Abstract
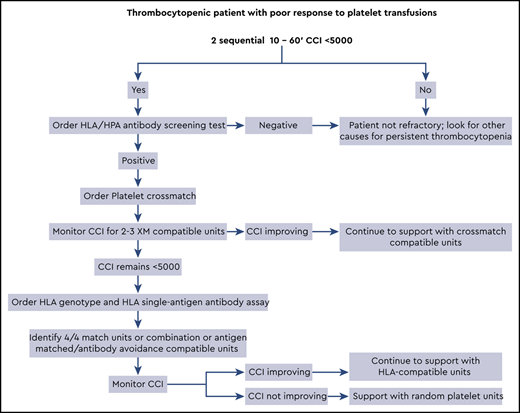
Platelet refractoriness continues to be a problem for thrombocytopenic patients because the risk of a major spontaneous or life-threatening bleed significantly increases when platelet counts drop below 10 × 109/L. The majority of patients have nonimmune causes driving the refractoriness, such as bleeding, medications, or diffuse intravascular coagulation; however, this article is dedicated to the diagnosis and support of patients with immune-based platelet refractoriness. Antibodies to class I HLA molecules (A and B alleles) are responsible for most immune-based refractory cases, with antibodies to platelet antigens seen much less frequently. Patients may be supported with either crossmatch-compatible or HLA-matched/compatible platelet units. When trying to select HLA units it can be difficult to find a perfect “4 of 4” match for the patient’s class IA and IB alleles. In these cases, it is better to use the antibody specificity prediction method, which identifies compatible units that lack antigens recognized by the patient’s anti-HLA antibodies. For an algorithmic approach to the patient with platelet refractoriness, see Visual Abstract.
Learning Objectives
Describe the role of immune-based factors in causing platelet refractoriness
Describe how to diagnose platelet refractoriness
Understand the availability and relative advantages of different compatible platelet products for a patient with platelet refractoriness
Clinical case
A 60-year-old woman with newly diagnosed acute myeloid leukemia is admitted for induction therapy. She has a history significant for multiple pregnancies (G5P5). Her platelet count on admission is 14,000/μL (150,000/μL to 450,000/μL). She reports a minor nosebleed the day before, lasting for <5 minutes. She reports no visible blood in her sputum, urine, or stools. Her physical examination is remarkable only for a few scattered petechiae on both arms. A peripheral smear reveals normal red cell morphology, with no spherocytes or schistocytes, and marked thrombocytopenia. Urinalysis and stool guaiac for blood are negative. The next day her platelet count is 5,000/μL. No new bleeding is identified. The clinical team orders a platelet transfusion and notes that her posttransfusion platelet count (taken the next morning) is 4,000/μL. Over the next 2 days this trend of lower-than-expected platelet increments continues. Her team requests a clinical consult with the transfusion service to better understand her refractory state and develop a plan to correct her thrombocytopenia.
Background
Platelet refractoriness is defined as a repeated suboptimal response to platelet transfusions with lower-than-expected posttransfusion count increments. Refractoriness can be caused by immune and nonimmune factors, with nonimmune factors (Table 1) responsible for 60% to 80% of cases.1 Immune factors, which play a role in 10% to 25% of patients with platelet refractoriness, include antibodies against four antigen classes: HLA class I, human platelet antigens (HPAs), ABO, and drug-dependent antibodies. In most cases HLA antibodies have been implicated.1 Antibodies against HLA arise because of pregnancy, solid organ transplantation, or blood transfusions. It is the residual white blood cells found in cellular blood components that cause HLA alloimmunization.2 Before the widespread use of leukoreduction, platelet refractoriness was seen in 30% to 70% of patients with bone marrow failure3 ; however, a Canadian study found that leukoreduction lowered HLA alloimmunization from 19% to 7% and alloimmune platelet refractoriness from 14% to 4% for patients undergoing chemotherapy for acute leukemia or stem cell transplantation (SCT).4 Despite this reduction, platelet refractoriness is still an important clinical problem in SCT and for patients with hematologic disorders.
Immune and nonimmune causes of platelet refractoriness
| Nonimmune causes . | Immune-mediated causes . |
|---|---|
| Fever, infection, or sepsis | Antibodies against HLA class I |
| Bleeding | ABO-mismatched platelets |
| Accelerated platelet consumption (DIC, microangiopathic hemolytic anemia) | Antibodies against human platelet antigens |
| Drugs (amphotericin B, vancomycin, ATG, interferons) | Antibodies against drug–platelet glycoprotein complex |
| Splenic sequestration | |
| Graft-versus-host disease | |
| Poor platelet quality or greater storage age |
| Nonimmune causes . | Immune-mediated causes . |
|---|---|
| Fever, infection, or sepsis | Antibodies against HLA class I |
| Bleeding | ABO-mismatched platelets |
| Accelerated platelet consumption (DIC, microangiopathic hemolytic anemia) | Antibodies against human platelet antigens |
| Drugs (amphotericin B, vancomycin, ATG, interferons) | Antibodies against drug–platelet glycoprotein complex |
| Splenic sequestration | |
| Graft-versus-host disease | |
| Poor platelet quality or greater storage age |
ATG, antithymoglobulin; DIC, diffuse intravascular coagulation.
Defining immune-based refractoriness
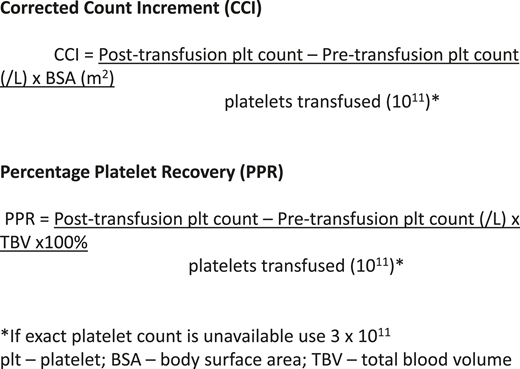
To define platelet refractoriness, one must follow posttransfusion platelet increments in a systematic fashion. The corrected count increment (CCI) and the percent platelet response (PPR) are the most frequently used formulas for tracking the posttransfusion increment adjusted for the size of the patient and the dosage administered.5 In both cases the pretransfusion platelet count is subtracted from the posttransfusion count and divided by the number of platelets transfused (Figure 1). The important difference is that the CCI uses the patient’s body surface area to normalize the calculation, whereas the PPR uses the patient’s blood volume. Most studies define refractoriness as a CCI of <5,000 after 2 sequential transfusions.2 However, a CCI of <7,500 or a PPR of <30% are also accepted values.6
Confirming immune-based refractoriness
When we suspect that a patient is refractory to platelet transfusions, we try to answer two questions: Is the patient truly refractory? And is the refractoriness caused by immune or nonimmune factors? To begin a workup, 2 posttransfusion platelet counts are taken within 10 to 60 minutes after the transfusion is completed. Studies have shown that platelets need at least 60 minutes to equilibrate within the intravascular space.7 Logistically however, a 1-hour posttransfusion count can be difficult to obtain. As a result, many use a 10-minute postcount for the CCI calculation.8 Some believe that a reduced 1-hour CCI points to an immune cause for refractoriness. However, the evidence to support this concept is confounded and highly variable.5,9-12
Once refractoriness has been confirmed, immune and nonimmune factors should be considered (Table 1) A good history and physical examination are usually sufficient to rule out nonimmune factors such as active bleeding, diffuse intravascular coagulation, and drug-induced thrombocytopenia. Sepsis and fever can also contribute to the nonimmune refractory state; however, these comorbidities are often present in patients with acute leukemia or SCT. Because most chronically thrombocytopenic patients with hematologic disorders are complicated, some immune and nonimmune factors may be present simultaneously.13,14
Antibody specificity
Platelets display class I HLA molecules, platelet-specific glycoproteins, and a low level of ABO on their surface membrane. These antibodies can bind to cognate antigens on the surface of transfused platelets and remove them from the patient’s circulation.
Class I HLA
The HLA system is composed of highly polymorphic cell surface proteins that are responsible for distinguishing self from nonself in the immune response. Class I HLA molecules are present on platelets and most nucleated cells in the body, whereas class II molecules are mostly restricted to cells involved in antigen presentation. Class I consists of three loci: HLA-A, HLA-B, and HLA-C; however, platelets predominantly express HLA-A and HLA-B alleles, and antibodies against HLA-C are not a significant cause of immune-based refractoriness. HLA antigens are highly immunogenic: The risk of alloimmunization is 11% with 1 pregnancy, 32% with ≥4 pregnancies,15 and 23% for multiply transfused patients.16
Platelet-specific antigens
There are 35 known human platelet antigens (HPAs). The HPA system has less antigenic variability when compared with the HLA system, which may be why far fewer antibodies against HPA are implicated in immune-based platelet refractory cases. Alloimmunization to HPA antigens has been reported in 2% to 8% of multiply transfused thrombocytopenic patients,2,16 and refractoriness due to HPA antibodies is rarely seen.17-19 Indeed, antibodies to HPA are usually found in combination with HLA antibodies.20
ABO
If a patient has high titers of anti-A or anti-B antibodies, then substantial clearance of donor platelets bearing cognate ABO antigens could occur. This problem may be avoided by transfusing ABO-identical platelets, because these units typically cause a better platelet increment than ABO-nonidentical units.21,22 For a full exploration of this topic, see the accompanying article by Dunbar.41
Drug-induced antibodies
Although several mechanisms for drug-induced antibody formation have been described, most clinically relevant drug-dependent platelet antibodies are thought to result when a drug interacts with platelet membrane glycoproteins.23,24 Drugs commonly implicated are listed in Table 2.25,26 Drug-induced antibodies can cause a rapid onset of thrombocytopenia that usually resolves within 3 to 4 days after drug discontinuation. There are also non–drug-dependent antibodies that do not require the continued presence of the drug for reactivity.
Drugs reported to cause drug-dependent platelet antibodies26
| Drugs . |
|---|
| Abciximab |
| Carbamazepine |
| Ceftriaxone |
| Eptifibatide |
| Heparin |
| Oxaliplatin |
| Phenytoin |
| Piperacillin |
| Piperacillin/tazobactam |
| Quinidine |
| Quinine |
| Rifampin |
| Sulfamethoxazole/trimethoprim |
| Tirofiban |
| Vancomycin |
| Drugs . |
|---|
| Abciximab |
| Carbamazepine |
| Ceftriaxone |
| Eptifibatide |
| Heparin |
| Oxaliplatin |
| Phenytoin |
| Piperacillin |
| Piperacillin/tazobactam |
| Quinidine |
| Quinine |
| Rifampin |
| Sulfamethoxazole/trimethoprim |
| Tirofiban |
| Vancomycin |
These drugs were associated with drug-dependent antibodies in ≥10 patients.
Testing for antibodies
When faced with a new patient, we often begin with a screening test that confirms the presence of HLA or HPA antibodies. At our institution we use a standard enzyme-linked immunosorbent assay that is rapid and, if negative, will save the time and expense needed for a full refractory workup. This assay determines only whether HLA or HPA antibodies are present; other tests are needed to define antibody specificity.
HLA antibody testing was initially performed with a lymphocytotoxic assay, which consists of incubating serum from a potential recipient with lymphocytes from prospective donors. Antibody binding caused complement-mediated lysis, indicating incompatibility. By using a panel of HLA phenotyped donors that were representative of the regional ethnic pool, the number of compatible donors could be assessed and the percent panel reactivity could be calculated. Variations of this test were used for >50 years, but the advent of solid phase testing has largely supplanted it.
Solid phase testing (also known as single-antigen bead or Luminex assay; Luminex Corp., Austin, TX) uses beads coated with individual HLA antigens. Antibody binding is detected by staining with fluorescently labeled antihuman globulin, and the level of antibody is characterized via flow cytometry, flow microarrays, or enzyme-linked immunosorbent assay. The strength (avidity) or amount of antibody binding is expressed as the mean fluorescent intensity (MFI). Single-antigen assays allow identification of multiple HLA antibody specificities that could not be readily distinguished via cytotoxic assays. The assay results in a list of antibody specificities and their MFIs. A calculated panel reactivity can also be used for an overall estimate of alloimmunization.
The increased sensitivity of the solid phase assay can create problems, because the clinical significance of low-level antibodies (MFI <500 to 1,000) is unclear. One study showed that weak to moderate HLA antibodies detectable by solid phase assay (but negative by lymphocytotoxic assay) were not associated with platelet refractoriness.27 This finding creates a problem for HLA labs, which must determine an MFI threshold that corresponds to a “positive” or clinically significant antibody. Unfortunately, there is wide interlaboratory variability, with a range of 500 to 6,000 MFI used as a cutoff value.28
In an effort to identify clinically significant antibodies, an adaptation of the solid phase assay was developed that targets complement-fixing antibodies. Although some platelets are removed from the circulation by macrophages stimulated by antigen–antibody interactions, a subset is bound by the CIq protein, which activates the classic complement cascade, ending with direct platelet lysis.29 The evidence is mixed as to the clinical importance of complement-fixing antibodies. For solid organ transplants, C1q-binding anti-HLA antibodies appear to be correlated with antibody-mediated rejection.30 However, no similar association was found for platelet-refractory patients with weak to moderate HLA antibody levels.31 More studies are needed to confirm or dismiss the utility of this assay for platelet-refractory patients.
Key question 1
Why are the patient’s posttransfusion increments lower than expected?
Answer
The patient’s epistaxis and petechiae are not likely to be significant contributing factors to her refractory state, and a review of her medications was noncontributory. Because there are no other obvious nonimmune causes, a workup for immune-based refractoriness should begin. A screening test should be ordered to confirm alloimmunization against HLA or HPA. If antibodies are present, the next step involves identification of compatible platelet units.
Identifying compatible platelet units
Finding a compatible platelet unit for an alloimmunized patient depends on the tests available, the frequency of the patient’s HLA type relative to the pool of HLA-matched donors, and the level of alloimmunization. Different methods can be used to identify a compatible unit (Table 3). Platelet crossmatching and HLA matching are frequently used, and the success rate of these strategies is comparable.32-34
Comparison of methods used to identify compatible platelet units for alloimmunized patients
| . | Crossmatched . | HLA matched . | HLA compatible . |
|---|---|---|---|
| Method | Test patient’s serum against a panel of platelets to determine compatibility | Identify platelet donors with perfect (4/4) match for patient’s HLA class IA and IB alleles | ASP: Use antibody specificities to select donor units that lack corresponding antigens |
| Pros | • Rapid turnaround-time | • 4/4 match ensures HLA compatibility | • Larger donor pool |
| • Obtain compatible units without HLA genotype or HLA antibody testing | • Reduced risk of future alloimmunization | • Reduced risk of future alloimmunization | |
| • Compatible with HLA and HPA antibodies | |||
| Cons | • Difficult to find compatible units for highly alloimmunized patients | • HLA genotyping required | • Not useful for HPA antibodies |
| • Risk of alloimmunization for mismatched HLA antigens | • Limited donor pool for some patients | • HLA antibody testing required |
| . | Crossmatched . | HLA matched . | HLA compatible . |
|---|---|---|---|
| Method | Test patient’s serum against a panel of platelets to determine compatibility | Identify platelet donors with perfect (4/4) match for patient’s HLA class IA and IB alleles | ASP: Use antibody specificities to select donor units that lack corresponding antigens |
| Pros | • Rapid turnaround-time | • 4/4 match ensures HLA compatibility | • Larger donor pool |
| • Obtain compatible units without HLA genotype or HLA antibody testing | • Reduced risk of future alloimmunization | • Reduced risk of future alloimmunization | |
| • Compatible with HLA and HPA antibodies | |||
| Cons | • Difficult to find compatible units for highly alloimmunized patients | • HLA genotyping required | • Not useful for HPA antibodies |
| • Risk of alloimmunization for mismatched HLA antigens | • Limited donor pool for some patients | • HLA antibody testing required |
Table adapted from Forest and Hod.1
ASP, antibody specificity prediction.
Platelet crossmatch
This method is usually the fastest and easiest way to obtain a compatible unit. The solid phase red cell adherence assay mixes a panel of donor platelets with the patient’s serum. Antibodies against HLA or HPA that bind to the platelets are visualized with indicator red cells coated with anti–immunoglobulin G. The crossmatch approach is commonly used because compatible units usually can be obtained within 24 hours, and both HLA and HPA compatibility issues are covered with no additional testing. Despite these advantages, HLA-matched units are sometimes preferred because the provision of HLA-matched platelets may reduce future alloimmunization. Most importantly, platelet crossmatching tends to be problematic with highly alloimmunized patients, which can make it difficult to find enough compatible units.
HLA match
Patients with platelet refractoriness can be supported with apheresis platelets from donors whose HLA-A and HLA-B antigens match those of the patient. However, obtaining a supply of 4/4 matches is possible only for blood centers that have a large number of HLA-typed donors and a well-organized inventory. Blood centers that cannot track the HLA type of units in inventory must resort to recruiting known donors, which causes delays in obtaining units and usually allows a very limited supply of platelet units.
When exact matches are unavailable, one can use the antibody profile determined by the single-antigen bead test to select donor units that lack the corresponding cognate antigens (ie, HLA compatible).13,14,35 This antibody specificity prediction (ASP) method is equivalent to HLA matching in terms of efficacy. In addition, the ASP method increases the pool of compatible donors when compared with the number available under traditional HLA-matching criteria, making it easier to support patients with platelet refractoriness.35 It is important to note that an HLA-compatible unit carries a potential risk for further alloimmunization, similar to a crossmatch-compatible unit.
HLAMatchmaker is another method for identifying HLA-compatible platelet units.36 Using the derived amino acid sequence of HLA class I alleles, Duquesnoy et al36 developed software that predicts epitopes within alloantibody-accessible regions of HLA molecules. These epitopes, or “eplets,” consist of clusters of amino acids that are brought together by the tertiary structure of the HLA molecule. The HLAMatchmaker algorithm (http://www.hlamatchmaker.net) predicts compatibility based on these defined epitopes. Studies have found that the HLAMatchmaker approach successfully identified donors associated with good transfusion outcomes in refractory recipients.37
The older antigen match grade system (A, BU, B2U, BX, C, and D matches) has been rendered obsolete by molecular typing methods and the specificity of the single-bead assay, and it should no longer be used.38 Compatibility across cross-reactive groups is also less of a concern in selecting compatible platelet units, because the single-antigen assay allows the specific delineation of relevant antibodies.
HPA match
Although the incidence of HPA antibodies causing transfusion refractoriness is small, this possibility should be investigated when most of the crossmatches are incompatible or when HLA-matched transfusions fail. If antibodies against HPA are present, then donors of known platelet antigen phenotype may be recruited. The patient’s relatives, who may share the patient’s phenotype, should also be tested.
Other options
Family members can sometimes provide directed platelet units that are a good match. However, if the patient is scheduled for SCT from a related donor, antibodies against minor-HLA antigens can develop and possibly create problems with engraftment.39 The complement inhibitor eculizumab has been used to increase the CCI in a limited number of transfusion refractory patients with severe thrombocytopenia.40 Finally, in extreme cases when a patient’s count must be increased for a procedure, we have resorted to an “in vivo adsorption” strategy: If a patient has a strong antibody against HLA-A2, we repeatedly transfuse an HLA-A2 positive unit to deplete the antibody, then drip-in an A2 positive unit during the procedure.
Key question 2
What steps can the clinical team take to manage the patient’s thrombocytopenia?
Answer
The clinical team must first order 10- to 60-minute posttransfusion platelet counts on 2 sequential transfusions to confirm refractoriness. The next step is a trial of crossmatch-compatible platelets, closely monitored with 10- to 60-minute CCI. If this trial fails, then HLA-matched or HLA-compatible platelets should be tested. See Visual Abstract for greater detail.
Clinical recap
For our 60-year-old thrombocytopenic patient, the first step in management would be calculating CCIs for the next 2 successive platelet transfusions and ruling out nonimmune factors for her refractory state (Visual Abstract). Given her multiparous history, it is reasonable to assume that she has HLA antibodies; however, an initial screen for HLA/HPA antibodies can be used to confirm this.
With an immune-driven refractory state high on the differential, the next step is a limited trial of crossmatch-compatible units. Two or three crossmatch-compatible platelet transfusions with 10- to 60-minute CCI can indicate the success or failure of this strategy. If the CCI improves, then continuing with crossmatch-compatible units is the best way to manage her thrombocytopenia. However, if the patient continues to have poor posttransfusion increments, then switching to an HLA matching strategy is indicated. For this patient, an HLA genotype and single-bead assay for class I HLA antibodies must be ordered. The chance of finding several units that are a perfect match for the patient is unlikely; therefore, a mixed strategy of HLA matching and antibody avoidance is probably necessary.
To keep things moving quickly, we order the required HLA tests if the first crossmatch unit fails to increase the CCI. We may find a few perfect 4/4 matches, but usually we turn to the ASP method to identify additional compatible units. If the patient is highly alloimmunized and we cannot find fully compatible units, we work closely with the HLA laboratory to decide which antibodies to honor. A 10- to 60-minute CCI should be obtained with the transfusion of each HLA-matched/compatible unit. For some patients, an extended trial of HLA-selected units does little to improve the CCI. In these cases, we return to transfusing random units, because obtaining HLA-matched units is not worth the time and expense. In most cases, however, the platelet count begins to rebound when either engraftment occurs or chemotherapy abates.
Conflict-of-interest disclosure
The author has received honoraria from Octapharma and Terumo Corporation. No other conflicts of interest to disclose.
Off-label drug use
None disclosed.
Correspondence
Claudia S. Cohn, University of Minnesota, D242 Mayo Building, MMC 609, 420 Delaware St, Minneapolis, MN, 55455; email: cscohn@umn.edu.