.5% intermediate dose) for up to 14 days. The primary outcome of survival to hospital discharge without organ support (as defined above) through 21 days occurred in 76.4% of participants receiving the usual care and increased by 4.6% with therapeutic-dose anticoagulation, with a median adjusted odds ratio of 1.29% and a 99% probability of the therapeutic dose being effective. The probability of survival to hospital discharge with therapeutic-dose heparin was 87.1%, with a median absolute improvement of 1.3%. Major bleeding occurred in 1.9% and 0.9% of therapeutic-dose and usual care participants, respectively. A prespecified analysis based on D-dimer levels showed a slightly better probability of superiority in patients with high levels (97.3%) vs low levels (92.9%).
The ACTION trial randomized 615 hospitalized patients (who were primarily stable and not critically ill) in 31 Brazilian sites with elevated D-dimer levels above the upper limit of normal from 24 June 2020 to 26 February 2021 to therapeutic doses of rivaroxaban (20 mg or renally adjusted 15 mg daily) for 30 days or standard VTE prophylaxis.31 Clinically unstable patients (10%) randomized to rivaroxaban first received enoxaparin or unfractionated heparin (1 patient) followed by rivaroxaban when clinically stable. Patients randomized to standard prophylaxis primarily received enoxaparin (84%), and 13% continued postdischarge prophylaxis at their clinician's discretion. The hierarchical analysis of time to death, duration of hospitalization, and duration of supplemental oxygen was performed using the win ratio, and there was no difference in efficacy with a win ratio of 0.86; P = .40. There was also no difference in each component of this composite outcome or thromboembolic event. However, major or clinically relevant nonmajor bleeding was increased with therapeutic anticoagulation (8%) compared to standard dosing (2%) with a relative risk of 3.64 (P = .001).
Until the 3 mpRCTs are fully published, it is reasonable to use either therapeutic or prophylactic doses of LMWH (preferred if renal function is acceptable) or heparin. Direct oral anticoagulants (DOACs) should not be used unless there is another indication, such as atrial fibrillation.
How we treat noncritically ill hospitalized patients
We discuss the risks and benefits of prophylactic vs therapeutic doses of anticoagulation and their impact on the need for organ support in noncritically ill patients hospitalized with COVID-19. We use therapeutic dosing of LMWH or unfractionated heparin based on these early results and eagerly await peer-reviewed publication and guideline updates.
CLINICAL CASE (continued)
Oxygen requirement increased for our patient, and a high flow was needed with transfer to the ICU, where LMWH was increased to intermediate dose. Several days later, intubation and mechanical ventilation were required. Meanwhile, the patient's father was also critically ill with COVID-19, and his clinicians used therapeutic-dose LMWH: 2 patients with the same last name, 1 floor apart, with different doses to prevent the same complications—all were searching for evidence.
Critically ill patients
ASH guidelines suggest prophylactic- over intermediate-dose anticoagulation in critically ill patients, and recommendations for therapeutic dose vs prophylactic dose are forthcoming (Figure 1).
ASH recommendations for VTE prophylaxis in COVID patients with critical illness. Reproduced with permission from Cuker et al.28
ASH recommendations for VTE prophylaxis in COVID patients with critical illness. Reproduced with permission from Cuker et al.28
Therapeutic dose
Data for critically ill participants randomized while receiving organ support as previously described in the 3 mpRCTs were posted on 12 March 2021.29 This prespecified cohort stopped recruitment for critically ill patients on 19 December 2020 because of futility. The primary outcome of survival until discharge and the median number of organ-support-free days was 3 in those randomized to therapeutic-dose LMWH or heparin and 5 days with the usual care (41% prophylactic-dose and 51% intermediate-dose LMWH or heparin; adjusted odds ratio, 0.87; 95% credible interval, 0.70-1.08; likelihood of not achieving meaningful relative improvement of at least 20%, 99.8%).
Intermediate dose
Among 600 randomized COVID-19 patients in the ICU, 562 were included in the primary analysis of the INSPIRATION trial.32 Intermediate enoxaparin doses of 1 mg/kg once daily vs 40 mg daily (with adjustment for weight and creatinine clearance) were compared for the primary composite efficacy outcome of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality. No differences were noted in 30-day outcomes between intermediate and prophylactic doses (45.7% vs 44.1%; odds ratio, 1.06; P = .70). Major bleeding occurred in 2.5% and 1.4% of patients who received intermediate and prophylactic dosing, respectively, and the risk difference failed to meet the noninferiority margin. Severe thrombocytopenia occurred in 6 patients, all of whom received an intermediate dose.
How we treat critically ill patients
We use prophylactic- and not intermediate- or therapeutic- dose LMWH or heparin in critically ill patients with COVID-19.
CLINICAL CASE (continued)
After a couple of weeks in the ICU with respiratory failure and septic shock, our patient was extubated but suffered a small PE while his central line was being removed. He was treated for 3 months with a DOAC, which precluded the decision regarding post-hospital discharge anticoagulant prophylaxis.
Postdischarge prophylaxis
This patient had symptoms of PE with acute chest pain and worsened hypoxemia and was stable for computed tomography, rendering a straightforward workup. We only pursue diagnosing VTE based on clinical suspicion and do not screen or use D-dimer thresholds to screen in accordance with guidance statements.33
As discussed previously, rates of VTE are relatively low post hospitalization; however, mitigation of arterial and VTE along with reductions in all-cause mortality deserves investigation. A prospective registry of 4906 patients with COVID-19 were contacted a mean of 92 days after discharge, and 1.55% developed VTE, arterial thromboembolic events (ATE) occurred in 1.71%, and all-cause mortality was 4.83%. There was a 46% relative reduction in the composite outcome of VTE, ATE, and mortality (P = .0046) in the multivariable analysis.34 The ACTIV-4b National Institutes of Health-sponsored trial is investigating whether apixaban at prophylactic or therapeutic doses and low-dose aspirin, compared to placebo, can reduce these complications after hospitalization. The studies are summarized in Table 2.
List of selected anticoagulation trials in COVID-19
| Patient population . | Trial name and trial identifier . | Intervention . | Comparison . | Outcome . | Status . |
|---|---|---|---|---|---|
| Hospitalized (non-ICU) | ACTION Trial31 | Therapeutic rivaroxaban or LMWH | Usual care thromboprophylaxis | Mortality, length of stay, and oxygen use | Published |
| ATTACC30 NCT04372589 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| ACTIV-4a30 NCT04505774 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| REMAP-CAP30 NCT02735707 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| Hospitalized (ICU) | ATTACC29 NCT04372589 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint |
| ACTIV-4a29 NCT04505774 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| REMAP-CAP29 NCT02735707 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| INSPIRATION32 NCT04486508 | Intermediate-dose heparin or LMWH | Prophylactic-dose heparin or LMWH | Composite of VTE, arterial thrombosis, or ECMO | Published | |
| Post discharge | ACTIV-4 Convalescent NCT04650087 | Prophylactic-dose apixaban | Placebo | Composite outcome of symptomatic DVT, PE, other VTE, ischemic stroke, acute MI, other ATE, and all-cause mortality | Recruiting |
| Outpatient | ACTIV-4c NCT04498273 | Prophylactic-dose apixaban, therapeutic- dose apixaban, ASA | Placebo | Composite of symptomatic DVT or PE, ATE, MI, ischemic stroke, hospitalization for cardiovascular/pulmonary events, and all-cause mortality | Recruiting |
| PREVENT-HD NCT04508023 | Prophylactic-dose rivaroxaban | Placebo | Composite of symptomatic VTE, MI, ischemic stroke, systemic embolism, acute limb ischemia, all-cause hospitalization, and all-cause mortality | Recruiting |
| Patient population . | Trial name and trial identifier . | Intervention . | Comparison . | Outcome . | Status . |
|---|---|---|---|---|---|
| Hospitalized (non-ICU) | ACTION Trial31 | Therapeutic rivaroxaban or LMWH | Usual care thromboprophylaxis | Mortality, length of stay, and oxygen use | Published |
| ATTACC30 NCT04372589 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| ACTIV-4a30 NCT04505774 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| REMAP-CAP30 NCT02735707 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| Hospitalized (ICU) | ATTACC29 NCT04372589 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint |
| ACTIV-4a29 NCT04505774 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| REMAP-CAP29 NCT02735707 | Therapeutic heparin or LMWH | Usual care thromboprophylaxis | Hospital discharge without need for organ support | Preprint | |
| INSPIRATION32 NCT04486508 | Intermediate-dose heparin or LMWH | Prophylactic-dose heparin or LMWH | Composite of VTE, arterial thrombosis, or ECMO | Published | |
| Post discharge | ACTIV-4 Convalescent NCT04650087 | Prophylactic-dose apixaban | Placebo | Composite outcome of symptomatic DVT, PE, other VTE, ischemic stroke, acute MI, other ATE, and all-cause mortality | Recruiting |
| Outpatient | ACTIV-4c NCT04498273 | Prophylactic-dose apixaban, therapeutic- dose apixaban, ASA | Placebo | Composite of symptomatic DVT or PE, ATE, MI, ischemic stroke, hospitalization for cardiovascular/pulmonary events, and all-cause mortality | Recruiting |
| PREVENT-HD NCT04508023 | Prophylactic-dose rivaroxaban | Placebo | Composite of symptomatic VTE, MI, ischemic stroke, systemic embolism, acute limb ischemia, all-cause hospitalization, and all-cause mortality | Recruiting |
ASA, aspirin; ECMO, extracorporeal membrane oxygenation; MI, myocardial infarction.
The US Food and Drug Administration has approved rivaroxaban and betrixaban for postdischarge VTE prophylaxis; however, betrixaban is not commercially available. The prophylactic dose of 10 mg of rivaroxaban for a total of 31 to 39 days has been approved but should not be used in patients with creatine clearance less than 30 mL/min, with drug-drug interactions and high bleeding-risk conditions.27
How we treat patients after discharge
We only use postdischarge prophylaxis in selected patients in accordance with US Food and Drug Administration approval for medically ill patients without increased bleeding risk using a DOAC.
“Prehospitalization” prophylaxis
The trial results above suggest that higher doses of anticoagulation are not effective late in the disease course, and it is a reasonable hypothesis that anticoagulation very early in the disease may be beneficial to decrease immuno-micro pulmonary thrombosis. Two trials with DOACs are underway to help answer this question (Table 2).
How we treat nonhospitalized outpatients
We do not use anticoagulation prophylaxis early in COVID-19 for patients who do not require hospitalization.
Postvaccination: vaccine-induced immune thrombotic thrombocytopenia
The approval and widespread administration of vaccines for COVID-19 brought yet another element into the discussion of COVID-19 and thrombosis. Vaccine-induced immune thrombotic thrombocytopenia (VITT) or thrombosis with thrombocytopenia syndrome is the name given to the thrombotic complications currently being reported across the world following the administration of the adenovirus vector vaccines (ChAdOx1 nCoV-19 [AstraZeneca/COVIDSHIELD] and Ad26.COV2.S [Johnson and Johnson/Janssen]) against SARS-CoV-2.35 The underlying pathophysiology of VITT is not fully understood. One hypothesis is that the polyanionic constituents of the adenovirus vector vaccines cause the formation of antibodies to PF4. These antibodies against PF4 then induce platelet activation, causing both thrombocytopenia and thrombosis. VITT is similar to autoimmune heparin-induced thrombocytopenia (aHIT), with the presence of anti-PF4 polyanion antibodies inducing platelet activation in the absence of and independently of heparin exposure; however, VITT and aHIT differ in the distribution of the thrombi.36,37
VITT generally presents between 4 to 30 days after initial vaccination with either adenovirus vector vaccine.35 Early reports showed that VITT primarily presented in younger females. Data on the incidence range from 1 case per 26 000 to 1 case per 127 000 doses of ChAdOx1 nCoV-19 vaccine administered and 1 case per 500 000 doses of the Ad26.COV2.S. No specific risk factors have been identified. In VITT, thrombosis tends to manifest in rare sites, most notably in the cerebral venous sinuses and in the splanchnic and hepatic veins.35,38
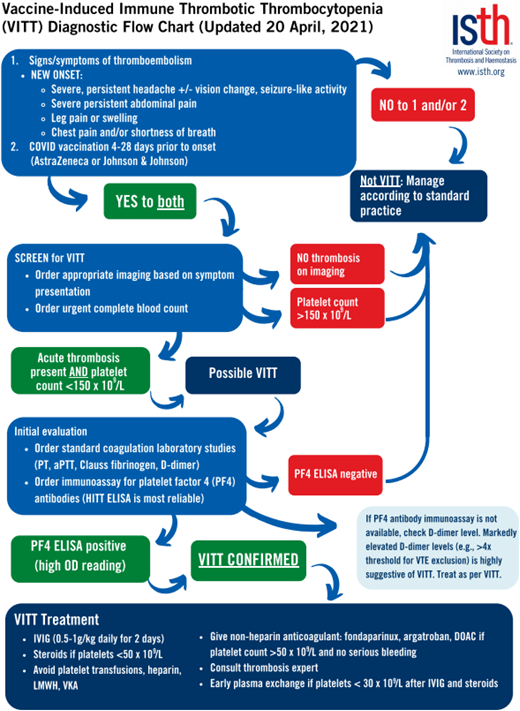
On presentation, laboratory testing generally shows varying levels of thrombocytopenia, high D-dimer levels, and low fibrinogen levels.35,38,39 Diagnostic testing for PF4 antibodies via enzyme-linked immunosorbent assay (ELISA) results is one of the more sensitive testing modalities for VITT, while the rapid HIT tests such as the chemiluminescent immunoassay and latex immunoturbidometric assay are not sensitive and can yield false-negative results.38 Principles of management mirror those of aHIT, with the goal of inhibiting Fcγ receptor-mediated platelet activation through the use of intravenous immune globulin (with suggested daily dosing of 0.5-1 g/kg of ideal body weight for at least 2 days) and the use of either oral or parental nonheparin anticoagulants.37 (See Figure 2 for management suggestions from the International Society on Thrombosis and Hemostasis [ISTH].)40 Vaccination with Ad26.COV2.S carries a warning about the potential development of VITT, especially in women aged 18 to 49.36
VITT diagnostic flowchart from ISTH interim guidance published 20 April 2021. aPTT, activated partial thromboplastin time; IVIG, intravenous immunoglobulin, PT, prothrombin time. Reproduced with permission from the ISTH.41
VITT diagnostic flowchart from ISTH interim guidance published 20 April 2021. aPTT, activated partial thromboplastin time; IVIG, intravenous immunoglobulin, PT, prothrombin time. Reproduced with permission from the ISTH.41
Conclusion
The thromboembolic burden of COVID-19 seems to be decreasing as the pandemic evolves, and a multitude of mechanisms are purported to explain the elevated risk with this infection. Key to understanding the timing, dosing, and patient setting for prophylaxis is an appreciation of immune-induced inflammatory microthrombosis. Robust randomized trials are the best tools to understand the appropriate use of anticoagulation to prevent thromboembolic complications, and the final results of many ongoing studies are eagerly awaited.
Conflict-of-interest disclosure
Bright Thilagar: no competing financial interest to declare.
Mohamad Beidoun: no competing financial interest to declare.
Ruben Rhoades: no competing financial interest to declare.
Scott Kaatz: research funding: Janssen, BMS, Osmosis Research, National Institutes of Health; consultancy: Janssen, BMS, Alexion/Portola, Novartis, CSL Behring, Gilead.
Off-label drug use
Bright Thilagar: nothing to disclose.
Mohamad Beidoun: nothing to disclose.
Ruben Rhoades: nothing to disclose.
Scott Kaatz: nothing to disclose.
Scott Kaatz’s institution received research support from Osmosis Research for one of the multi-platform trials using therapeutic dose anticoagulation prophylaxis.