Abstract
Heavy menstrual bleeding (HMB) is a common complication of anticoagulation, affecting ∼70% of menstruating women receiving oral anticoagulants. The risk of HMB is lower with apixaban and/or dabigatran than with rivaroxaban. HMB can result in iron deficiency with or without anemia, increased need for medical interventions, decreased quality of life, and missed school/work. Mainstays of treatment include hormone therapies such as the levonorgestrel intrauterine system, subdermal implant, and other progesterone-based therapies, which can result in decreased blood loss and, in some cases, amenorrhea. Combined hormone therapies can be used while patients continue receiving anticoagulation and are also highly effective for decreasing menstrual blood loss. Rarely, procedure-based interventions such as endometrial ablation may be required. Patients should be evaluated for iron deficiency and anemia and offered supportive therapies as needed. Abbreviating the course of anticoagulation or skipping doses can increase the risk of recurrent venous thromboembolism by as much as fivefold, but switching oral anticoagulants may be considered. Awareness of HMB and careful history taking at each visit are crucial to avoid a missed diagnosis.
Learning Objectives
Understand the risks, signs/symptoms, and consequences of heavy menstrual bleeding in women treated with various oral anticoagulants
Review options for the treatment of heavy menstrual bleeding in the anticoagulated woman
Clinical case
A 27-year-old woman presented to a clinic with fatigue and decreased exercise tolerance. Her past medical history was remarkable for deep vein thrombosis (DVT) of the left lower extremity, which had been diagnosed ∼2 months ago in the setting of oral contraceptive pill (OCP) use. She was undergoing treatment with rivaroxaban 20 mg daily and had discontinued the OCPs upon diagnosis of her DVT. She denied chest pain, dyspnea at rest, and lightheadedness. The symptoms of her DVT, lower extremity pain, and swelling resolved within 2 weeks of initiating therapy. She denied epistaxis, melena, hematochezia, or other bleeding but described her menses as “very heavy.” Since discontinuing her OCPs and initiating rivaroxaban therapy, her periods had been lasting 10 to 14 days, and, because of a need to change a pad or tampon every 30 minutes, she was now wearing both at once and changing protection every 45 minutes on her heaviest day. On further questioning, she also reported passing multiple clots up to 1.5 inches in diameter each cycle. A complete blood count (CBC) revealed that her hemoglobin concentration was 8.0 g/dL, her mean corpuscular volume was 72.0 fL, and her other parameters were normal. At the time of her DVT diagnosis, her hemoglobin was 10.8 g/dL, and her mean corpuscular volume was 81.0 fL.
Diagnosing heavy menstrual bleeding
Heavy menstrual bleeding (HMB) is defined as menstrual blood loss (MBL) of >80 mL per cycle.1 For research purposes, the Pictorial Blood Loss Assessment Chart, a chart with which patients can report the number of hygiene products used and the degree of saturation throughout a menstrual cycle, is a commonly used and accurate diagnostic tool with a sensitivity and specificity of >80% for scores >100.2 Although this tool is also available in the clinical setting, administration may not be practical, and therefore many clinicians must rely on a snapshot history and physical examination in combination with laboratory findings.
The authors of the landmark study Menorrhagia 1 identified 3 key predictors of MBL >80 mL/cycle that can be captured in a single visit. These include patient-reported passing of clots >1 inch in diameter, low ferritin, and need to change protection more often than hourly during the heaviest days of menses.3 Subject description of her menses as “very heavy” as opposed to “moderate” or “heavy” was significantly associated with increased MBL (64 mL vs 40 mL; P < .001), suggesting that patient reporting is more accurate than previously considered. Additional factors associated with increased mean MBL are listed in Table 1. Ultimately, there is no gold standard outside of measuring blood loss and/or using the Pictorial Blood Loss Assessment Chart, and thus a combination of all these factors and clinical judgment are required. It is also important to note that, in addition to medical outcomes such as iron deficiency, HMB is associated with decreased quality of life and missed time from work and school,4 which impact overall well-being.
Clinical and laboratory features associated with heavy menstrual bleeding and/or increased menstrual blood loss (MBL)
| Clinical and laboratory features . | Odds ratio (95% CI) or P value . |
|---|---|
| Features associated with MBL >80 mL3 | |
| Finding | |
| Changing protection more often than hourly | 3.08 (1.4-68) |
| Clots >1.1 inch in diameter | 4.80 (1.9-12.2) |
| Low ferritin | 5.71 (1.9-17.4) |
| Features associated with increased mean MBL3 | |
| Finding | |
| Subjective report of “very heavy” periods | <.001 |
| Hemoglobin <12.0 g/dL | .002 |
| Need to change protection during thenight | <.001 |
| Leaking through protection | <.001 |
| Need to wear double protection | <.001 |
| Clinical and laboratory features . | Odds ratio (95% CI) or P value . |
|---|---|
| Features associated with MBL >80 mL3 | |
| Finding | |
| Changing protection more often than hourly | 3.08 (1.4-68) |
| Clots >1.1 inch in diameter | 4.80 (1.9-12.2) |
| Low ferritin | 5.71 (1.9-17.4) |
| Features associated with increased mean MBL3 | |
| Finding | |
| Subjective report of “very heavy” periods | <.001 |
| Hemoglobin <12.0 g/dL | .002 |
| Need to change protection during thenight | <.001 |
| Leaking through protection | <.001 |
| Need to wear double protection | <.001 |
CI, confidence interval.
HMB while receiving anticoagulation
Up to one-third of women will meet criteria for HMB at some point in their lifetime. For women receiving anticoagulation, the risk of HMB or otherwise abnormal uterine bleeding (AUB) increases to ∼70% and varies on the basis of choice of anticoagulant,5 although comparisons between agents can be challenging because of inconsistency in outcome definitions throughout the literature (Table 2). Standard major bleeding (MB) and clinically relevant nonmajor bleeding (CRNMB) definitions, used almost exclusively in large trials of direct oral anticoagulants, are problematic because they fail to account for the chronic and recurrent nature of HMB. Although patients with HMB rarely require transfusions or experience a rapid decline in hemoglobin, they often develop iron deficiency, sometimes severe, over the course of multiple cycles and may hesitate to seek needed medical attention. Subsequently, true HMB is underreported and underappreciated in virtually all trials of anticoagulants.
Definitions of terms used to capture heavy or otherwise abnormal menstrual bleeding in the literature
| Term . | Definition . |
|---|---|
| Heavy menstrual bleeding (HMB)/menorrhagia | Menstrual blood loss (MBL) of >80 mL/cycle or Pictorial Blood Loss Assessment Chart score >100 |
| Abnormal uterine bleeding (AUB) | Menstrual bleeding of abnormal quantity, duration, or schedule. Includes heavy bleeding; excessively frequent, infrequent, or irregular bleeding; and intermenstrual bleeding. |
| Clinically relevant non-major bleeding (CRMNB) | Uterine bleeding that requires medical intervention by a health care professional, leads to hospitalization or increased level of care, or prompts a face-to-face evaluation |
| Major bleeding (MB) | Uterine bleeding that is fatal or causes a fall in hemoglobin level of ≥20 g/L (≥2 g/dL) or leading to transfusion of ≥2 units of whole blood or red cells |
| Term . | Definition . |
|---|---|
| Heavy menstrual bleeding (HMB)/menorrhagia | Menstrual blood loss (MBL) of >80 mL/cycle or Pictorial Blood Loss Assessment Chart score >100 |
| Abnormal uterine bleeding (AUB) | Menstrual bleeding of abnormal quantity, duration, or schedule. Includes heavy bleeding; excessively frequent, infrequent, or irregular bleeding; and intermenstrual bleeding. |
| Clinically relevant non-major bleeding (CRMNB) | Uterine bleeding that requires medical intervention by a health care professional, leads to hospitalization or increased level of care, or prompts a face-to-face evaluation |
| Major bleeding (MB) | Uterine bleeding that is fatal or causes a fall in hemoglobin level of ≥20 g/L (≥2 g/dL) or leading to transfusion of ≥2 units of whole blood or red cells |
Vitamin K antagonists
When evaluating specifically for AUB and/or HMB, rates as high as 67% are reported among subjects using vitamin K antagonists such as warfarin.5 Other focused studies report somewhat lower but still impressive rates ranging from 18% to 39%.6,7 Large trials using MB and CRNMB criteria, however, report much lower rates, consistently <10%,8,9 suggesting that most HMB goes unreported and undiagnosed in the anticoagulated population.
Anti-Xa agents
Analysis of data from the large registry trials of each of the 3 oral anti-Xa agents has demonstrated a greater than a twofold increased risk of uterine MB or CRNMB in female subjects of all ages using rivaroxaban as compared with warfarin and low-molecular-weight heparin. Users of apixaban were no more likely to meet such criteria than users of warfarin/low-molecular-weight heparin. Data from registry trials of edoxaban, as reported in product monographs, demonstrated uterine MB and CRNMB rates similar to those seen with rivaroxaban (9%). Subjects receiving warfarin in these studies, however, reported higher rates of uterine bleeding events than those seen in trials of rivaroxaban or apixaban, ultimately resulting in a relative risk of 1.26 for all-age female users of edoxaban as compared with warfarin (Table 3).8
Relative risk of heavy menstrual bleeding by choice of oral anticoagulant in women aged ≥18 years
| OAC . | Incidence of uterine CRNMB/MB . | Relative risk . |
|---|---|---|
| Warfarin | 4.5%-9.6% | Reference |
| Apixaban | 5.4% | 1.18 |
| Edoxaban | 9.0% | 1.26 |
| Rivaroxaban | 9.5% | 2.10* |
| Dabigatran | 4.7% | 0.53* |
| OAC . | Incidence of uterine CRNMB/MB . | Relative risk . |
|---|---|---|
| Warfarin | 4.5%-9.6% | Reference |
| Apixaban | 5.4% | 1.18 |
| Edoxaban | 9.0% | 1.26 |
| Rivaroxaban | 9.5% | 2.10* |
| Dabigatran | 4.7% | 0.53* |
OAC, oral anticoagulant.
Statistically significant, P < 0.01.
Although additional data on uterine bleeding events in these registry trials have been published in the form of post hoc analyses, comparison is challenging because of inclusion of different populations (based on age group or menopausal status) in each analysis. Post hoc analysis of the Hokusai-VTE study,10 when excluding subjects >50 years of age, reported an incidence of 15 cases of uterine MB or CRNMB per 100 person-years (95% confidence interval [CI], 11-19), translating to a 1.7-fold (95% CI, 1.1-2.5) increased risk with edoxaban as compared with warfarin.11 Post hoc analysis of a slightly older (≤60 years of age) group of women included in the EINSTEIN trials demonstrated a hazard ratio of 2.13 (95% CI, 1.57-2.89) for uterine bleeding events in users of rivaroxaban vs warfarin. Incidence densities of uterine bleeding events were 28.9% or 30.7%/year for users of rivaroxaban and 15.5% or 13.4%/year for users of warfarin who were or were not receiving hormone therapy, respectively.12 Age-specific rates of uterine MB or CRNMB events from the AMPLIFY studies were not published, although one post hoc analysis reported the odds ratio of a combined outcome of intermenstrual bleeding, HMB, and anemia to be 1.3 (95% CI, 0.2-7.3) for premenopausal women using apixaban vs warfarin.13
Studies using actual AUB and HMB definitions are much rarer, but rates from 20% to 73%5,14 have been reported with rivaroxaban. Women using rivaroxaban have also been demonstrated to experience more prolonged menses and to be more likely to require medical or surgical intervention, unscheduled contact with a medical provider, and/or modification of anticoagulation for menstrual bleeding.5 Furthermore, HMB in users of rivaroxaban resulted in an increased risk of recurrent venous thromboembolism (VTE) as high as fivefold, potentially because of increased rates of modification of anticoagulation, including abbreviated courses and missed doses.6 One observational study of women receiving apixaban reported the incidence of true HMB to be 9.3%,15 but studies using such definitions in users of edoxaban are lacking.
Dabigatran
Post hoc analysis of the RE-COVER and RE-MEDY studies suggested that dabigatran carries a lower risk of uterine MB and CRNMB (4.7%) than warfarin, although AUB occurred more frequently in the warfarin arm than in any other study (9.6%).
Evaluation for HMB while receiving anticoagulation
Observational data suggest that HMB is underrecognized in anticoagulated women, in part because of an absence of discussion of symptoms. The first assessment for HMB should be conducted at the time of initial oral anticoagulant prescription and should include both current and past symptoms, particularly for women whose VTE occurred in the setting of use of OCPs or other hormone agents that may have temporarily improved symptoms. Women who report symptoms consistent with HMB or increased MBL (Table 1) should undergo a CBC and a ferritin check. For women who report additional bleeding symptoms beyond HMB, completion of the International Society on Thrombosis and Haemostasis/Scientific and Standardization Committee Bleeding Assessment Tool followed by workup for any potential underlying bleeding disorders, such as von Willebrand disease, should be considered.
Patients who do not immediately report symptoms of HMB should be educated on the signs of it and instructed to notify the prescriber if these symptoms develop. Patients should be asked about symptoms again at subsequent evaluations, at least annually for those women receiving long-term anticoagulation, keeping in mind that HMB can develop at any point, particularly during perimenopausal years, when bleeding becomes more irregular and can be heavier. Periodic laboratory monitoring, including CBC and ferritin checks, is also appropriate. Ongoing and open dialogue is critical because patients may be hesitant to report symptoms promptly due to embarrassment or the belief that nothing can be done to manage them. Once HMB is identified, there are many options for management.
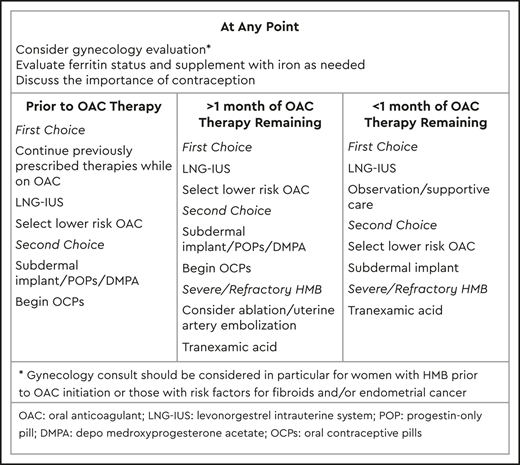
Management of HMB while receiving anticoagulation
Hormone therapies
Although somewhat counterintuitive, hormone therapies are a first-line option for managing HMB in this population. In addition to being effective for the reduction of bleeding, hormone therapies provide contraception, which is particularly important in women receiving teratogenic agents, such as warfarin, and in women with a recent history of VTE due to the dramatically increased risk of recurrence with pregnancy.
The levonorgestrel intrauterine system (LNG-IUS) can be incredibly effective for management of HMB, boasting a 44% amenorrhea rate at 6 months of use, increasing to 50% by 1 year,16 as well as >99% effectiveness for prevention of pregnancy. Individuals who do not achieve amenorrhea can still be expected to experience a reduction in median MBL of 80% by 4 months, accompanied by an increase in hemoglobin of 7.8%.17 Of note, the LNG-IUS has been associated with an increased risk of ovarian cysts, particularly in the first 12 months of use.18 The subdermal implant can result in amenorrhea in ∼20% of cases, although irregular spotting is common and can be troublesome.19 The subdermal implant also has a >99% efficacy for prevention of pregnancy.
Depo-medroxyprogesterone acetate results in amenorrhea in 55% of women at 1 year and in 68% at 2 years,20 and it is >99% effective for prevention of pregnancy when used perfectly. However, it is associated with a 2.2- to threefold increased risk of VTE and therefore is not recommended in the absence of anticoagulation.21 Progestin-only pills also result in amenorrhea in 5% to 10% of women but frequently cause other menstrual irregularities22 and require precise adherence to avoid reduction in contraceptive efficacy.
Combined contraceptives, including both an estrogen and a progesterone component, are highly effective for the management of HMB. OCPs are considered by many to be the first-line treatment of HMB and provide the option of prescribing with or without scheduled interruptions in hormone exposure, allowing the potential to induce amenorrhea. Because of the increased risk of VTE associated with estrogen, many consider estrogen-containing therapies to be contraindicated in women with a history of thrombosis. However, no difference in rates of recurrent VTE were found retrospectively in a comparison of women who were and were not prescribed combined contraceptives while receiving anticoagulation,12 suggesting that, for many women, the benefits of OCPs outweigh the risks while receiving anticoagulation. High-quality prospective studies of the safety and efficacy of combined contraceptives in anticoagulated women are urgently needed. In particular, those women who develop VTE while receiving OCPs for treatment of HMB, such as the patient in our case study, will be especially vulnerable to heavy bleeding with the additive effects of anticoagulation and withdrawal of hormone therapy. Strong consideration should be given to either continuing the current hormone therapy regimen, with appropriate risk–benefit counseling, in the setting of anticoagulation or rapidly transitioning to an alternative therapy, such as a progestin-based option. OCPs should be discontinued before anticoagulation withdrawal, preferably in the setting of a transition to an alternative agent, such as the LNG-IUS. If an estrogen-based therapy is newly initiated in the setting of a recent clot, ensuring therapeutic anticoagulation is already in place before the first administration is imperative.
Procedural therapies
In extreme cases, surgical therapies such as endometrial ablation or uterine artery embolization may be considered. Such therapies should, in general, be limited to refractory bleeding in women who either require long-term anticoagulation or who have HMB even in the absence of anticoagulation. These options are also limited to women who have completed childbearing, because they either guarantee infertility or dramatically increase the odds of morbidity with future pregnancies. Therapeutic options include endometrial ablation, uterine artery embolization, and hysterectomy. Approximately 87% of women undergoing endometrial ablation for HMB will perceive improvement in symptoms at 1 year, although 12% will require further surgery for HMB.23 Although uterine artery embolization is typically limited to women with fibroids, women who meet this qualification may enjoy up to a 90% reduction in MBL.24 Hysterectomy, a true permanent solution, requires discontinuation of anticoagulation in the perioperative setting, carries an increased risk of both bleeding and VTE, and thus is a last resort.
Antifibrinolytics
Antifibrinolytics, such as tranexamic acid, have established efficacy in the management of HMB in non-anticoagulated populations, including women with bleeding disorders. Although antifibrinolytics have historically been considered to be contraindicated in women with a history of thrombosis, large studies of patients at high risk of VTE, including postpartum women,25 trauma patients,26 and patients undergoing orthopedic surgery,27 have failed to demonstrate increased incidence of VTE. One study of patients with gastrointestinal bleeding noted a very small increased risk (0.4%) of VTE with high-dose (4 g over 24 hours) intravenous tranexamic acid compared with placebo.28 The vast majority (90%) of these patients were not anticoagulated, and the difference in rates of thrombosis between treatment groups was most notable in patients with underlying cirrhosis.
No studies have been done of the combination of anticoagulants and antifibrinolytics, and, although use in the immediate post-VTE setting would be inadvisable because of the desire for ongoing fibrinolysis of the thrombosis, the benefit may outweigh the risks in specific situations, particularly if it enables continuation of anticoagulation without interruptions. Antifibrinolytic use was reported in a few subjects in the EINSTEIN DVT and pulmonary embolism cohorts, although not in association with VTE outcomes.12 As with use for HMB in other circumstances, antifibrinolytics should be prescribed during only the heaviest days of the cycle.
Modification of anticoagulation
In the setting of HMB, temporary or early discontinuation of anticoagulation may be tempting. However, because this has been shown to result in increased risk of recurrent VTE,6 alternative approaches are strongly recommended. Although high-quality data, such as randomized controlled trials, comparing HMB between agents are lacking, a preponderance of observational data seem to suggest increased rates of HMB with rivaroxaban. Therefore, consideration of alternative agents, such as dabigatran or apixaban, in women at high risk for, or perhaps even those who experience, HMB while receiving anticoagulation is reasonable in addition to the above recommended approaches. Data on temporary dose reduction (eg, to prophylactic doses) during menses are lacking, and this approach is not currently recommended outside of the research setting.
Adjunctive therapies
An important but often forgotten aspect of the care of patients with HMB is treatment of iron deficiency anemia. All women reporting HMB should be tested with a CBC and a ferritin level measurement. Iron therapy, either oral or intravenous, should be administered as necessary.
Gynecological evaluation
Patients receiving anticoagulation are vulnerable to all the same gynecological causes of bleeding as non-anticoagulated patients, and therefore the possibility of underlying causes beyond anticoagulation must be considered. Postmenopausal bleeding is worrisome for a potential diagnosis of endometrial cancer, and periods that are both heavy and painful can be indicative of other problems, such as fibroids or endometriosis, which may improve with targeted management strategies offered by gynecologists.
Clinical case continued
On the basis of her reported history of changing protection more frequently than hourly, passing clots >1 inch, and reported “very heavy” bleeding, our patient can be diagnosed with HMB. Management options, including hormone therapies and switching from rivaroxaban to an alternative agent, should be offered. Because this patient is likely to discontinue anticoagulation in 1 month, progesterone-based therapies, in particular the LNG-IUS, are preferred to OCPs, although continuing OCPs at the time of diagnosis and later transitioning to a progesterone-based therapy may have reduced the severity of the clinical picture. This patient almost certainly has iron deficiency and, after a ferritin check, should be offered iron therapy. She should also be counseled on the importance of using effective contraception, regardless of menstrual management strategy, until and unless pregnancy is desired, in which case preconception counseling by a hematologist and/or obstetrician experienced with the use of anticoagulation in pregnancy is strongly advised.
In summary, an ideal approach to HMB while receiving anticoagulation includes addressing it at the time of anticoagulation initiation, counseling patients about the fact that they may experience new or worsened HMB, and asking specifically about menstrual bleeding at subsequent visits. A key aspect of prevention and management is having detailed risk–benefit discussions about choice of anticoagulant (rivaroxaban vs other) and the potential to continue estrogen-based therapies or start progestin-only therapy for the management of HMB. In general, hormone therapy is the most effective strategy to manage HMB while allowing continued anticoagulation and is the one I most often recommend.
Correspondence
Bethany Samuelson Bannow, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, OC14HO, Portland, OR 97239; e-mail: samuelsb@ohsu.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug disclosure: None disclosed.